
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Georgia Saxami | -- | 5123 | 2023-10-19 08:52:30 | | | |
| 2 | Camila Xu | -53 word(s) | 5070 | 2023-10-19 09:45:57 | | |
Video Upload Options
The human gut microbiota (GM) is a complex microbial ecosystem that colonises the gastrointestinal tract (GIT) and is comprised of bacteria, viruses, fungi, and protozoa. The GM has a symbiotic relationship with its host that is fundamental for body homeostasis. The GM is not limited to the scope of the GIT, but there are bidirectional interactions between the GM and other organs, highlighting the concept of the “gut–organ axis”. Any deviation from the normal composition of the GM, termed ”microbial dysbiosis”, is implicated in the pathogenesis of various diseases. Only a few studies have demonstrated a relationship between GM modifications and disease phenotypes, and it is still unknown whether an altered GM contributes to a disease or simply reflects its status. Restoration of the GM with probiotics and prebiotics has been postulated, but evidence for the effects of prebiotics is limited. Prebiotics are substrates that are “selectively utilized by host microorganisms, conferring a health benefit”.
1. Introduction
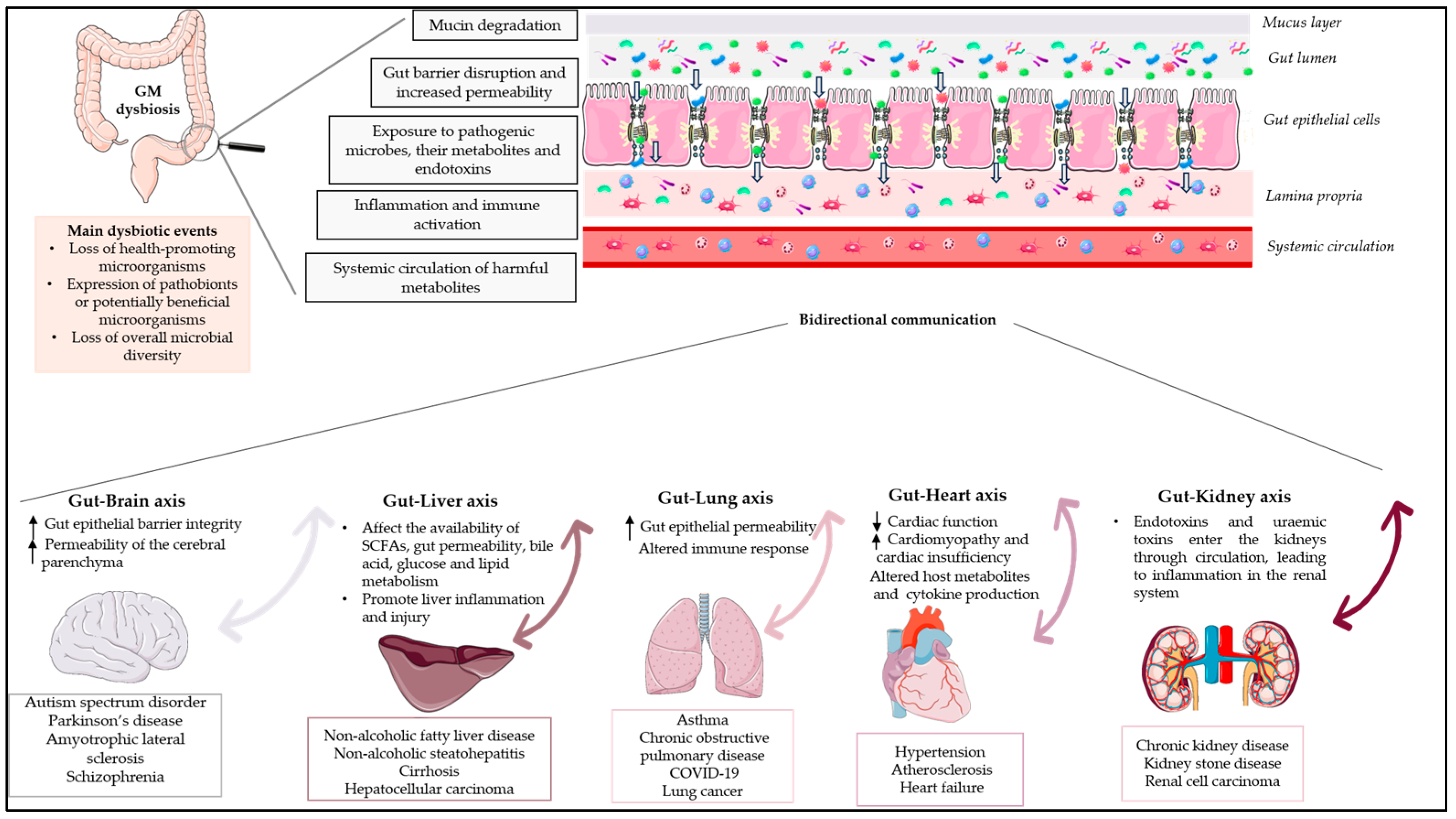
2. The Gut–Brain Axis
2.1. Gut Dysbiosis in Neurologic Diseases
2.1.1. Dysbiosis in Autism Spectrum Disorder
2.1.2. Dysbiosis in Parkinson’s Disease
2.1.3. Dysbiosis in Amyotrophic Lateral Sclerosis (ALS)
2.1.4. Dysbiosis in Schizophrenia
2.2. The Role of Prebiotics in Neurological Diseases
| Neurodegenerative Disease |
Main Dysbiotic Events in GM | Reference |
|---|---|---|
| Autism spectrum disorder (ASD) |
|
[15][26][29] |
| Parkinson’s disease (PD) |
|
[38][39] |
| Amyotrophic lateral sclerosis (ALS) |
|
[51][53] |
| Schizophrenia |
|
[60][62][63][64] |
| Disease | Study Design | Population | Prebiotic Compound |
Effects on the Disease | Beneficial Effects on GM |
Reference |
|---|---|---|---|---|---|---|
| Neurological diseases | Randomised, double-blind, placebo-controlled study |
30 children diagnosed with ASD were categorised into two groups, A and B, based on their dietary habits. Group A consisted of children with unrestricted diets (n = 18), while Group B comprised those following an exclusion diet (n = 12). Subsequently, within each of these groups, children were assigned randomly to two feeding subgroups using a random number system. Group I received a placebo, while Group II was administered B-GOS® | B-GOS® mixture (Bimuno®; Clasado Biosciences Ltd., Reading, UK) 1.8 g: 80% GOS content for a 6-week feeding period | Improvement in social behaviour scores | The administration of B-GOS led to modulation of the GM composition in autistic children following unrestricted diets. This modulation primarily affected bifidobacterial populations and also influenced other bacterial groups, including members of the Lachnospiraceae family such as Coprococcus spp., Dorea formicigenerans, and Oribacterium spp. | [74] |
| Cohort study | 13 ASD children aged 4–9 years |
Partially hydrolysed guar gum (6 g/day) for two months or longer | Decrease the behavioural irritability | The relative prevalence of Acidaminococcus and Blautia increased, whereas the relative prevalence of Streptococcus, Odoribacter, and Eubacterium decreased | [75] | |
| Open-label, non-randomised study | 20 participants with PD, consisting of 10 newly diagnosed, non-medicated individuals with PD and 10 individuals who were already receiving treatment for PD | Prebiotics in the form of a bar containing resistant starch, rice bran, resistant maltodextrin, and inulin for 10 days (one bar = 10 g fibre) |
Unified Parkinson’s Disease Rating Scale improved with treatment | The consumption of prebiotics resulted in a reduction in the relative abundance of potentially pro-inflammatory bacteria, such as Proteobacteria and Escherichia coli, while increasing the relative abundance of bacteria known to produce SCFAs, including Faecalibacterium prausnitzii | [76] | |
| Monocentric, prospective, open-label clinical trial |
The study included 87 subjects distributed across three study arms: 32 PD patients who received resistant starch, 30 control subjects who also received resistant starch, and 25 PD patients who were provided with dietary instructions only | 5 g of resistant starch twice per day orally over a period of 8 weeks | Reduction in non-motor symptom load in the PD patients who received resistant starch | Stabilised faecal microbial diversity | [77] | |
| 1 female subject with schizophrenia | A prebiotic preparation of lactosucrose (OligoOne®) 3.0 g/day was administered, with the medication unchanged | Improvement of psychotic symptoms | After three months of lactosucrose administration, there was a significant decrease in the abundance of Clostridium and an increased Bifidobacterium to Clostridium ratio. Additionally, improvements were observed in bowel movements, and there was a reduction in constipation | [78] | ||
| Liver diseases | Placebo-controlled, randomised pilot trial | 14 individuals with liver-biopsy-confirmed NASH | The subjects were randomised to receive oligofructose (8 g/day for 12 weeks followed by 16 g/day for 24 weeks) or isocaloric placebo for 9 months | Prebiotic improved liver steatosis relative to placebo and improved overall NAS score | Oligofructose supplementation led to an increase in Bifidobacterium levels, while it resulted in a reduction of bacteria belonging to Clostridium cluster XI and I | [79] |
| Small cohort single-centre study | Twenty-four subjects with histologically confirmed liver cirrhosis and a body mass index (BMI) of 25.78 kg/m2 were compared to 29 healthy controls | In the patient group, lactitol was administered orally at a dosage of 5 g three times daily, and samples were collected after four weeks of treatment | All clinical parameters, including MELD, showed no difference between pre- and post-lactitol treatment groups | After the lactitol intervention, there was an increase in the levels of health-promoting lactic acid bacteria, such as Bifidobacterium longum, B. pseudo-catenulatum, and Lactobacillus salivarius. Additionally, there was a significant decrease in the pathogen Klebsiella pneumonia and the associated antibiotic-resistant genes and virulence factors | [80] | |
| Heart diseases | Randomised, placebo-controlled, double-blind cross-over trial | Untreated individuals with hypertension, being of either sex, 18–70 years of age, and having a BMI of 18.5–35 kg/m2 | Participants were initially assigned to either Diet A or Diet B for a duration of 3 weeks. Diet A included HAMSAB (prebiotic acetylated and butyrylated high amylose maize starch) administered at a daily dosage of 40 g, while Diet B consisted of a daily intake of 40 g of a placebo over the same 3-week period. After a 3-week washout period, participants switched to the opposite diet arm for another 3 weeks | Reduction in ambulatory systolic blood pressure | HAMSAB intervention promoted the growth of the commensal bacteria P. distasonis and R. gauvreauii and supported the restoration of local production of SCFAs by these microbes | [81] |
| Kidney diseases | Double-blind, parallel, randomised, placebo-controlled trial | 20 patients with end-stage CKD undergoing haemodialysis | The participants were randomised to two groups: one received biscuits containing 20 g/d of high-amylose maize-resistant starch type 2 (HAM-RS2), an insoluble, fermentable fibre, while the other received regular wheat flour (placebo) for the first month and 25 g/d during the second month | Decrease in in systemic inflammation (serum urea, IL-6, TNFα, and malondialdehyde) | Supplementation of amylose-resistant starch, HAM-RS2, in patients with CKD led to an increase in Faecalibacterium | [82] |
| Randomised controlled clinical trial | 32 patients with CKD in stages 3 and 4 were recruited and randomly assigned to intervention (n = 16) and control (n = 16) groups |
Patients in intervention group received 30 mm lactulose syrup three timesa day for an 8-week period. Control group received placebo 30 mm three times a day | Creatinine significantly decreased in intervention group | Lactulose administration increase faecal Bifidobacteria and Lactobacillus counts in CKD patients |
[83] | |
| Randomised, double-blind, placebo-controlled, crossover study | 12 patients undergoing haemodialysis | Patients were randomised to consume inulin (10 g/d for females; 15 g/d for males) or maltodextrin (6 g/d for females; 9 g/d for males) for 4 weeks, with a 4-week washout period | Inulin did not reduce faecal p-cresol or indoles, or plasma concentrations of p-cresyl sulphate or indoxyl sulphate | Inulin increased the relative abundance of the phylum Verrucomicrobia and its genus Akkermansia. In addition, inulin and maltodextrin resulted in an increased relative abundance of the phylum Bacteroidetes and its genus Bacteroides | [84] | |
| Randomised single-centre, single-blinded control trial | 59 predialysis participants with CKD in stages 3 to 5 were randomised | 59 participants were randomised to either the β-glucan prebiotic intervention group (13.5 g of β-glucan prebiotic fibre supplement containing 6 g of fibre, of which 3 g was β-glucan per serving) daily (n = 30) or the control group (n = 29) for 14 weeks |
Supplementation of β-glucan fibre resulted in reduced plasma levels of the free fraction of colon-derived uremic toxins, without a change in kidney function over the 14-week study period |
High prevalence of Bacteroides 2 in the CKD population | [85] |
References
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135.
- Chen, Y.; Zhou, J.; Wang, L. Role and mechanism of gut microbiota in human disease. Front. Cell. Infect. Microbiol. 2021, 11, 86.
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001.
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533.
- Matzaras, R.; Nikopoulou, A.; Protonotariou, E.; Christaki, E. Gut microbiota modulation and prevention of dysbiosis as an alternative approach to antimicrobial resistance: A narrative review. Yale J. Biol. Med. 2022, 95, 479.
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203.
- Van de Wouw, M.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-gut-brain axis: Modulator of host metabolism and appetite. J. Nutr. 2017, 147, 727–745.
- Auchtung, T.A.; Fofanova, T.Y.; Stewart, C.J.; Nash, A.K.; Wong, M.C.; Gesell, J.R.; Auchtung, J.M.; Ajami, N.J.; Petrosino, J.F. Investigating colonization of the healthy adult gastrointestinal tract by fungi. MSphere 2018, 3, e00092-18.
- Matijašić, M.; Meštrović, T.; Čipčić Paljetak, H.; Perić, M.; Barešić, A.; Verbanac, D. Gut microbiota beyond bacteria—Mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668.
- Ahlawat, S.; Asha; Sharma, K. Gut–organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668.
- Guo, Y.; Chen, X.; Gong, P.; Li, G.; Yao, W.; Yang, W. The Gut–Organ-Axis Concept: Advances the Application of Gut-on-Chip Technology. Int. J. Mol. Sci. 2023, 24, 4089.
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033.
- Das, B.; Nair, G.B. Homeostasis and dysbiosis of the gut microbiome in health and disease. J. Biosci. 2019, 44, 117.
- Ng, Q.X.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Soh, A.Y.S.; Yeo, W.S. A systematic review of the role of prebiotics and probiotics in autism spectrum disorders. Medicina 2019, 55, 129.
- Saxami, G.; Mitsou, E.K.; Kerezoudi, E.N.; Mavrouli, I.; Vlassopoulou, M.; Koutrotsios, G.; Mountzouris, K.C.; Zervakis, G.I.; Kyriacou, A. In Vitro Fermentation of Edible Mushrooms: Effects on Faecal Microbiota Characteristics of Autistic and Neurotypical Children. Microorganisms 2023, 11, 414.
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017, 179, 223–244.
- Naveed, M.; Zhou, Q.-G.; Xu, C.; Taleb, A.; Meng, F.; Ahmed, B.; Zhang, Y.; Fukunaga, K.; Han, F. Gut-brain axis: A matter of concern in neuropsychiatric disorders…! Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 104, 110051.
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013.
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 2018, 9, 44.
- Miri, S.; Yeo, J.; Abubaker, S.; Hammami, R. Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol. 2023, 14, 1098412.
- Yarandi, S.S.; Peterson, D.A.; Treisman, G.J.; Moran, T.H.; Pasricha, P.J. Modulatory effects of gut microbiota on the central nervous system: How gut could play a role in neuropsychiatric health and diseases. J. Neurogastroenterol. Motil. 2016, 22, 201.
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut microbe to brain signaling: What happens in vagus. Neuron 2019, 101, 998–1002.
- Intili, G.; Paladino, L.; Rappa, F.; Alberti, G.; Plicato, A.; Calabrò, F.; Fucarino, A.; Cappello, F.; Bucchieri, F.; Tomasello, G. From Dysbiosis to Neurodegenerative Diseases through Different Communication Pathways: An Overview. Biology 2023, 12, 195.
- Zhu, J.; Guo, M.; Yang, T.; Lai, X.; Tang, T.; Chen, J.; Li, L.; Li, T. Nutritional Status and Symptoms in Preschool Children With Autism Spectrum Disorder: A Two-Center Comparative Study in Chongqing and Hainan Province, China. Front. Pediatr. 2020, 8, 469.
- Bougeard, C.; Picarel-Blanchot, F.; Schmid, R.; Campbell, R.; Buitelaar, J. Prevalence of Autism Spectrum Disorder and Co-morbidities in Children and Adolescents: A Systematic Literature Review. Front. Psychiatry 2021, 12, 744709.
- Andreo-Martínez, P.; García-Martínez, N.; Sánchez-Samper, E.; González, A. An approach to gut microbiota profile in children with autism spectrum disorder. Environ. Microbiol. Rep. 2019, 12, 115–135.
- Yitik Tonkaz, G.; Esin, I.S.; Turan, B.; Uslu, H.; Dursun, O.B. Determinants of Leaky Gut and Gut Microbiota Differences in Children With Autism Spectrum Disorder and Their Siblings. J. Autism Dev. Disord. 2022, 53, 2703–2716.
- Alharthi, A.; Alhazmi, S.; Alburae, N.; Bahieldin, A. The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 1363.
- Iglesias-Vázquez, L.; Van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792.
- Chakraborty, P.; Carpenter, K.L.H.; Major, S.; Deaver, M.; Vermeer, S.; Herold, B.; Franz, L.; Howard, J.; Dawson, G. Gastrointestinal problems are associated with increased repetitive behaviors but not social communication difficulties in young children with autism spectrum disorders. Autism 2021, 25, 405–415.
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712.
- Faruqui, N.A.; Prium, D.H.; Mowna, S.A.; Rahaman, T.I.; Dutta, A.R.; Akter, M.F. Identification of Common Molecular Signatures Shared between Alzheimer’s and Parkinson’s Diseases and Therapeutic Agents Exploration: An Integrated Genomics Approach. bioRxiv 2021.
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflammation 2019, 16, 53.
- Stopińska, K.; Radziwoń-Zaleska, M.; Domitrz, I. The Microbiota-Gut-Brain Axis as a Key to Neuropsychiatric Disorders: A Mini Review. J. Clin. Med. 2021, 10, 4640.
- Elfil, M.; Kamel, S.; Kandil, M.; Koo, B.B.; Schaefer, S.M. Implications of the gut microbiome in Parkinson’s disease. Mov. Disord. 2020, 35, 921–933.
- Grochowska, M.; Laskus, T.; Radkowski, M. Gut microbiota in neurological disorders. Arch. Immunol. Ther. Exp. 2019, 67, 375–383.
- Lin, A.; Zheng, W.; He, Y.; Tang, W.; Wei, X.; He, R.; Huang, W.; Su, Y.; Huang, Y.; Zhou, H.; et al. Gut microbiota in patients with Parkinson’s disease in southern China. Park. Relat. Disord. 2018, 53, 82–88.
- Romano, S.; Savva, G.; Bedarf, J.; Charles, I.; Hildebrand, F.; Narbad, A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. npj Park. Dis. 2021, 7, 27.
- Guo, T.; Chen, L. Gut microbiota and inflammation in Parkinson’s disease: Pathogenetic and therapeutic insights. Eur. J. Inflamm. 2022, 20, 1721727X221083763.
- Lin, C.-H.; Chen, C.-C.; Chiang, H.-L.; Liou, J.-M.; Chang, C.-M.; Lu, T.-P.; Chuang, E.Y.; Tai, Y.-C.; Cheng, C.; Lin, H.-Y. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammation 2019, 16, 129.
- Scheperjans, F.; Aho, V.; Pereira, P.A.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358.
- Vascellari, S.; Melis, M.; Palmas, V.; Pisanu, S.; Serra, A.; Perra, D.; Santoru, M.L.; Oppo, V.; Cusano, R.; Uva, P. Clinical phenotypes of Parkinson’s disease associate with distinct gut microbiota and metabolome enterotypes. Biomolecules 2021, 11, 144.
- Hashish, S.; Salama, M. The Role of an Altered Gut Microbiome in Parkinson’s Disease: A Narrative Review. Appl. Microbiol. 2023, 3, 429–447.
- Yang, D.; Zhao, D.; Ali Shah, S.Z.; Wu, W.; Lai, M.; Zhang, X.; Li, J.; Guan, Z.; Zhao, H.; Li, W. The role of the gut microbiota in the pathogenesis of Parkinson’s disease. Front. Neurol. 2019, 10, 1155.
- Boddy, S.L.; Giovannelli, I.; Sassani, M.; Cooper-Knock, J.; Snyder, M.P.; Segal, E.; Elinav, E.; Barker, L.A.; Shaw, P.J.; McDermott, C.J. The gut microbiome: A key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. 2021, 19, 13.
- Gotkine, M.; Kviatcovsky, D.; Elinav, E. Amyotrophic lateral sclerosis and intestinal microbiota—Toward establishing cause and effect. Gut Microbes 2020, 11, 1833–1841.
- McCombe, P.A.; Henderson, R.D.; Lee, A.; Lee, J.D.; Woodruff, T.M.; Restuadi, R.; McRae, A.; Wray, N.R.; Ngo, S.; Steyn, F.J. Gut microbiota in ALS: Possible role in pathogenesis? Expert Rev. Neurother. 2019, 19, 785–805.
- Zeng, Q.; Shen, J.; Chen, K.; Zhou, J.; Liao, Q.; Lu, K.; Yuan, J.; Bi, F. The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci. Rep. 2020, 10, 12998.
- Maskovic, J.; Ilic, A.; Zugic, V.; Stevic, Z.; Stjepanovic, M.I. What is the right moment for noninvasive ventilation in amyotrophic lateral sclerosis? Arch. Med. Sci. 2023, 19, 337.
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol. Ther. 2022, 231, 107988.
- Fang, X.; Wang, X.; Yang, S.; Meng, F.; Wang, X.; Wei, H.; Chen, T. Evaluation of the microbial diversity in amyotrophic lateral sclerosis using high-throughput sequencing. Front. Microbiol. 2016, 7, 1479.
- Hertzberg, V.S.; Singh, H.; Fournier, C.N.; Moustafa, A.; Polak, M.; Kuelbs, C.A.; Torralba, M.G.; Tansey, M.G.; Nelson, K.E.; Glass, J.D. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 91–99.
- Di Gioia, D.; Bozzi Cionci, N.; Baffoni, L.; Amoruso, A.; Pane, M.; Mogna, L.; Gaggìa, F.; Lucenti, M.A.; Bersano, E.; Cantello, R. A prospective longitudinal study on the microbiota composition in amyotrophic lateral sclerosis. BMC Med. 2020, 18, 153.
- Insel, T.R. Rethinking schizophrenia. Nature 2010, 468, 187–193.
- Howes, O.D.; Murray, R.M. Schizophrenia: An integrated sociodevelopmental-cognitive model. Lancet 2014, 383, 1677–1687.
- Howes, O.D.; McCutcheon, R.; Owen, M.J.; Murray, R.M. The role of genes, stress, and dopamine in the development of schizophrenia. Biol. Psychiatry 2017, 81, 9–20.
- Kahn, R.; Sommer, I. The neurobiology and treatment of first-episode schizophrenia. Mol. Psychiatry 2015, 20, 84–97.
- Kahn, R.S.; Keefe, R.S. Schizophrenia is a cognitive illness: Time for a change in focus. JAMA Psychiatry 2013, 70, 1107–1112.
- Menezes, N.; Arenovich, T.; Zipursky, R. A systematic review of longitudinal outcome studies of first-episode psychosis. Psychol. Med. 2006, 36, 1349–1362.
- Zheng, P.; Zeng, B.; Liu, M.; Chen, J.; Pan, J.; Han, Y.; Liu, Y.; Cheng, K.; Zhou, C.; Wang, H. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019, 5, eaau8317.
- Gokulakrishnan, K.; Nikhil, J.; Viswanath, B.; Thirumoorthy, C.; Narasimhan, S.; Devarajan, B.; Joseph, E.; David, A.K.D.; Sharma, S.; Vasudevan, K. Comparison of gut microbiome profile in patients with schizophrenia and healthy controls-A plausible non-invasive biomarker? J. Psychiatr. Res. 2023, 162, 140–149.
- Shen, Y.; Xu, J.; Li, Z.; Huang, Y.; Yuan, Y.; Wang, J.; Zhang, M.; Hu, S.; Liang, Y. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study. Schizophr. Res. 2018, 197, 470–477.
- Pan, R.; Zhang, X.; Gao, J.; Yi, W.; Wei, Q.; Su, H. Analysis of the diversity of intestinal microbiome and its potential value as a biomarker in patients with schizophrenia: A cohort study. Psychiatry Res. 2020, 291, 113260.
- Ma, X.; Asif, H.; Dai, L.; He, Y.; Zheng, W.; Wang, D.; Ren, H.; Tang, J.; Li, C.; Jin, K. Alteration of the gut microbiome in first-episode drug-naïve and chronic medicated schizophrenia correlate with regional brain volumes. J. Psychiatr. Res. 2020, 123, 136–144.
- Yang, C.; Lin, X.; Wang, X.; Liu, H.; Huang, J.; Wang, S. The schizophrenia and gut microbiota: A bibliometric and visual analysis. Front. Psychiatry 2022, 13, 1022472.
- Kao, A.C.C.; Harty, S.; Burnet, P.W.J. Chapter Two—The Influence of Prebiotics on Neurobiology and Behavior. In Gut Microbiome and Behavior; Cryan, J.F., Clarke, G., Eds.; International Review of Neurobiology; Academic Press: Cambridge, MA, USA, 2016; Volume 131, pp. 21–48.
- He, Q.; Si, C.; Sun, Z.; Chen, Y.; Zhang, X. The intervention of prebiotics on depression via the Gut–Brain axis. Molecules 2022, 27, 3671.
- Liu, X.; Cao, S.; Zhang, X. Modulation of gut microbiota–brain axis by probiotics, prebiotics, and diet. J. Agric. Food Chem. 2015, 63, 7885–7895.
- Franco-Robles, E.; Ramírez-Emiliano, J.; López-Briones, J.S.; Balcón-Pacheco, C.D. Prebiotics and the Modulation on the Microbiota-GALT-Brain Axis. In Prebiotics and Probiotics-Potential Benefits in Nutrition and Health; IntechOpen: London, UK, 2019.
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C. The microbiota–gut–brain axis: Pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell. Mol. Life Sci. 2022, 79, 80.
- Lee, S.H.; Ahmad, S.R.; Lim, Y.C.; Zulkipli, I.N. The use of probiotic therapy in metabolic and neurological diseases. Front. Nutr. 2022, 9, 887019.
- Sharma, V.; Kaur, S. The Effect of Probiotic Intervention in Ameliorating the Altered Central Nervous System Functions in Neurological Disorders: A Review. Open Microbiol. J. 2020, 14, 18–29.
- Babu, C.S.; Chethan, N.; Rao, B.S.; Bhat, A.; Bipul, R.; Tousif, A.; Mahadevan, M.; Sathiya, S.; Manivasagam, T.; Thenmozhi, A.J. Probiotics, prebiotics, and synbiotics on neurological disorders: Relevance to Huntington’s disease. In Food for Huntington’s Disease; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2018; pp. 105–140.
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Leigh Gibson, E.; Nielsen, D.S.; Costabile, A. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 2018, 6, 133.
- Inoue, R.; Sakaue, Y.; Kawada, Y.; Tamaki, R.; Yasukawa, Z.; Ozeki, M.; Ueba, S.; Sawai, C.; Nonomura, K.; Tsukahara, T.; et al. Dietary supplementation with partially hydrolyzed guar gum helps improve constipation and gut dysbiosis symptoms and behavioral irritability in children with autism spectrum disorder. J. Clin. Biochem. Nutr. 2019, 64, 217–223.
- Hall, D.; Voigt-Zuwala, R.; Jungles, T.; Hamaker, B.; Engen, P.; Shaikh, M.; Raeisi, S.; Green, S.; Naqib, A.; Forsyth, C.; et al. An open label, non-randomized study assessing a prebiotic fiber intervention in a small cohort of Parkinson’s disease participants. Nat. Commun. 2023, 14, 926.
- Becker, A.; Schmartz, G.; Gröger, L.; Grammes, N.; Galata, V.; Philippeit, H.; Weiland, J.; Ludwig, N.; Meese, E.; Tierling, S.; et al. Effects of Resistant Starch on Symptoms, Fecal Markers and Gut Microbiota in Parkinson’s Disease—The RESISTA-PD Trial. Genom. Proteom. Bioinform. 2021, 20, 274–287.
- Ido, Y.; Nagamine, T.; Okamura, T.; Tasaki, M.; Fukuo, K. Prebiotic lactosucrose may improve not only constipation but also psychotic symptoms of Schizophrenia. Int. Med. J. 2017, 24, 305–306.
- Bomhof, M.; Parnell, J.; Ramay, H.; Crotty, P.; Rioux, K.; Probert, C.; Jayakumar, S.; Raman, M.; Reimer, R. Histological improvement of non-alcoholic steatohepatitis with a prebiotic: A pilot clinical trial. Eur. J. Nutr. 2019, 58, 1735–1745.
- Lu, H.; Chen, L.; Pan, X.; Yao, Y.; Huan, Z.; Zhu, X.; Lou, X.; Zhu, C.; Wang, J.; Li, L.; et al. Lactitol Supplementation Modulates Intestinal Microbiome in Liver Cirrhotic Patients. Front. Med. 2021, 8, 762930.
- Jama, H.; Rhys-Jones, D.; Nakai, M.; Yao, C.; Climie, R.; Sata, Y.; Anderson, D.; Creek, D.; Head, G.; Kaye, D.; et al. Prebiotic intervention with HAMSAB in untreated essential hypertensive patients assessed in a phase II randomized trial. Nat. Cardiovasc. Res. 2023, 2, 35–43.
- Laffin, M.; Park, H.; Laffin, L.; Madsen, K.; Kafil, H.; Abedi, B.; Shiralizadeh, S.; Vaziri, N. Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: Microbial analysis from a randomized placebo-controlled trial. Hemodial. Int. 2019, 23, 343–347.
- Tayebi-Khosroshahi, H.; Habibzadeh, A.; Niknafs, B.; Ghotaslou, R.; Yeganeh Sefidan, F.; Ghojazadeh, M.; Moghaddaszadeh, M.; Parkhide, S. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial. J. Ren. Inj. Prev. 2016, 5, 162–167.
- Biruete, A.; Cross, T.-W.; Allen, J.; Kistler, B.; Loor, H.; Evenepoel, P.; Fahey, G.; Bauer, L.; Swanson, K.; Wilund, K. Effect of Dietary Inulin Supplementation on the Gut Microbiota Composition and Derived Metabolites of Individuals Undergoing Hemodialysis: A Pilot Study. J. Ren. Nutr. 2021, 31, 512–522.
- Ebrahim, Z.; Proost, S.; Tito Tadeo, R.; Raes, J.; Glorieux, G.; Moosa, R.; Blaauw, R. The Effect of ß-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial. Nutrients 2022, 14, 805.




