Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eleonora Russo | -- | 1450 | 2023-10-23 17:57:28 | | | |
| 2 | Sirius Huang | Meta information modification | 1450 | 2023-10-25 03:08:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Russo, E.; Alberti, G.; Corrao, S.; Borlongan, C.V.; Miceli, V.; Conaldi, P.G.; Di Gaudio, F.; La Rocca, G. Extracellular Vesicles from Human Perinatal Stem Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/50694 (accessed on 07 February 2026).
Russo E, Alberti G, Corrao S, Borlongan CV, Miceli V, Conaldi PG, et al. Extracellular Vesicles from Human Perinatal Stem Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/50694. Accessed February 07, 2026.
Russo, Eleonora, Giusi Alberti, Simona Corrao, Cesar V. Borlongan, Vitale Miceli, Pier Giulio Conaldi, Francesca Di Gaudio, Giampiero La Rocca. "Extracellular Vesicles from Human Perinatal Stem Cells" Encyclopedia, https://encyclopedia.pub/entry/50694 (accessed February 07, 2026).
Russo, E., Alberti, G., Corrao, S., Borlongan, C.V., Miceli, V., Conaldi, P.G., Di Gaudio, F., & La Rocca, G. (2023, October 23). Extracellular Vesicles from Human Perinatal Stem Cells. In Encyclopedia. https://encyclopedia.pub/entry/50694
Russo, Eleonora, et al. "Extracellular Vesicles from Human Perinatal Stem Cells." Encyclopedia. Web. 23 October, 2023.
Copy Citation
The potential of perinatal tissues to provide cellular populations to be used in different applications of regenerative medicine is well established. Efforts of researchers are being addressed regarding the evaluation of cell products (secreted molecules or extracellular vesicles, EVs) to be used as an alternative to cellular infusion. The data regarding the effective recapitulation of most perinatal cells’ properties by their secreted complement point in this direction. EVs secreted from perinatal cells exhibit key therapeutic effects such as tissue repair and regeneration, the suppression of inflammatory responses, immune system modulation, and a variety of other functions.
mesenchymal stromal cells
human perinatal tissues
extracellular vesicles
1. Introduction
In the last decade, significant advances have been made to fully assess the biology of mesenchymal stromal cells (MSCs) derived from perinatal tissues. The term “perinatal” defines birth-associated tissues and other fetal annexes (e.g., amnion, chorion, and the umbilical cord), obtained immediately after birth. To date, many applications of perinatal MSCs for clinical purposes have been studied with promising results, mainly due to their unique immune and differentiative properties [1]. Despite this, perinatal MSC-based therapy still presents problems related to different aspects, such as the difficulty of developing specific methods of isolation and characterization, as well as the identification of an optimal protocol for ex vivo expansion and the often observed efficacy post transplant [2][3]. Nevertheless, it has been suggested that the perinatal MSCs’ therapeutic efficacy may be fully recapitulated by their secretome or conditioned medium (e.g., soluble proteins, lipids, and extracellular vesicles) [4]. Thus, a new definition was introduced by Silini et al. namely “perinatal derivatives (PnD)” which includes all birth-associated tissues, the cells they are composed of, and all the biomolecules secreted [5]. Therapeutic approaches based on MSC secretomes may have different advantages over the use of transplanted MSCs, due to the minimal effect on immunogenicity, the higher yields of bioactive molecules, and easier application. Perinatal MSC secretomes seem to exhibit the same anti-inflammatory, immunomodulatory, and regenerative properties as parental MSCs [6][7][8], and appear to be effective in treating the side effects of ischemia–reperfusion injury [9][10]. Furthermore, the use of secretomes provides a suitable strategy to enhance MSCs’ therapeutic potential and standardize the production of MSC-derived products intended for clinical use [11]. Therefore, the utilization of secretomes could contribute to developing a novel, cell-free therapeutic approach.
The secretome is enriched with extracellular vesicles (EVs), secreted by cells through the development of multivesicular bodies or by cell membrane shedding [12][13]. They are classified into different types, based on their size, mechanism of biogenesis, functions, and tissue of origin, into: exosomes, microvesicles, ectosomes, and oncosomes [14]. Importantly, the cells (including the perinatal MSCs) are able to release various types of EVs [15]. EVs are present in almost every type of body fluid (e.g., plasma, urine, saliva, amniotic fluid, and others) and are therefore easily accessible. Once in the extracellular space, EVs facilitate cell-to-cell communication, between neighboring and distant cells, through transferring their molecular cargo (enriched in proteins, RNAs, and DNAs), both in physiological and pathological conditions [16][17][18]. The ISEV (International Society for Extracellular Vesicles) suggested using “extracellular vesicle” as the common term for all the vesicles released from cells to avert uncertainty within this complex field [19].
The increasing interest in the therapeutic benefits obtained by using the EVs released by parental MSCs in various diseases reinforces the hypothesis of the usefulness of MSC secretomes as a new therapeutic strategy.
2. Properties of Human Perinatal Tissue and Their Possible Therapeutic Application as Sources of EVs
Perinatal tissues represent a plentiful source of promising cell types. Among the organs that may be used as a source of perinatal cells, the placenta is of key importance for its role in fetal nutrition and development, acting as a functional interface between the mother and fetus, either via the exchange of nutrients or via its immune and endocrine roles [20]. The formation of the placenta is the result of a series of processes that start in the moment of embryo implantation at the end of the first week of development. Then, the organ assists in the development of the embryo and then the fetus until delivery [21]. At delivery, the placenta features a diameter of 20–22 cm, with a thickness of up to 2.5 cm [22]. Structurally, the human placenta is a highly specialized essential organ with a distinction between the maternal-uterine part and the fetal part. Research related to perinatal MSCs began over a decade ago and has grown exponentially with the isolation and characterization of cells from different perinatal tissues [23][24][25] (Figure 1).
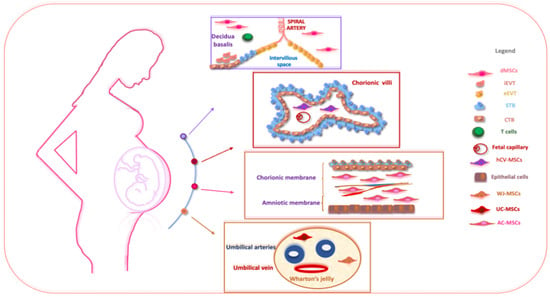
Figure 1. The schematic structure of the placenta and a description of perinatal MSCs. The placenta is an organ in which interactions between maternal and fetal cells, necessary for the development of the fetus, take place. Specifically, the placenta is made up of structures of fetal origin, such as the placental disc, the fetal membranes, divided into amniotic and chorionic membranes, and the umbilical cord, as well as a membrane of maternal origin called the decidua which originates from the endometrium. The chorionic villosity that forms the boundary between maternal and fetal blood during pregnancy represents the functional unit of the placenta. The different structures of the placenta are enriched by MSCs particularly useful in regenerative medicine and for the treatment of various pathologies. Abbreviations: dMSCs: decidua mesenchymal stromal cells; iEVT: inner extravillous trophoblast; eEVT: external extravillous trophoblast; STB: syncytiotrophoblast; CTB: cytotrophoblast; hCV-MSCs: human chorionic villi mesenchymal stromal cells; WJ-MSCs: Wharton’s jelly mesenchymal stromal cells; UC-MSCs: umbilical cord mesenchymal stromal cells; AC-MSCs: amnio-chorion mesenchymal stromal cells.
The placenta is usually discarded post-partum and easily obtained with a virtually limitless availability, free from ethical issues, and provides efficient MSC recovery without invasive procedures [26]. Different perinatal MSC populations are present and useful in regenerative medicine for the treatment of various pathologies, namely human decidua-MSCs (hD-MSCs), human syncytiotrophoblast and human cytotrophoblast (hSTB-MSCs and hCTB-MSCs), hAC-MSCs of the human amnio-chorionic membrane, as well as the MSCs present in the human umbilical cord (hUC-MSCs) and human amniotic fluid (hAF-MSCs).
MSCs are involved dynamically and actively in feto-maternal communication by releasing several molecules into the maternal circulation (including hormones and proteins) as well as EVs [27]. The therapeutic application of perinatal MSCs’ secretomes may present some advantages over the direct application of cells [28]. Indeed, it is now known that the therapeutic effects of perinatal cells are largely mediated by the secretion of EVs [11][29][30][31]. According to their size and biogenesis, EVs are distinguished into: (i) exosomes (30–150 nm), (ii) microvesicles (50 to 1000 nm, MV), and (iii) apoptotic bodies (1000–5000 nm) [32][33][34]. Of note, MSC-derived EVs were first isolated in 2010 in mice in a model of myocardial ischemia–reperfusion [35]. MSC-derived EVs are enriched with important bioactive molecules, such as mRNA and proteins, which regulate various biological processes. They express conventional markers of EVs (such as Hsp70, CD63, Flotillin-1, and TSG101) [22]. Nevertheless, they do not express costimulatory molecules, such as CD80 and CD86, thus conferring immune tolerance [36]. Proteomic analyses have allowed researchers to highlight the fact that perinatal-derived EVs are enriched in key proteins involved in several processes (e.g., immune, metabolic, and regenerative pathways) when compared to adult MSCs [37]. Currently, a few studies have attempted to characterize the miRNA profile of EVs isolated from perinatal MSCs [38][39], although some evidence is already available from experimental animal models [40][41]. One of these studies highlighted the presence of a few specific and highly expressed miRNAs, such as miR-145, miR-181c-5p, miR-Let-7e, and others [42]. Bulati et al. revealed that nine miRNAs found in human amnion MSC-derived EVs (hAMSC-derived EVs) were able to regulate the key proteins that control both T cell activation and monocyte differentiation [43]. Additionally, it has been reported that human umbilical cord MSC-derived EVs (hUCMSC-derived EVs) express high levels of miR16 and miR-Let-c which are involved in the regulation of T lymphocytes [44]. Perinatal MSC-derived EVs possess a lipid bilayer membrane also enriched with cholesterol, sphingomyelin, ceramide, and lipid raft proteins, which are involved in facilitating the trafficking and fusion of the membrane, bypassing any biological barrier [26]. Due to their specific properties, a number of studies have focused on EVs released by perinatal MSCs, highlighting their potential for cell-free therapy in clinical practice. Perinatal MSC-derived EVs are safer and have a longer shelf life than the MSCs themselves [45]. It has been observed that perinatal MSC-derived EVs have shown encouraging therapeutic effects in preclinical studies; five of these studies are reported in the www.ClinicalTrials.gov database accessed on 30 July 2023. One of these (NCT04213248) investigates the ability of umbilical cord-derived EVs to reduce dry eye symptoms in patients with chronic graft-versus-host disease (cGVHD). Another study (NCT04202770) evaluates the potential of liquid amniotic-derived EVs in the treatment of depression and neurodegenerative dementia, while another study (NCT04384445) evaluates their ability to suppress the activation of cytokines or intervene in other adverse events in COVID-19 patients with severe symptoms.
References
- Li, S.; Wang, J.; Jiang, B.; Jiang, J.; Luo, L.; Zheng, B.; Si, W. Mesenchymal stem cells derived from different perinatal tissues donated by same donors manifest variant performance on the acute liver failure model in mouse. Stem Cell Res. Ther. 2022, 13, 231.
- Beeravolu, N.; McKee, C.; Alamri, A.; Mikhael, S.; Brown, C.; Perez-Cruet, M.; Chaudhry, G.R. Isolation and Characterization of Mesenchymal Stromal Cells from Human Umbilical Cord and Fetal Placenta. J. Vis. Exp. 2017, 122, 55224.
- Wu, M.; Zhang, R.; Zou, Q.; Chen, Y.; Zhou, M.; Li, X.; Ran, R.; Chen, Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci. Rep. 2018, 8, 5014.
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084.
- Silini, A.R.; Di Pietro, R.; Lang-Olip, I.; Alviano, F.; Banerjee, A.; Basile, M.; Borutinskaite, V.; Eissner, G.; Gellhaus, A.; Giebel, B.; et al. Where Do We Stand? A Roadmap of the Human Placenta and Consensus for Tissue and Cell Nomenclature. Front. Bioeng. Biotechnol. 2020, 8, 610544.
- Alberti, G.; Russo, E.; Corrao, S.; Anzalone, R.; Kruzliak, P.; Miceli, V.; Conaldi, P.G.; Di Gaudio, F.; La Rocca, G. Current Perspectives on Adult Mesenchymal Stromal Cell-Derived Extracellular Vesicles: Biological Features and Clinical Indications. Biomedicines 2022, 10, 2822.
- Russo, E.; Corrao, S.; Di Gaudio, F.; Alberti, G.; Caprnda, M.; Kubatka, P.; Kruzliak, P.; Miceli, V.; Conaldi, P.G.; Borlongan, C.V.; et al. Facing the Challenges in the COVID-19 Pandemic Era: From Standard Treatments to the Umbilical Cord-Derived Mesenchymal Stromal Cells as a New Therapeutic Strategy. Cells 2023, 12, 1664.
- Lo Nigro, A.; Gallo, A.; Bulati, M.; Vitale, G.; Paini, D.S.; Pampalone, M.; Galvagno, D.; Conaldi, P.G.; Miceli, V. Amnion-Derived Mesenchymal Stromal/Stem Cell Paracrine Signals Potentiate Human Liver Organoid Differentiation: Translational Implications for Liver Regeneration. Front. Med. 2021, 8, 746298.
- Miceli, V.; Bertani, A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells 2022, 11, 826.
- Miceli, V.; Bertani, A.; Chinnici, C.M.; Bulati, M.; Pampalone, M.; Amico, G.; Carcione, C.; Schmelzer, E.; Gerlach, J.C.; Conaldi, P.G. Conditioned Medium from Human Amnion-Derived Mesenchymal Stromal/Stem Cells Attenuating the Effects of Cold Ischemia-Reperfusion Injury in an In Vitro Model Using Human Alveolar Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 510.
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763.
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727.
- Heusermann, W.; Hean, J.; Trojer, D.; Steib, E.; von Bueren, S.; Graff-Meyer, A.; Genoud, C.; Martin, K.; Pizzato, N.; Voshol, J.; et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J. Cell Biol. 2016, 213, 173–184.
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797.
- Cargnoni, A.; Papait, A.; Masserdotti, A.; Pasotti, A.; Stefani, F.R.; Silini, A.R.; Parolini, O. Extracellular Vesicles From Perinatal Cells for Anti-inflammatory Therapy. Front. Bioeng. Biotechnol. 2021, 9, 637737.
- Alberti, G.; Sánchez-López, C.M.; Andres, A.; Santonocito, R.; Campanella, C.; Cappello, F.; Marcilla, A. Molecular Profile Study of Extracellular Vesicles for the Identification of Useful Small “Hit” in Cancer Diagnosis. Appl. Sci. 2021, 11, 10787.
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232.
- Alberti, G.; Mazzola, M.; Gagliardo, C.; Pitruzzella, A.; Fucarini, A.; Giammanco, M.; Tomasello, G.; Carini, F. Extracellular vesicles derived from gut microbiota in inflammatory bowel disease and colorectal cancer. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2021, 165, 233–240.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Burton, G.J.; Jauniaux, E. Development of the Human Placenta and Fetal Heart: Synergic or Independent? Front. Physiol. 2018, 9, 373.
- Cross, J.C.; Werb, Z.; Fisher, S.J. Implantation and the placenta: Key pieces of the development puzzle. Science 1994, 266, 1508–1518.
- Plitman Mayo, R. Advances in Human Placental Biomechanics. Comput. Struct. Biotechnol. J. 2018, 16, 298–306.
- Anzalone, R.; Lo Iacono, M.; Corrao, S.; Magno, F.; Loria, T.; Cappello, F.; Zummo, G.; Farina, F.; La Rocca, G. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: Immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010, 19, 423–438.
- Portmann-Lanz, C.B.; Schoeberlein, A.; Huber, A.; Sager, R.; Malek, A.; Holzgreve, W.; Surbek, D.V. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am. J. Obstet. Gynecol. 2006, 194, 664–673.
- La Rocca, G.; Anzalone, R.; Corrao, S.; Magno, F.; Loria, T.; Lo Iacono, M.; Di Stefano, A.; Giannuzzi, P.; Marasà, L.; Cappello, F.; et al. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: Differentiation potential and detection of new markers. Histochem. Cell Biol. 2009, 131, 267–282.
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605.
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496.
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852.
- Thomi, G.; Surbek, D.; Haesler, V.; Joerger-Messerli, M.; Schoeberlein, A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res. Ther. 2019, 10, 105.
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062.
- Miceli, V.; Pampalone, M.; Vella, S.; Carreca, A.P.; Amico, G.; Conaldi, P.G. Comparison of Immunosuppressive and Angiogenic Properties of Human Amnion-Derived Mesenchymal Stem Cells between 2D and 3D Culture Systems. Stem Cells Int. 2019, 2019, 7486279.
- Wang, J.; Bonacquisti, E.E.; Brown, A.D.; Nguyen, J. Boosting the Biogenesis and Secretion of Mesenchymal Stem Cell-Derived Exosomes. Cells 2020, 9, 660.
- Revenfeld, A.L.; Bæk, R.; Nielsen, M.H.; Stensballe, A.; Varming, K.; Jørgensen, M. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin. Ther. 2014, 36, 830–846.
- Riazifar, M.; Pone, E.J.; Lötvall, J.; Zhao, W. Stem Cell Extracellular Vesicles: Extended Messages of Regeneration. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 125–154.
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222.
- Antounians, L.; Tzanetakis, A.; Pellerito, O.; Catania, V.D.; Sulistyo, A.; Montalva, L.; McVey, M.J.; Zani, A. The Regenerative Potential of Amniotic Fluid Stem Cell Extracellular Vesicles: Lessons Learned by Comparing Different Isolation Techniques. Sci. Rep. 2019, 9, 1837.
- Wang, Z.G.; He, Z.Y.; Liang, S.; Yang, Q.; Cheng, P.; Chen, A.M. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 511.
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439.
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.; Lu, M.; Zhu, Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40.
- Lange-Consiglio, A.; Lazzari, B.; Perrini, C.; Pizzi, F.; Stella, A.; Cremonesi, F.; Capra, E. MicroRNAs of Equine Amniotic Mesenchymal Cell-derived Microvesicles and Their Involvement in Anti-inflammatory Processes. Cell Transpl. 2018, 27, 45–54.
- Lange-Consiglio, A.; Lazzari, B.; Pizzi, F.; Stella, A.; Girani, A.; Quintè, A.; Cremonesi, F.; Capra, E. Different Culture Times Affect MicroRNA Cargo in Equine Amniotic Mesenchymal Cells and Their Microvesicles. Tissue Eng. C 2018, 24, 596–604.
- Meng, X.; Xue, M.; Xu, P.; Hu, F.; Sun, B.; Xiao, Z. MicroRNA profiling analysis revealed different cellular senescence mechanisms in human mesenchymal stem cells derived from different origin. Genomics 2017, 109, 147–157.
- Bulati, M.; Miceli, V.; Gallo, A.; Amico, G.; Carcione, C.; Pampalone, M.; Conaldi, P.G. The Immunomodulatory Properties of the Human Amnion-Derived Mesenchymal Stromal/Stem Cells Are Induced by INF-γ Produced by Activated Lymphomonocytes and Are Mediated by Cell-To-Cell Contact and Soluble Factors. Front. Immunol. 2020, 11, 54.
- Zou, X.Y.; Yu, Y.; Lin, S.; Zhong, L.; Sun, J.; Zhang, G.; Zhu, Y. Comprehensive miRNA Analysis of Human Umbilical Cord-Derived Mesenchymal Stromal Cells and Extracellular Vesicles. Kidney Blood Press. Res. 2018, 43, 152–161.
- Fujita, Y.; Kadota, T.; Araya, J.; Ochiya, T.; Kuwano, K. Clinical Application of Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapeutics for Inflammatory Lung Diseases. J. Clin. Med. 2018, 7, 355.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
681
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
25 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




