Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adrian KORYCKI | -- | 2941 | 2023-10-19 10:22:39 | | | |
| 2 | Sirius Huang | Meta information modification | 2941 | 2023-10-20 02:58:28 | | | | |
| 3 | Sirius Huang | -170 word(s) | 2771 | 2023-11-24 10:13:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Korycki, A.; Carassus, F.; Tramis, O.; Garnier, C.; Djilali, T.; Chabert, F. Polyaryletherketone Based Blends. Encyclopedia. Available online: https://encyclopedia.pub/entry/50516 (accessed on 07 February 2026).
Korycki A, Carassus F, Tramis O, Garnier C, Djilali T, Chabert F. Polyaryletherketone Based Blends. Encyclopedia. Available at: https://encyclopedia.pub/entry/50516. Accessed February 07, 2026.
Korycki, Adrian, Fabrice Carassus, Olivier Tramis, Christian Garnier, Toufik Djilali, France Chabert. "Polyaryletherketone Based Blends" Encyclopedia, https://encyclopedia.pub/entry/50516 (accessed February 07, 2026).
Korycki, A., Carassus, F., Tramis, O., Garnier, C., Djilali, T., & Chabert, F. (2023, October 19). Polyaryletherketone Based Blends. In Encyclopedia. https://encyclopedia.pub/entry/50516
Korycki, Adrian, et al. "Polyaryletherketone Based Blends." Encyclopedia. Web. 19 October, 2023.
Copy Citation
Polyaryletherketone-based thermoplastic blends (PAEK) are high-performance copolymers able to replace metals in many applications including those related to the environmental and energy transition. PAEK lead to the extension of high-performance multifunctional materials to target embedded electronics, robotics, aerospace, medical devices and prostheses. Blending PAEK with other thermostable thermoplastic polymers is a viable option to obtain materials with new affordable properties.
polymer blend
miscibility
thermal transition
crystallization
1. Introduction
High-performance thermoplastic (HPT) polymers lead the extension of advanced tailored applications such as embedded electronics, robotics, aerospace, medical devices and prostheses. In addition, HPT research and growth are driven by their use in structural composite materials, in which thermoplastics are an option for the matrix, offering significant weight savings and time-to-market reduction compared to thermoset composites or incumbent steel. Other widespread applications of HPT are as membranes from the barrier, filtration, osmosis and as templates for chemical reactions, such as proton exchange membrane fuel cells.
Thermostable polymers are defined as materials with heat resistance in continuous uses at 200 °C or above and resistance to aging in a thermo-oxidative environment. Only a few thermoplastics reach these outstanding properties, their common feature being the presence of aromatic groups in their chemical backbone. Among them, polyaryletherketones have been developed since the 1980s and have demonstrated some of the longest lifespans when submitted to thermo-oxidative aging [1]. PAEK are semicrystalline polymers displaying a large range of melting temperatures (Tm) from 320 °C for polyetheretheretherketone (PEEEK) to 390 °C for polyetherketoneketone (PEKK), with their crystalline morphology, the kinetics of crystallization and properties. The most popular is polyetheretherketone (PEEK). PEEK has a glass transition temperature (Tg) of 143 °C and a melting temperature of 335 °C. Its maximum operating temperature is from 250 °C to 260 °C and its processing temperature is from 370 °C to 400 °C [2]. The maximum achievable crystallinity is 48% [3]. Due to the fast kinetics of crystallization, it was reported that the amorphous state of pure PEEK can be obtained only when cooled at very high cooling rates, nearly 1000 K·min−1 [4].
All PAEKs offer a compromise between thermal stability, mechanical properties, chemical resistance and durability. The enhancement of these features can lead to the development of cutting-edge applications [5]. Ideally, one material should fit all the targeted properties, namely “multi-functional” material. Blending two polymers may be the easiest option to design such new materials. Indeed, two or more thermoplastics, often chosen such as their properties complement one another, yield a new material with synergetic properties.
2. Presentation of Blends Components
In this section, the polymer blends are classified according to their miscibility and then the chemical structures and properties of PAEKs and other high-performance thermoplastics are briefly presented.
2.1. Classes of Blends
Many attempts have been constructed to classify the polymer blends: miscible, immiscible, compatible, incompatible, partially miscible, etc. The goal here is not to review terminology or to discuss which definition is best suited to each situation. This text will use the following terms to classify the PAEK/HPT blends: miscible, partially miscible and immiscible. Miscible blends behave in a single phase and exhibit a single glass transition temperature. Partially miscible blends exhibit miscibility to an extent, as a function of blend composition or process parameters. It is worth mentioning that, whatever the polymer added to PAEK, the miscibility depends on the molecular mass. However, this information is barely provided in the reviewed articles. Finally, the blends that do not fall into the previous categories are classified as immiscible blends. Table 1 shows the common PAEK/HPT blends found in the literature. It is noteworthy that PEI is the only HPT that forms a fully miscible blend with various PAEK in the melted state. However, PEI comes in different conformations, and some of them are not miscible with PAEK.
Table 1. Classification of the most common HPT blended with PAEK as a function of their miscibility and method of blending.
| Blending Method | Miscible Blends |
Partially Miscible Blends |
Immiscible Blends |
|---|---|---|---|
| Melt-mixing | PAEK/meta-PEI | PAEK/PAI | PEEK/para-PEI |
| PAEK/PES | PEEK/ortho-PEI | ||
| PEKK/TPI PEK/TPI | PEEK/TPI | ||
| PAEK/LCP | PEEK/PBI | ||
| PAEK/PTFE | |||
| Solution blending | SPEEK/PAI | PEKK/PBI | |
| SPEEK/PEI | PEEK/PES | ||
| SPEEK/PBI | |||
| SPEEK/PES |
2.2. Presentation of PAEK
Polyaryletherketones are semicrystalline high-performance thermoplastics with strong molecular rigidity of their repeating units. They demonstrate high-temperature stability, chemical resistance and high mechanical strength over a wide temperature range. PEEK (Figure 1), PEKK (Figure 2), PEK (Figure 3) and others have similar crystalline structures of two-chain orthorhombic packing [6] but not the same ether/ketone content [7].
Figure 1. Structural formula of PEEK.
Figure 2. Chemical structure of PEKK.
Figure 3. Scheme of PEK unit.
Polyetheretherketone combines the strength of 98 MPa and the stiffness of 125 MPa with a very good tensile fatigue of 97 MPa, thermal and chemical resistance (including the majority of organic solvents, oils and acids). Its mechanical properties remain stable up to temperatures of about 240 °C [8].
Polyetherketoneketone is a polymer with high heat resistance above 300 °C, chemical resistance and an ability to withstand high mechanical loads from 88 MPa to 112 MPa [9][10][11]. This polymer is synthesized in various formulations with individually unique properties. The PEKK formulations are expressed by the ratio of the percent of terephthaloyl (T) to isophthaloyl (I) moieties used during the synthesis that created the polymer. The T/I ratio affects the melting point ranging from 305 °C to 360 °C, the glass transition temperature from 160 °C to 165 °C and the crystallization kinetics [12].
Polyetherketone is characterized by very good material properties such as a tensile strength of 110 MPa, an elastic modulus of 4200 MPa and an elongation at break of 35%. It has a high resistance to abrasion and increased compression strength of 180 MPa at higher temperatures [13].
The properties of polyaryletherketones are due to the occurrence of phenylene rings linked via oxygen bridges (ether, R–O–R) and carbonyl groups (ketone, R-CO-R) in different configurations and proportions. The glass transition temperature, Tg, and the melting temperature, Tm, of the polymer depend on the ratio and sequence of ethers and ketones. They also affect its heat resistance and processing temperature. The lower the ratio of ether/ketone, the more rigid the polymer chain is and the higher the Tg and Tm as seen in Figure 4. As a consequence, the processing temperature ranges from 350 °C to 400 °C [14]. The main suppliers are Victrex, Arkema, Solvay, Evonik, Sabic and Gharda.
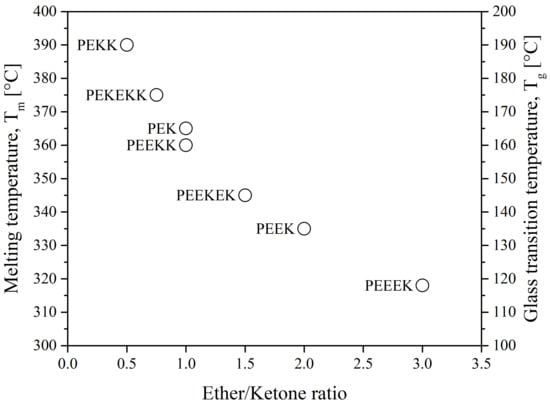
Figure 4. The melting temperature and glass transition temperature of PAEK as a function of ether/ketone ratio [15].
Another type of PAEK was launched recently on the market by Victrex, with a lower melting temperature while keeping the same glass transition, maned as LM-PAEK (LM: low melting). The typical trend for Tg and Tm as seen in Figure 4 is not valid anymore, due to the higher rigidity of its monomers. Furthermore, to increase the hydrophilicity of PEEK materials, charged groups are introduced using sulfuric acid into the polymer chains to make them ion-exchangeable. Sulfonation aids in the transport of cations and increases the hydrophilicity of the polymer. Sulfonated PEEK is presented in Figure 5.
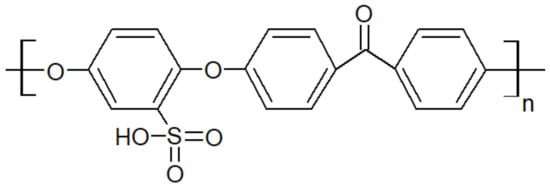
Figure 5. Sulfonated PEEK structure.
2.3. Presentation of HPT Usually Blended with PAEK
Polyetherimide is an amorphous polymer whose chemical structure of grade Ultem 1000 from Sabic is seen in Figure 6. It is renowned for being inherently flame retardant. Ultem 1000 resin in an unreinforced general-purpose grade offers a high strength of 105 MPa and a modulus of 3.2 GPa and a broad chemical resistance up to high temperatures of 170 °C while maintaining stable electrical properties over a wide range of frequencies [16][17]. The ketone groups in its backbone render it more flexible than polyimides, hence better processability. Similar to other amorphous thermoplastics, its mechanical strength decreases fast above its Tg at 215 °C. PAEK/PEI blends have been reported to be miscible, especially the binary blend PEEK/PEI. The latter is commonly used in PEEK composite parts [18][19][20], where the PEI is a joining agent, a method referred to as “Thermabond” [21]. Also, PEI is used as an energy director, meaning interfacial film, to assemble carbon fiber/PAEK composites through ultrasonic welding [22]. PEEK/PEI blends have also found application as tribological material [23], biomedical implants [24] and lightweight foam structures [25]. Since this blend has been reported to be miscible at all compositions in the amorphous state, it has attracted considerable attention to further the fundamental understanding of miscible blends [26][27][28][29][30].
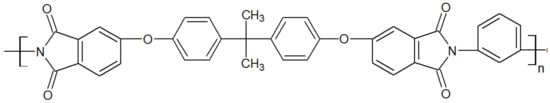
Figure 6. Schematic structure of PEI Ultem 1000.
Most studies with polyamideimides mention Torlon® 4000T from Solvay, whose chemical structure is presented in Figure 7, as well as other PAI Torlon®. Torlon® 4000T is the unfilled PAI powder mainly for adhesive applications [31]. Some authors have synthesized their PAI [32] with specific properties. Their mechanical, thermal and oxidative properties make them suitable for various applications thanks to a high glass transition temperature of around 275 °C [33].
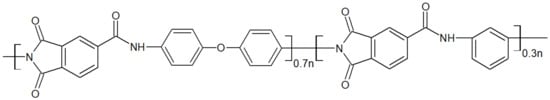
Figure 7. Chemical structure of the PAI Torlon® 4000T.
The chemical structure of polybenzimidazole is presented in Figure 8. Its rigid structure gives excellent thermal and mechanical properties, such as a melting point above 600 °C [34] and a glass transition of 435 °C [35]. It has the highest tensile strength among high-performance polymers, up to 145 MPa, and offers good chemical resistance [34][36][37][38]. However, it has a large water uptake of 15 wt.%, and the imidazole ring in the repeat unit may be subjected to hydrolysis, reducing its lifetime in applications such as fuel cells [39].
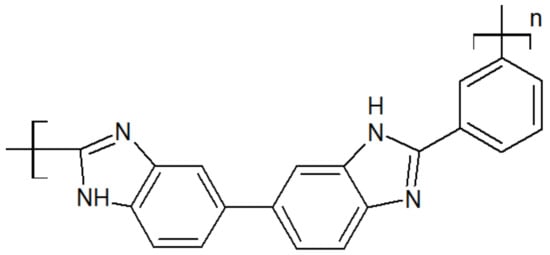
Figure 8. Scheme of a PBI unit.
Polyethersulfone, whose chemical structure is sketched in Figure 9, is an amorphous thermoplastic with a Tg between 190 °C and 230 °C, yielding high thermal stability. It also offers high mechanical rigidity and creep resistance, which make it a good candidate to blend with PAEK [40].
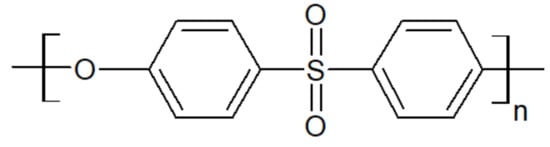
Figure 9. The repeat unit of a PES.
Thermoplastic polyimides, whose structure is depicted in Figure 10, display high mechanical properties of 128.7 MPa at tensile strength and 14.2% at elongation at break for TPI-4 [41]. They have one of the highest continuous operating temperatures for an unfiled thermoplastic of 240 °C [42]. Their Tg is at 250 °C. The main drawbacks of TPI are their high melt viscosity and low chemical resistance, making their processing challenging. Modification of the aromatic backbone by the inclusion of flexible functional bonds has enabled the synthesis of polyimide with a lower melt viscosity [43][44]. While TPIs are amorphous (Matrimid 5218, LaRC-TPI, Extem XH/UH), semicrystalline TPIs (with a crystallinity at 20%) were developed as an alternative, such as the so-called “New-TPI” by Mitsui Toatsu Chemical, Inc. (Tokyo, Japan) [45], later renamed “Aurum” or “Regulus” [46] depending on its end-use. Both amorphous and semicrystalline TPI suffer from low processability, even though the N-TPI has better overall mechanical properties and chemical resistance than TPI. Blending TPI or N-TPI with PAEK is an interesting way to improve the processability of the former, despite their immiscibility with PAEK.
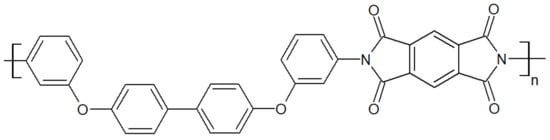
Figure 10. TPI repeat unit.
Polytetrafluoroethylene is a synthetic fluorocarbon with a high molecular weight compound consisting of carbon and fluorine. Their structure is shown in Figure 11. It is highly hydrophobic, biocompatible and widely used as a solid lubricant thanks to its low friction coefficient. Concerning engineering applications, PEEK is wear-resistant but may suffer from wear loss or high friction coefficient at elevated temperatures. Blending PEEK with PTFE is a way to improve the tribological performances of PAEK-based blends. But PTFE is extremely viscous in the melted state, which complicates its processability. Its glass transition temperature is around 114 °C and its melting point is 320 °C and it starts deteriorating above 260 °C, making it difficult to blend with PAEK.
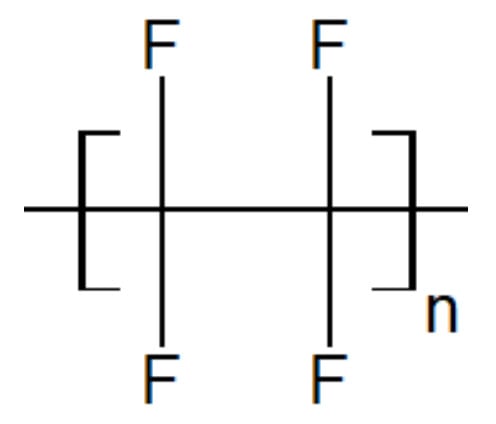
Figure 11. Schematic structure of the PTFE unit.
Liquid crystal polymers are characterized by the properties of liquid crystal, which can be those of conventional liquids or those of solid crystals. For example, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. Those thermoplastics showed up on the market in the 1980s. Thermotropic LCP, shown in Figure 12, is melt processable, while lyotropic LCP with a higher chain rigidity and intermolecular bonding is spun from a solution such as sulfuric acid. During melt processing, the rigid molecules with units disrupt chain linearity and pack slightly to reduce the melting point. It has been reported that the blends with LCP often tend to be immiscible but have a range of useful properties. TLCP have been blended with many thermoplastics including PEEK [47].
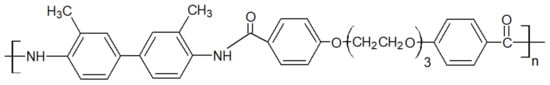
Figure 12. Repeat unit of TLCP.
3. Thermodynamics of Miscibility and Morphologies
Polymer–polymer theory of miscibility is based on the Flory–Huggins theory for a solution, for which, ideally, each part of the solute fills a space in the solution that it is soluble in, e.g., the so-called “lattice model” [48]. Polymers, and especially HPT, are at best semirigid due to their aromatic backbone. The Flory–Huggins theory still serves to describe the polymer–polymer mixture. The free energy of mixing depends on combinatorial entropy since the polymer of the mixture cannot fill all the space offered by the lattice due to its restricted degree of freedom. This effect is taken into account by considering the volume of the polymer, i.e., steric effects [49]. The free energy of mixing depends also on the interaction between like and unlike pairs of monomers, which is an enthalpic contribution. Usually, since macromolecules contain a large number of monomers, the repulsion between macromolecules is large [50]. The strength of this interaction, as the difference between like and unlike pairs, is defined as the interaction Flory–Huggins parameter, χ. The free energy of mixing is usually positive, i.e., unfavorable to mixing. However, strong specific interactions (dipole–dipole, ion–dipole, hydrogen bonding, acid-base or charge transfer) [51] may occur, and these will counterbalance the unfavorable entropic (steric) and enthalpic (van der Walls) interactions to give miscibility. Additionally, the free energy of mixing must have a positive curvature (in other words, increased differential growth at the center, for example, the surface of the sphere) at any concentration to give a miscible blend concerning the negative enthalpic term. Usually, this is accomplished by specific interactions, such as dipole–dipole and hydrogen bonding [52]. Such interactions are highly dependent on temperature; as a consequence, the miscibility depends on the temperature. This dependency is evidenced by miscibility diagrams depicting an upper critical solution temperature (UCST) or a lower critical solution temperature (LCST).
Partially miscible blends have a negative curvature (in other words, increased differential growth at the edges, for example, “saddle”) for some concentrations on a miscibility diagram [53]. For example, PEEK/PES blends are partially miscible; their miscibility depends on the processing method. A probable cause, in view of the discussion above, would be that their respective conformation does not allow the macromolecules to interact attractively. However, sulfonated PEEK is fully miscible with PES, mainly due to the presence of sulfonate groups, which would specifically interact with the likes on the PES backbone. Another example would be PEEK/PBI blends, where steric repulsion would be the main reason for their immiscibility. Nevertheless, SPEEK is fully miscible with PBI, mainly due to the acid-base interaction between the basic, amide groups of the PBI and the acid, sulfonate groups of the SPEEK.
When functionalization is not possible, immiscible polymers may be compatibilized. The addition of a compatibilizer is the main route to obtain melt-mixed polymer blends [54][55]. The compatibilizers may be classified into two categories:
-
Block copolymers: For instance, PET/PP blends are often compatibilized by PP-b-MA (MA: maleic anhydride) copolymers, where the block MA is miscible with PET. Gao et al. [58] compatibilized PEEK/PI blends with PEEK-b-PI block copolymers. In any case, the length of the blocks and the number of copolymers control the stability and the final morphology of the blends. It is worth noting that the choice of copolymers available to compatibilize PAEK with another HPT is very narrow.
Immiscible blends exhibit a two-phase morphology, where each phase is a rich phase of one component, whereas miscible blends should behave as a single-phase material. Compatibilization leads to fine dispersion of one of the phases into the other, depending on the composition. Partially miscible blends have a spectrum of morphologies depending on the composition and processing parameters, as in Figure 13. Of interest is obtaining a co-continuous structure [59]. Indeed, even if the blend has an immiscible state, a co-continuous morphology may give a blend with mechanical properties better than expected. Thus, if blending two polymers leads to an immiscible blend, but they have complementary properties (for instance, thermal stability or solvent resistance), one may want to process them with a set that gives a co-continuous morphology to obtain enhanced properties. Such morphologies are expected when blending PAEK with other thermoplastics, likewise with conventional polymer blends.
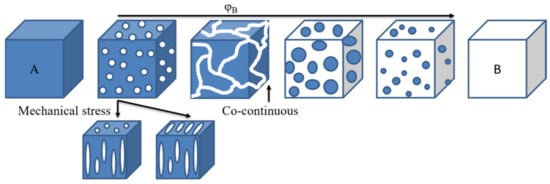
Figure 13. Basic types of phase structures in polymer blends.
Moreover, due to the crystallization of PAEK, the resulting morphology depends on the crystallinity generated in the blend. In the PAEK/HPT blends, the HPT are mostly amorphous, so the semicrystalline/amorphous blends may develop three main configurations [27][28][60][61].
References
- Lu, S.X.; Cebe, P.; Capel, M. Thermal stability and thermal expansion studies of PEEK and related polyimides. Polymer 1996, 37, 2999–3009.
- Blundell, D.J.; Osborn, B.N. The morphology of poly(aryl-ether-ether-ketone). Polymer 1983, 24, 953–958.
- Rigby, R.B. Polyetheretherketone PEEK. Polym. News 1984, 9, 325.
- Velisaris, C.N.; Seferis, J.C. Crystallization kinetics of polyetheretherketone (PEEK) matrices. Polym. Eng. Sci. 1986, 26, 1574–1581.
- Parameswaranpillai, J.; Thomas, S.; Grohens, Y. Polymer blends: State of the art, new challenges, and opportunities. In Characterization of Polymer Blends; Thomas, S., Grohens, Y., Jyotishkumar, P., Eds.; Wiley: Weinheim, Germany, 2014; pp. 1–6. ISBN 978-3-527-64560-2.
- Dosière, M.; Villers, D.; Zolotukhin, M.G.; Koch, M.H.J. Comparison of the structure and thermal properties of a poly(aryl ether ketone ether ketone naphthyl ketone) with those of poly(aryl ether ketone ether ketone ketone). e-Polymers 2007, 7, 130.
- Harsha, A.P.; Tewari, U.S. Tribo performance of polyaryletherketone composites. Polym. Test. 2002, 21, 697–709.
- Schmidt, M.; Pohle, D.; Rechtenwald, T. Selective laser sintering of PEEK. CIRP Ann. 2007, 56, 205–208.
- Technical data—Kepstan 6000. Arkema Innovative Chemistry: Colombes, France, 2013.
- Technical data—Kepstan 7000. Arkema Innovative Chemistry: Colombes, France, 2012.
- Technical data—Kepstan 8000. Arkema Innovative Chemistry: Colombes, France, 2013.
- Villar Montoya, M. Procédé de soudage laser de polymères haute performance: Établissement des relations entre les paramètres du procédé, la structure et la morphologie du polymère et les propriétés mécaniques de l’assemblage. Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2018.
- Technical data—Victrex HT G45. Victrex High Performance Polymers: Lancashire, UK, 2014.
- Team Xomertry Polyether Ether Ketone (PEEK): Characteristics, Features, and Process. Available online: https://www.xometry.com/resources/materials/polyether-ether-ketone/ (accessed on 27 July 2023).
- Friedrich, K.; Lu, Z.; Hager, A.M. Recent advances in polymer composites’ tribology. Wear 1995, 190, 139–144.
- Heath, D.R.; Pittsfield; Mass; Wirth, J.G. Schenectady Polyetherimides. Patent US003847867, 1974.
- Technical data—Sabic ULTEMTM Resin 1000—Europe. Sabic: Sittard, The Netherlands, 2018.
- Frigione, M.; Naddeo, C.; Acierno, D. The rheological behavior of polyetheretherketone (PEEK)/polyetherimide (PEI) blends. J. Polym. Eng. 1996, 16, 14.
- Ramani, R.; Alam, S. Composition optimization of PEEK/PEI blend using model-free kinetics analysis. Thermochim. Acta 2010, 511, 179–188.
- Briscoe, B.J.; Stuart, B.H. A fourier transform raman specroscopy study of the crystallization behaviour of poly (ether ether ketone)/poly (ether imide) blends. Spectrochim. Acta 1993, 49A, 753–758.
- Meakin, P.J.; Cogswell, F.N.; Halbritter, A.J.; Smiley, A.J.; Staniland, P.A. Thermoplastic interlayer bonding of aromatic polymer composites—Methods for using semi-crystallized polymers. Compos. Manuf. 1991, 2, 86–91.
- Korycki, A.; Garnier, C.; Bonmatin, M.; Laurent, E.; Chabert, F. Assembling of carbon fibre/PEEK composites: Comparison of ultrasonic, induction, and transmission laser welding. Materials 2022, 15(18), 6365.
- Yoo, J.H.; Eiss, N.S., Jr. Tribological behavior of blends of polyether ether ketone and polyether imide. Wear 1993, 162–164, 418–425.
- Díez Pascual, A.M.; Díez Vicente, A.L. Nano-TiO2 reinforced PEEK/PEI blends as biomaterials for load-bearing implant applications. ACS Appl. Mater. Interfaces 2015, 7, 5561–5573.
- Cafiero, L.; Alfano, O.; Iannone, M.; Esposito, F.; Iannace, S.; Sorrentino, L. Microcellular foams from PEEK/PEI miscible blends. In Proceedings of the Regional Conference Graz, Graz, Austria, 6–10 September 2015; AIP Publishing: Graz, Austria, 2016; Volume 1779, p. 090009_1-5.
- Harris, J.E.; Robeson, L.M. Miscible blends of poly(aryl ether ketone)s and polyetherimides. J. Appl. Polym. Sci. 1988, 35, 1877–1891.
- Hudson, S.D.; Davis, D.D.; Lovinger, A.J. Semicrystalline morphology of poly(aryl ether ether ketone)/poly(ether imide) blends. Macromolecules 1992, 25, 1759–1765.
- Hsiao, B.S.; Sauer, B.B. Glass transition, crystallization, and morphology relationships in miscible poly(aryl ether ketones) and poly(ether imide) blends. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 901–915.
- Sauer, B.B.; Hsiao, B.S. Miscibility of three different poly(aryl ether ketones) with a high melting thermoplastic polyimide. Polymer 1993, 34, 3315–3318.
- Nemoto, T.; Takagi, J.; Ohshima, M. Nanocellular foamscell structure difference between immiscible and miscible PEEK/PEI polymer blends. Polym. Eng. Sci. 2010, 50, 2408–2416.
- Mark, J.E. Polymer Data Handbook; Oxford University Press: New York, NY, USA, 1999.
- Wu, H.L.; Ma, C.C.M.; Li, C.H.; Lee, T.M.; Chen, C.Y.; Chiang, C.L.; Wu, C. Sulfonated poly(ether ether ketone)/poly(amide imide) polymer blends for proton conducting membrane. J. Membr. Sci. 2006, 280, 501–508.
- Wang, Y.; Goh, S.H.; Chung, T.S. Miscibility study of Torlon® polyamide-imide with Matrimid® 5218 polyimide and polybenzimidazole. Polymer 2007, 48, 2901–2909.
- Jones, D.J.; Rozière, J. Recent advances in the functionalisation of polybenzimidazole and polyetherketone for fuel cell applications. J. Membr. Sci. 2001, 185, 41–58.
- Liu, P.; Mullins, M.; Bremner, T.; Browne, J.A.; Sue, H.J. Hygrothermal behavior of polybenzimidazole. Polymer 2016, 93, 88–98.
- Brooks, N.W.; Duckett, R.A.; Rose, J.; Ward, I.M.; Clements, J. An n.m.r. study of absorbed water in polybenzimidazole. Polymer 1993, 34, 4038–4042.
- Chung, T.S. A critical review of polybenzimidazoles. Polym. Revs. 1997, 37, 277–301.
- Zhang, H.; Li, X.; Zhao, C.; Fu, T.; Shi, Y.; Na, H. Composite membranes based on highly sulfonated PEEK and PBI: Morphology characteristics and performance. J. Membr. Sci. 2008, 308, 66–74.
- Linkous, C. Development of solid polymer electrolytes for water electrolysis at intermediate temperatures. Int. J. Hydrog. Energy 1993, 18, 641–646.
- Yu, X.; Zheng, Y.; Wu, Z.; Tang, X.; Jiang, B. Study on the compatibility of the blend of poly(aryl ether ether ketone) with poly(aryl ether sulfone). J. Appl. Polym. Sci. 1990, 41, 2649–2654.
- Liu, J.; Li, J.; Wang, T.; Huang, D.; Li, Z.; Zhong, A.; Liu, W.; Sui, Y.; Liu, Q.; Niu, F.; et al. Organosoluble thermoplastic polyimide with improved thermal stability and UV absorption for temporary bonding and debonding in ultra-thin chip package. Polymer 2022, 244, 124660.
- Tsutsumi, T.; Nakakura, T.; Takahashi, T.; Morita, A.; Gotoh, Y.; Oochi, H. Polyimide based resin composition. Patent US005516837A, 1996.
- Hou, T.H.; Reddy, R.M. Thermoplastic Polyimide New-TPI; NASA: Hampton, VA, USA, 1990.
- Yang, H.; Liu, J.; Ji, M.; Yang, S. Novel thermoplastic polyimide composite materials. In Thermoplastic—Composite Materials; Intech Open Science: London, UK, 2012; pp. 1–11.
- Tsutsumi, T.; Nakakura, T.; Morikawa, S.; Shimamura, K.; Takahashi, T.; Morita, A.; Koga, N.; Yamaguchi, A.; Ohta, M.; Gotoh, Y.; et al. Polyimide based resin composition. Patent US5312866A, 1994.
- Kemmish, D.J. High performance engineering plastics; Report 86; Smithers Rapra Publishing: Shawbury, UK, 1995.
- Kiss, G. In situ composites: Blends of isotropic polymers and thermotropic liquid crystalline polymers. Polym. Eng. Sci. 1987, 27, 410–423.
- Sariban, A.; Binder, K. Citical properties of the Flory–Huggins lattice model of polymer mixtures. J. Chem. Phys. 1987, 86, 5859–5873.
- Patterson, D.; Robard, A. Thermodynamics of polymer compatibility. Macromolecules 1978, 11, 690–695.
- Helfand, E.; Tagami, Y. Theory of the interface between immiscible polymers. II. J. Chem. Phys. 1972, 56, 3592–3601.
- Olabisi, O. Process for the moulding of plastic structural web and the resulting articles. Patent US004136220, 1979.
- DeMeuse, M.T. High temperature polymer blends: An overview of the literature. Polym. Adv. Technol. 1995, 6, 76–82.
- Higgins, J.S.; Lipson, J.E.G.; White, R.P. A simple approach to polymer mixture miscibility. Philos. Trans. R. Soc. A 2010, 368, 1009–1025.
- Scott, C.E.; Macosko, C.W. Morphology development during the initial stages of polymer-polymer blending. Polymer 1995, 36, 461–470.
- Sundararaj, U.; Macosko, C.W. Drop breakup and coalescence in polymer blends: The effects of concentration and compatibilization. Macromolecules 1995, 28, 2647–2657.
- Faria, J.; Ruiz, M.P.; Resasco, D.E. Phase-selective catalysis in emulsions stabilized by janus silica-nanoparticles. Adv. Synth. Catal. 2010, 352, 2359–2364.
- Raphaël, E. Equilibre d’un “grain janus” à une interface eau/huil. CR Acad. Sci. Paris Série II 1988, 307, 9–12.
- Gao, C.; Zhang, S.; Li, X.; Zhu, S.; Jiang, Z. Synthesis of poly(ether ether ketone)-block-polyimide copolymer and its compatibilization for poly(ether ether ketone)/thermoplastic polyimide blends. Polymer 2014, 55, 119–125.
- Pötschke, P.; Paul, D.R. Formation of co-continuous structures in melt-mixed immiscible polymer blends. J. Macromol. Sci. Part C Polym. Rev. 2003, 43, 87–141.
- Chen, H.L.; Porter, R.S. Phase and crystallization behavior of solution-blended poly(ether ether ketone) and poly(ether imide). Polym. Eng. Sci. 1992, 32, 1870–1875.
- Crevecoeur, G.; Groeninckx, G. Binary blends of poly(ether ether ketone) and poly(ether imide). Miscibility, crystallization behavior and semicrystalline morphology. Macromolecules 1991, 24, 1190–1195.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
24 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




