Wheat dwarf disease (WDD) is an important disease of monocotyledonous species, including economically important cereals. The causative pathogen, wheat dwarf virus (WDV), is persistently transmitted mainly by the leafhopper Psammotettix alienus and can lead to high yield losses. Due to climate change, the periods of vector activity increased, and the vectors have spread to new habitats, leading to an increased importance of WDV in large parts of Europe. In the light of integrated pest management, cultivation practices and the use of resistant/tolerant host plants are currently the only effective methods to control WDV.
- wheat dwarf virus
- WDV
- resistance
- mastrevirus
1. Introduction
2. Wheat Dwarf Virus (WDV)
2.1. Classification and Genomic Organization of WDV
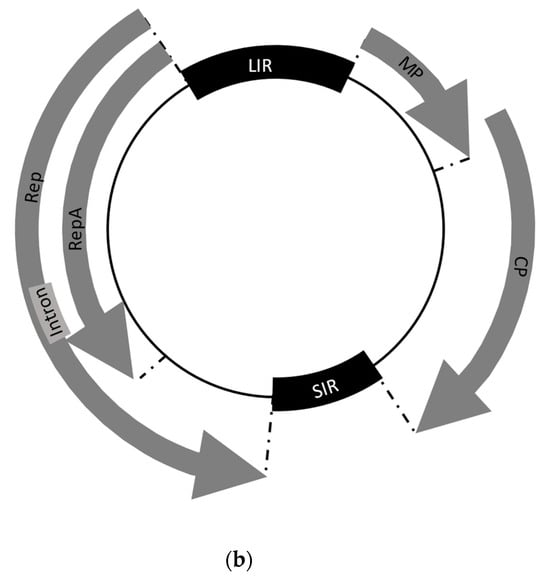
2.2. Life Cycle of the Virus
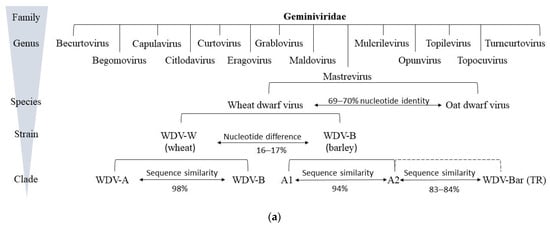
2.3. Phylogenetics
3. Wheat Dwarf Disease (WDD)
3.1. History
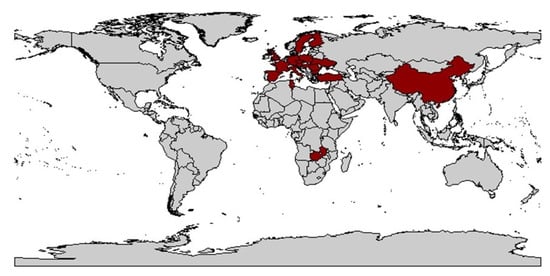
3.2. Host Range
3.3. Symptoms of WDD

This entry is adapted from the peer-reviewed paper 10.3390/plants12203633
References
- Buck, K.W. Geminiviruses (Geminiviridae). In Encyclopedia of Virology; Granoff, A., Webster, R.G., Eds.; Academic Press: Cambridge, MA, USA, 1999; pp. 597–606.
- Canto, T.; Aranda, M.A.; Fereres, A. Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran-borne plant viruses. Glob. Chang. Biol. 2009, 15, 1884–1894.
- Habekuß, A.; Riedel, C.; Schliephake, E.; Ordon, F. Breeding for resistance to insect-transmitted viruses in barley—An emerging challenge due to global warming. J. Für Kult. 2009, 61, 53–61.
- Roos, J.; Hopkins, R.; Kvarnheden, A.; Dixelius, C. The impact of global warming on plant diseases and insect vectors in Sweden. Eur. J. Plant Pathol. 2011, 129, 9–19.
- Ziesche, T.M.; Bell, J.; Ordon, F.; Schliephake, E.; Will, T. Long-term monitoring of insects in agricultural landscapes. Mitteilungen Der DGaaE 2020, 22, 101–106.
- Barnett, O.W.; Main, C.E. Plant Virus Disease—Economic Aspects. In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1318–1326.
- Waterworth, H.E.; Hadidi, A. Economic Losses due to Plant Viruses. In Plant Virus Disease control; APS Press: St. Paul, MN, USA, 1998.
- Fraser, R.S.S. Plant Resistance to Viruses|Natural Resistance. In Encyclopedia of Virology, 2nd ed.; Granoff, A., Webster, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1300–1307.
- van Regenmortel, M.H.; Fauquet, C.M.; Bishop, D.H.; Carstens, E.B.; Estes, M.K.; Lemon, S.M.; Maniloff, J.; Mayo, M.A.; McGeoch, D.J.; Pringle, C.R.; et al. Virus Taxonomy: Classification and Nomenclature of Viruses. In Seventh Report of the International Committee on Taxonomy of Viruses; Academic Press: New York, NY, USA, 2000.
- Vacke, J. Wheat dwarf virus disease. Biol. Plant 1961, 3, 228–233.
- Mehner, S.; Manurung, B.; Gruntzig, M.; Habekuss, A.; Witsack, W.; Fuchs, E. Investigations into the ecology of the Wheat dwarf virus (WDV) in Saxony-Anhalt, Germany. J. Plant Dis. Prot. 2003, 110, 313–323.
- Manurung, B.; Witsack, W.; Mehner, S.; Gruntzig, M.; Fuchs, E. Studies on biology and population dynamics of the leafhopper Psammotettix alienus Dahlb. (Homoptera: Auchenorrhyncha) as vector of Wheat dwarf virus (WDV) in Saxony-Anhalt, Germany. J. Plant Dis. Prot. 2005, 112, 497–507.
- Vacke, J. Host plants range and symptoms of wheat dwarf virus. Věd Pr Výz Ust. Rostl Výroby Praha-Ruzyně 1972, 17, 151–162.
- MacDowell, S.W.; Macdonald, H.; Hamilton, W.D.O.; Coutts, R.H.A.; Buck, K.W. The nucleotide sequence of cloned wheat dwarf virus DNA. EMBO J. 1985, 4, 2173–2180.
- Macdonald, H.; Coutts, R.H.A.; Buck, K.W. Characterization of a Subgenomic DNA Isolated from Triticum Aestivum Plants Infected with Wheat Dwarf. J. Gen. Virol. 1988, 69, 1339–1344.
- Schalk, H.J.; Matzeit, V.; Schiller, B.; Schell, J.; Gronenborn, B. Wheat dwarf virus, a geminivirus of graminaceous plants needs splicing for replication. EMBO J. 1989, 8, 359–364.
- Lindblad, M.; Waern, P. Correlation of wheat dwarf incidence to winter wheat cultivation practices. Agric. Ecosyst. Environ. 2002, 92, 115–122.
- Lemmetty, A.; Huusela-Veistola, E. First Report of Wheat dwarf virus in Winter Wheat in Finland. Plant Dis. 2005, 89, 912.
- Lindsten, K.; Lindsten, B.; Abdelmoeti, M.; Junti, N. Purification and some properties of wheat dwarf virus. In Proceedings of the 3rd Conference on Virus Diseases of Gramineae in Europe, Rothamsted, UK, 28–30 May 1980; pp. 27–31.
- Fauquet, C.M.; Briddon, R.W.; Brown, J.K.; Moriones, E.; Stanley, J.; Zerbini, M.; Zhou, X. Geminivirus strain demarcation and nomenclature. Arch. Virol. 2008, 153, 783–821.
- Bernardo, P.; Golden, M.; Akram, M.; Naimuddin, N.N.; Fernandez, E.; Granier, M.; Rebelo, A.G.; Peterschmitt, M.; Martin, D.P.; Roumagnac, P. Identification and characterisation of a highly divergent geminivirus: Evolutionary and taxonomic implications. Virus Res. 2013, 177, 35–45.
- Varsani, A.; Navas-Castillo, J.; Moriones, E.; Hernández-Zepeda, C.; Idris, A.; Brown, J.K.; Murilo Zerbini, F.; Martin, D.P. Establishment of three new genera in the family Geminiviridae: Becurtovirus, Eragrovirus and Turncurtovirus. Arch. Virol. 2014, 159, 2193–2203.
- Agrios, G.N. Plant Pathology, 3rd ed.; Academic Press: New York, NY, USA, 1988; pp. 3–39.
- Matzeit, V. Wheat Dwarf Virus—Ein Geminivirus Monokotyledoner Pflanzen-DNA-Sequenz, Replikation und Einsatz Seines Genoms zur Amplifikation und Expression Fremder Gene. Ph.D. Thesis, Universität zu Köln, Köln, Germany, 1988.
- Zhang, W.; Olson, N.H.; Baker, T.S.; Faulkner, L.; Agbandje-McKenna, M.; Boulton, M.I.; Davies, J.W.; McKenna, R. Structure of the Maize Streak Virus Geminate Particle. Virology 2001, 279, 471–477.
- Boulton, M.I. Functions and interactions of mastrevirus gene products. Physiol. Mol. Plant Pathol. 2002, 60, 243–255.
- Drews, G.; Adam, G.; Heinze, C. Molekulare Pflanzenvirologie; Springer: Berlin/Heidelberg, Germany, 2004.
- Adejare, G.O.; Coutts, R.H.A. The Isolation and Characterisation of a Virus from Nigerian Cassava Plants Affected by the Cassava Mosaic Disease, and Attempted Transmission of the Disease. J. Phytopathol. 1982, 103, 198–210.
- Harrison, B.D. Advances in Geminivirus Research. Annu. Rev. Phytopathol. 1985, 23, 55–82.
- Damsteegt, V.D.; Igwegbe, E.C.K. Epidemiology and Control of Maize streak disease. In Plant Virus Disease Control; APS Press: St. Paul, MN, USA, 1998; pp. 484–494.
- Moffat, A.S. Geminiviruses Emerge as Serious Crop Threat. Science 1999, 286446, 1835.
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717.
- Fiallo-Olivé, E.; Lett, J.-M.; Martin, D.P.; Roumagnac, P.; Varsani, A.; Zerbini, F.M.; Navas-Castillo, J. ICTV Virus Taxonomy Profile: Geminiviridae 2021. J. Gen. Virol. 2021, 1022, 001696.
- Family: Geminiviridae. Available online: https://ictv.global/report/chapter/geminiviridae/geminiviridae (accessed on 12 November 2022).
- Fauquet, C.M.; Bisaro, D.M.; Briddon, R.W.; Brown, J.K.; Harrison, B.D.; Rybicki, E.P.; Stenger, D.C.; Stanley, J. Virology division news: Revision of taxonomic criteria for species demarcation in the family Geminiviridae, and an updated list of begomovirus species. Arch. Virol. 2003, 148, 405–420.
- Muhire, B.; Martin, D.P.; Brown, J.K.; Navas-Castillo, J.; Moriones, E.; Zerbini, F.M.; Rivera-Bustamante, R.; Malathi, V.G.; Briddon, R.W.; Varsani, A. A Genome-Wide Pairwise-Identity-Based Proposal for the Classification of Viruses in the Genus Mastrevirus (Family Geminiviridae). Arch. Virol. 2013, 158, 1411–1424.
- Candresse, T.; Filloux, D.; Muhire, B.; Julian, C.; Galzi, S.; Fort, G.; Bernardo, P.; Daugrois, J.H.; Fernandez, E.; Martin, D.P.; et al. Appearances can be deceptive: Revealing a hidden viral infection with deep sequencing in a plant quarantine context. PLoS ONE 2014, 9, e102945.
- NCBI Virus. Available online: https://www.ncbi.nlm.nih.gov/labs/virus/vssi/#/virus?SeqType_s=Nucleotide&VirusLineage_ss=Mastrevirus,%20taxid:11212 (accessed on 22 September 2023).
- Morris, B.A.M.; Richardson, K.A.; Haley, A.; Zhan, X.; Thomas, J.E. The nucleotide sequence of the infectious cloned DNA component of tobacco yellow dwarf virus reveals features of geminiviruses infecting monocotyledonous plants. Virology 1992, 187, 633–642.
- Gutierrez, C. Geminivirus DNA replication. Mol. Life Sci. CMLS 1999, 56, 313–329.
- Thomas, J.E.; Parry, J.N.; Schwinghamer, M.W.; Dann, E.K. Two novel mastreviruses from chickpea (Cicer arietinum) in Australia. Arch. Virol. 2010, 155, 1777–1788.
- Zerbini, F.M.; Briddon, R.W.; Idris, A.; Martin, D.P.; Moriones, E.; Navas-Castillo, J.; Rivera-Bustamante, R.; Roumagnac, P.; Varsani, A. ICTV Virus Taxonomy Profile: Geminiviridae. J. Gen. Virol. 2017, 98, 131–133.
- Gafni, Y.; Epel, B.L. The role of host and viral proteins in intra- and inter-cellular trafficking of geminiviruses. Physiol. Mol. Plant Pathol. 2002, 60, 231–241.
- Ramsell, J.N.E. Genetic Variability of Wheat Dwarf Virus. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2007.
- Woolston, C.J.; Barker, R.; Gunn, H.; Boulton, M.I.; Mullineaux, P.M. Agroinfection and nucleotide sequence of cloned wheat dwarf virus DNA. Plant Mol. Biol. 1988, 11, 35–43.
- Bendahmane, M.; Schalk, H.J.; Gronenborn, B. Identification and characterization of wheat dwarf virus from France using a rapid method for geminivirus DNA preparation. Phytopathology 1995, 851, 1449–1455.
- Dickinson, V.J.; Halder, J.; Woolston, C.J. The Product of Maize Streak Virus ORF V1 Is Associated with Secondary Plasmodesmata and Is First Detected with the Onset of Viral Lesions. Virology 1996, 220, 51–59.
- Gutierrez, C. Geminiviruses and the plant cell cycle. Plant Mol. Biol. 2000, 43, 763–772.
- Gutierrez, C. DNA replication and cell cycle in plants: Learning from geminiviruses. EMBO J. 2000, 19, 792–799.
- Gutierrez, C.; Ramirez-Parra, E.; Mar Castellano, M.; Sanz-Burgos, A.P.; Luque, A.; Missich, R. Geminivirus DNA replication and cell cycle interactions. Vet. Microbiol. 2004, 98, 111–119.
- Rojas, M.R.; Hagen, C.; Lucas, W.J.; Gilbertson, R.L. Exploiting chinks in the plant’s armor: Evolution and emergence of geminiviruses. Annu. Rev. Phytopathol. 2005, 43, 361–394.
- Briddon, R.W.; Martin, D.P.; Owor, B.E.; Donaldson, L.; Markham, P.G.; Greber, R.S.; Varsani, A. A novel species of mastrevirus (family Geminiviridae) isolated from Digitaria didactyla grass from Australia. Arch. Virol. 2010, 155, 1529–1534.
- Hofer, J.M.I.; Dekker, E.L.; Reynolds, H.V.; Woolston, C.J.; Cox, B.S.; Mullineaux, P.M. Coordinate Regulation of Replication and Virion Sense Gene Expression in Wheat Dwarf Virus. Plant Cell 1992, 4, 213–223.
- Morris-Krsinich, B.A.M.; Mullineaux, P.M.; Donson, J.; Boulton, M.I.; Markham, P.G.; Short, M.N.; Davies, J.W. Bidirectional transcription of maize streak virus DNA and identification of the coat protein gene. Nucleic Acids Res. 1985, 130, 7237–7256.
- Dekker, E.L.; Woolston, C.J.; Xue, Y.; Cox, B.; Mullineaux, P.M. Transcript mapping reveals different expression strategies for the bicistronic RNAs of the geminivirus wheat dwarf virus. Nucleic Acids Res. 1991, 195, 4075–4081.
- Fenoll, C.; Black, D.M.; Howell, S.H. The intergenic region of maize streak virus contains promoter elements involved in rightward transcription of the viral genome. EMBO J. 1988, 7, 1589–1596.
- Accotto, G.P.; Donson, J.; Mullineaux, P.M. Mapping of Digitaria streak virus transcripts reveals different RNA species from the same transcription unit. EMBO J. 1989, 8, 1033–1039.
- Mullineaux, P.M.; Guerineau, F.; Accotto, G.-P. Processing of complementary sense RNAs of Digitariastreak virus in its host and in transgenic tobacco. Nucleic Acids Res. 1990, 184, 7259–7265.
- Wright, E.A.; Heckel, T.; Groenendijk, J.; Davies, J.W.; Boulton, M.I. Splicing features in maize streak virus virion- and complementary-sense gene expression. Plant J. 1997, 12, 1285–1297.
- Palmer, K.E.; Rybicki, E.P. The Molecular Biology of Mastreviruses. Adv. Virus Res. 1998, 50, 183–234.
- Wang, Y.; Mao, Q.; Liu, W.; Mar, T.; Wei, T.; Liu, Y.; Wang, X. Localization and Distribution of Wheat dwarf virus in Its Vector Leafhopper, Psammotettix alienus. Phytopathology 2014, 104, 897–904.
- Noueiry, A.O.; Lucas, W.J.; Gilbertson, R.L. Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell 1994, 76, 925–932.
- Liu, H.; Boulton, M.I.; Oparka, K.J.; Davies, J.W. Interaction of the movement and coat proteins of Maize streak virus: Implications for the transport of viral DNA. J. Gen. Virol. 2001, 82, 35–44.
- Liu, H.; Andrew, L.P.; Davies, J.W.; Boulton, M.I. A single amino acid change in the coat protein of Maize streak virus abolishes systemic infection, but not interaction with viral DNA or movement protein. Mol. Plant Pathol. 2001, 2, 223–228.
- Noris, E.; Vaira, A.M.; Caciagli, P.; Masenga, V.; Gronenborn, B.; Accotto, G.P. Amino Acids in the Capsid Protein of Tomato Yellow Leaf Curl Virus That Are Crucial for Systemic Infection, Particle Formation, and Insect Transmission. J. Virol. 1998, 722, 10050–10057.
- Liu, H.; Boulton, M.I.; Thomas, C.L.; Prior, D.A.M.; Oparka, K.J.; Davies, J.W. Maize Streak Virus Coat Protein Is Karyophyllic and Facilitates Nuclear Transport of Viral DNA. Mol. Plant Microb. Interact. 1999, 120, 894–900.
- Kotlizky, G.; Boulton, M.I.; Pitaksutheepong, C.; Davies, J.W.; Epel, B.L. Intracellular and Intercellular Movement of Maize Streak Geminivirus V1 and V2 Proteins Transiently Expressed as Green Fluorescent Protein Fusions. Virology 2000, 274, 32–38.
- Sunter, G.; Bisaro, D.M. Transactivation of Geminivirus AR1 and BR1 Gene Expression by the Viral AL2 Gene Product Occurs at the Level of Transcription. Plant Cell 1992, 40, 1321–1331.
- Hong, Y.; Saunders, K.; Hartley, M.R.; Stanley, J. Resistance to Geminivirus Infection by Virus-Induced Expression of Dianthin in Transgenic Plants. Virology 1996, 220, 119–127.
- Voinnet, O.; Pinto, Y.M.; Baulcombe, D.C. Suppression of gene silencing: A general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl. Acad. Sci. USA 1999, 964, 14147–14152.
- Shivaprasad, P.V.; Akbergenov, R.; Trinks, D.; Rajeswaran, R.; Veluthambi, K.; Hohn, T.; Pooggin, M.M. Promoters, Transcripts, and Regulatory Proteins of Mungbean Yellow Mosaic Geminivirus. J. Virol. 2005, 793, 8149–8163.
- Trinks, D.; Rajeswaran, R.; Shivaprasad, P.V.; Akbergenov, R.; Oakeley, E.J.; Veluthambi, K.; Hohn, T.; Pooggin, M.M. Suppression of RNA Silencing by a Geminivirus Nuclear Protein, AC2, Correlates with Transactivation of Host Genes. J. Virol. 2005, 79, 2517–2527.
- Wang, H.; Buckley, K.J.; Yang, X.; Buchmann, R.C.; Bisaro, D.M. Adenosine Kinase Inhibition and Suppression of RNA Silencing by Geminivirus AL2 and L2 Proteins. J. Virol. 2005, 792, 7410–7418.
- Chowda-Reddy, R.V.; Dong, W.; Felton, C.; Ryman, D.; Ballard, K.; Fondong, V.N. Characterization of the cassava geminivirus transcription activation protein putative nuclear localization signal. Virus Res. 2009, 145, 270–278.
- Castillo-González, C.; Liu, X.; Huang, C.; Zhao, C.; Ma, Z.; Hu, T.; Sun, F.; Zhou, X.; Wang, X.J.; Zhang, X. Geminivirus-Encoded TrAP Suppressor Inhibits the Histone Methyltransferase SUVH4/KYP to Counter Host Defense. eLife 2015, 4, e06671.
- Kumar, V.; Mishra, S.K.; Rahman, J.; Taneja, J.; Sundaresan, G.; Mishra, N.S.; Mukherjee, S.K. Mungbean yellow mosaic Indian virus encoded AC2 protein suppresses RNA silencing by inhibiting Arabidopsis RDR6 and AGO1 activities. Virology 2015, 486, 158–172.
- Kvarnheden, A.; Lindblad, M.; Lindsten, K.; Valkonen, J.P.T. Genetic diversity of Wheat dwarf virus. Arch. Virol. 2002, 147, 205–216.
- Koch, C. Die Bestimmung der DNA-Sequenz des Geminivirus WDV-ER Genoms und Versuche zur Übertragung des Virus auf Gerste mit Agrobacterium tumefaciens. Ph.D. Thesis, Universität Köln, Köln, Germany, 1990.
- Schubert, J.; Habekuß, A.; Rabenstein, F. Investigation of differences between wheat and barley forms of Wheat dwarf virus and their distribution in host plants. Plant Prot. Sci. Prague 2003, 38, 43–48.
- Jeske, H. Geminiviruses. In TT Viruses. Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 185–226.
- Hanley-Bowdoin, L.; Bejarano, E.R.; Robertson, D.; Mansoor, S. Geminiviruses: Masters at redirecting and reprogramming plant processes. Nat. Rev. Microbiol. 2013, 111, 777–788.
- Wu, B.; Shang, X.; Schubert, J.; Habekuß, A.; Elena, S.F.; Wang, X. Global-scale computational analysis of genomic sequences reveals the recombination pattern and coevolution dynamics of cereal-infecting geminiviruses. Sci. Rep. 2015, 5, 8153.
- Van Bel, A.J.E. The phloem, a miracle of ingenuity. Plant Cell Environ. 2003, 26, 125–149.
- Kammann, M.; Schalk, H.-J.; Matzeit, V.; Schaefer, S.; Schell, J.; Gronenborn, B. DNA replication of wheat dwarf virus, a geminivirus, requires two cis-acting signals. Virology 1991, 184, 786–790.
- Heyraud, F.; Matzeit, V.; Schaefer, S.; Schell, J.; Gronenborn, B. The conserved nonanucleotide motif of the geminivirus stem-loop sequence promotes replicational release of virus molecules from redundant copies. Biochimie 1993, 75, 605–615.
- Laufs, J.; Jupin, I.; David, C.; Schumacher, S.; Heyraud-Nitschke, F.; Gronenborn, B. Geminivirus replication: Genetic and biochemical characterization of Rep protein function, a review. Biochimie 1995, 770, 765–773.
- Hanley-Bowdoin, L.; Settlage, S.B.; Orozco, B.M.; Nagar, S.; Robertson, D. Geminiviruses: Models for Plant DNA Replication, Transcription, and Cell Cycle Regulation. Crit. Rev. Plant Sci. 1999, 18, 71–106.
- Bosque-Pérez, N.A. Eight decades of maize streak virus research. Virus Res. 2000, 71, 107–121.
- Astier, S.; Albouy, J.; Maury, Y.; Robaglia, C.; Lecoq, H. Principles of Plant Virology: Genome, Pathogenicity, Virus Ecology; Institut National de la Recherche Agronomique: Paris, France, 2007.
- Tomenius, K.; Oxelfelt, P. Preliminary Observations of Viruslike Particles in Nuclei in Cells of Wheat Infected with the Wheat Dwarf Disease. J. Phytopathol. 1981, 101, 163–167.
- Huth, W.; Lesemann, D.-E. Nachweis des wheat dwarf virus in Deutschland. Nachrichtenblatt Dtsch. Pflanzenschutzdienstes 1994, 46, 105–106.
- Hehnle, S.; Wege, C.; Jeske, H. Interaction of DNA with the Movement Proteins of Geminiviruses Revisited. J. Virol. 2004, 784, 7698–7706.
- Evert, R.F.; Russin, W.A.; Botha, C.E.J. Distribution and frequency of plasmodesmata in relation to photoassimilate pathways and phloem loading in the barley leaf. Planta 1996, 198, 572–579.
- Aoki, N.; Scofield, G.N.; Wang, X.-D.; Patrick, J.W.; Offler, C.E.; Furbank, R.T. Expression and localisation analysis of the wheat sucrose transporter TaSUT1 in vegetative tissues. Planta 2004, 219, 176–184.
- Crawford, K.M.; Zambryski, P.C. Non-Targeted and Targeted Protein Movement through Plasmodesmata in Leaves in Different Developmental and Physiological States. Plant Physiol. 2001, 125, 1802–1812.
- Peterschmitt, M.; Quiot, J.B.; Reynaud, B.; Baudin, P. Detection of maize streak virus antigens over time in different parts of maize plants of a sensitive and a so-called tolerant cultivar by ELISA. Ann. Appl. Biol. 1992, 121, 641–653.
- Mariano, A.C.; Andrade, M.O.; Santos, A.A.; Carolino, S.M.B.; Oliveira, M.L.; Baracat-Pereira, M.C.; Brommonshenkel, S.H.; Fontes, E.P.B. Identification of a novel receptor-like protein kinase that interacts with a geminivirus nuclear shuttle protein. Virology 2004, 318, 24–31.
- Maule, A.; Leh, V.; Lederer, C. The dialogue between viruses and hosts in compatible interactions. Curr. Opin. Plant Biol. 2002, 5, 279–284.
- Plant Resistance to Geminiviruses. Available online: https://biblio.iita.org/documents/S20InbkPatilPlantNothomDev.pdf-c1c85057a36d00c1bca8600d973d2cdc.pdf (accessed on 23 September 2023).
- Mehner, S. Zur Ökologie des Wheat Dwarf Virus (WDV) in Sachsen-Anhalt. Ph.D. Thesis, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany, 2005.
- Commandeur, U.; Huth, W. Differentiation of strains of Wheat dwarf virus in infected wheat and barley plants by means of polymerase chain reaction. J. Plant Dis. Prot. 1999, 106, 550–552.
- Lindsten, K.; Vacke, J. A possible barley adapted strain of wheat dwarf virus (WDV). Acta Phytopathol. Entomol. Hung. 1991, 26, 175–180.
- Schubert, J.; Habekuß, A.; Kazmaier, K.; Jeske, H. Surveying cereal-infecting geminiviruses in Germany—Diagnostics and direct sequencing using rolling circle amplification. Virus Res. 2007, 127, 61–70.
- Wu, B.; Melcher, U.; Guo, X.; Wang, X.; Fan, L.; Zhou, G. Assessment of codivergence of Mastreviruses with their plant hosts. BMC Evol. Biol. 2008, 8, 335.
- Mishchenko, L.T.; Dunich, A.A.; Mishchenko, I.A.; Dashchenko, A.V.; Kozub, N.O.; Kyslykh, T.M.; Molodchenkova, O.O. Wheat dwarf virus in Ukraine: Occurrence, molecular characterization and impact on the yield. J. Plant Dis. Prot. 2022, 129, 107–116.
- Shepherd, D.N.; Martin, D.P.; McGivern, D.R.; Boulton, M.I.; Thomson, J.A.; Rybicki, E.P. A three-nucleotide mutation altering the Maize streak virus Rep pRBR-interaction motif reduces symptom severity in maize and partially reverts at high frequency without restoring pRBR–Rep binding. J. Gen. Virol. 2005, 86, 803–813.
- Schubert, J.; Habekuß, A.; Wu, B.; Thieme, T.; Wang, X. Analysis of complete genomes of isolates of the Wheat dwarf virus from new geographical locations and descriptions of their defective forms. Virus Genes 2014, 48, 133–139.
- Köklü, G.; Ramsell, J.N.E.; Kvarnheden, A. The complete genome sequence for a Turkish isolate of Wheat dwarf virus (WDV) from barley confirms the presence of two distinct WDV strains. Virus Genes 2007, 34, 359–366.
- Ramsell, J.N.E.; Boulton, M.I.; Martin, D.P.; Valkonen, J.P.T.; Kvarnheden, A. Studies on the host range of the barley strain of Wheat dwarf virus using an agroinfectious viral clone. Plant Pathol. 2009, 58, 1161–1169.
- Wu, X.; Weigel, D.; Wigge, P.A. Signaling in plants by intercellular RNA and protein movement. Genes Dev. 2002, 16, 151–158.
- Owor, B.E.; Shepherd, D.N.; Taylor, N.J.; Edema, R.; Monjane, A.L.; Thomson, J.A.; Martin, D.P.; Varsani, A. Successful application of FTA® Classic Card technology and use of bacteriophage ϕ29 DNA polymerase for large-scale field sampling and cloning of complete maize streak virus genomes. J. Virol. Methods 2007, 140, 100–105.
- Jungner, J. Die Zwergzikade (Cicadula sexnotata Fall.) und ihre Bekämpfung; Deutsche landwirtschafts-gesellschaft: Berlin, Germany, 1906.
- Tullgren, A. Zur Morphologie und Systematik der Hemipteren I. Entomol. Tidskr. Entomol. Föreningen I Stockh. 1918, 1918, 113–133.
- Lindsten, K.; Vacke, J.; Gerhardson, B. A preliminary report on three cereal virus diseases new to Sweden spread by Macrosteles and Psammotettix leafhoppers. Medd. Fran Statens Vaxtskyddsanst. 1970, 1423, 285–297.
- Gaborjanyi, R.; Vacke, J.; Bisztray, G. Wheat Dwarf Virus: A New Cereal Pathogen in Hungary; Novenytermeles: Debrecen, Hungary, 1988.
- Lapierre, H.; Cousin, M.T.; Della Giustina, W.; Moreau, J.P.; Khogali, M.; Roux, J. Nanisme blé: Agent pathogéne et vecteur. Description, biologie, interaction. Phytoma 1991, 432, 26–28.
- Conti, M. Leafhopper-borne plant viruses in Italy. Mem. Della Soc. Entomol. Ital. 1993, 72, 541–547.
- Jilaveanu, A.; Vacke, J. Isolation and identification of wheat dwarf virus (WDV) in Romania. Probl. Prot. Plantelor. 1995, 23, 51–62.
- Najar, A.; Makkouk, K.M.; Boudhir, H.; Kumari, S.G.; Zarouk, R.; Bessai, R.; Othman, F.B. Viral Diseases of Cultivated Legume and Cereal Crops in Tunisia; Firenze University Press: Florence, France, 2000; pp. 1000–1010.
- Lindsten, K.; Lindsten, B. Wheat dwarf—An old disease with new outbreaks in Sweden. J. Plant Dis. Prot. 1999, 106, 325–332.
- Sandgren, M.; Lindblad, M. Field studies of Wheat dwarf virus. In Proceedings of the 7th International Congress of Plant Pathology, Edinburgh, UK, 9–16 August 1998.
- Lindblad, M. What happened to the wheat dwarf disease. Växtskyddsnotiser 2000, 64, 11–13.
- Lindsten, K. Wheat dwarf—An old disease caused by a unique and earlier unknown virus. Vaextskyddsnotiser 1980.
- Dlabola, J. Zur Schädlichkeit der Zikaden in Getreidefeldern. Nachrichtenblatt Dtsch. Pflanzenschutzd. 1961, 14, 120–122.
- Moreau, J.-P.; Lapierre, H.; Navarro, D.; Debray, P.; Fohrer, F.; Lebrun, I. Distinction des effets du nanisme et de la jaunisse sur le blé. Phytoma Défense Végétaux 1992, 443, 21–25.
- Lindsten, K.; Lindsten, B. Occurrence and transmission of Wheat dwarf virus (WDV) in France. In Proceedings of the Third International Conference on Pest in Agriculture, Montpellier, France, 7–9 December 1993; pp. 7–9.
- Giustina, W.D.; Lebrun, I.; Lapierre, H.; Lochon, S.; Groupe de Travail „Biologie et Écologie de, P. alienus “. Distribution géographique du vecteur et du virus. Phytoma Défense Végétaux 1991, 432, 30–34.
- Anonym. New Knowledges about wheat dwarf virus. Phytoma Défense Végétaux 1992, 443, 17–20.
- Vacher, C.; Felix, I.; Bonnand, E. Lutte contre Psammotettix alienus, Cicadelle vectrice de la maladie des pieds chétifs. Perspect. Agric. 1991, 162, 86–89.
- Bisztray, G.; Gaborjanyi, R.; Vacke, J. Isolation and characterization of wheat dwarf virus found for the first time in Hungary. J. Plant Dis. Prot. 1989, 96, 449–454.
- Jezewska, J. First report of Wheat dwarf virus occurring in Poland. Phytopathol. Pol. 2001, 21, 93–100.
- Achon, M.A.; Serrano, L.; Ratti, C.; Rubies-Autonell, C. First Detection of Wheat dwarf virus in Barley in Spain Associated with an Outbreak of Barley Yellow Dwarf. Plant Dis. 2006, 90, 970.
- Viršček Marn, M.; Mavrič Pleško, I. First Report of the Occurrence of Wheat dwarf virus Infecting Wheat in Slovenia. Plant Dis. 2017, 101, 1336.
- Behjatnia, S.A.A.; Afsharifar, A.R.; Tahan, V.; Motlagh, M.H.A.; Gandomani, O.E.; Niazi, A.; Izadpanah, K. Widespread occurrence and molecular characterization of Wheat dwarf virus in Iran. Australas. Plant Pathol. 2011, 40, 12–19.
- Kapooria, R.G.; Ndunguru, J. Occurrence of viruses in irrigated wheat in Zambia. EPPO Bull. 2004, 34, 413–419.
- Ekzayez, A.M.; Kumari, S.G.; Ismail, I. First Report of Wheat dwarf virus and Its Vector (Psammotettix provincialis) Affecting Wheat and Barley Crops in Syria. Plant Dis. 2011, 95, 76.
- Xie, J.; Wang, X.; Liu, Y.; Peng, Y.; Zhou, G. First Report of the Occurrence of Wheat dwarf virus in Wheat in China. Plant Dis. 2007, 91, 111.
- Wang, X.; Wu, B.; Wang, J.F. First report of Wheat dwarf virus infecting barley in Yunnan, China. J. Plant Pathol. 2008, 90, 400.
- Bivand, R.; Lewin-Koh, N.; Pebesma, E.; Archer, E.; Baddeley, A.; Bearman, N.; Golicher, D. Package ‘maptools’, Version 1.1–4. 2022.
- Felix, I.; Larcher, J.M.; Maraby, J.; Philippeau, G.; Vinatier, K. Risques d’attaques de cicadelles et conditions d’efficacité des insecticides. Perspect. Agric. 1992, 173, 98–106.
- ICTV. Report Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2012.
- Ramsell, J.N.E.; Lemmetty, A.; Jonasson, J.; Andersson, A.; Sigvald, R.; Kvarnheden, A. Sequence analyses of Wheat dwarf virus isolates from different hosts reveal low genetic diversity within the wheat strain. Plant Pathol. 2008, 57, 834–841.
- Vacke, J.; Cibulka, R. Silky bent grass (Apera spica-venti Beauv.)—A new host and reservoir of wheat dwarf virus. Plant Prot. Sci. 1999, 35, 47–50.
- Brunt, A.; Crabtree, K.; Dallwitz, M.; Gibbs, A.; Watson, L. Viruses of Plants; CAB International: Oxfordshire, UK, 1996.
- Fohrer, F.; Lebrun, I.; Lapierre, E.H. Acquisitions recéntes sur le virus du nanisme du blé. Phytoma Défense Végétaux 1992, 443, 18–20.
- Vacke, J.; Cibulka, R. Response of selected winter wheat varieties to wheat dwarf virus infection at an early growth stage. Czech J. Genet. Plant Breed. 2000, 36, 1–4.
- Manurung, B.; Witsack, W.; Mehner, S.; Gruntzig, M.; Fuchs, E. The epidemiology of Wheat dwarf virus in relation to occurrence of the leafhopper Psammotettix alienus in Middle-Germany. Virus Res. 2004, 100, 109–113.
- Širlová, L.; Vacke, J.; Chaloupková, M. Reaction of selected winter wheat varieties to autumnal infection with Wheat dwarf virus. Plant Prot. Sci 2005, 41, 1–7.
- Jones, R.A.C. Global Plant Virus Disease Pandemics and Epidemics. Plants 2021, 10, 233.
- Huth, W. Weizenverzwergung—Bisher übersehen? Pflanzenschutz Prax. 1994, 4, 37–39.
- Áy, Z.; Kerényi, Z.; Takács, A.; Papp, M.; Petróczi, I.; Gáborjányi, R.; Silhavy, D.; Pauk, J.; Kertész, Z. Detection of cereal viruses in wheat (Triticum aestivum L.) by serological and molecular methods. Cereal Res. Commun. 2008, 36, 215–224.
