The human gut microbiota (GM) is a complex microbial ecosystem that colonises the gastrointestinal tract (GIT) and is comprised of bacteria, viruses, fungi, and protozoa. The GM has a symbiotic relationship with its host that is fundamental for body homeostasis. The GM is not limited to the scope of the GIT, but there are bidirectional interactions between the GM and other organs, highlighting the concept of the “gut–organ axis”. Any deviation from the normal composition of the GM, termed ”microbial dysbiosis”, is implicated in the pathogenesis of various diseases. Only a few studies have demonstrated a relationship between GM modifications and disease phenotypes, and it is still unknown whether an altered GM contributes to a disease or simply reflects its status. Restoration of the GM with probiotics and prebiotics has been postulated, but evidence for the effects of prebiotics is limited. Prebiotics are substrates that are “selectively utilized by host microorganisms, conferring a health benefit”. This study highlights the bidirectional relationship between the gut and vital human organs and demonstrates the relationship between GM dysbiosis and the emergence of certain representative diseases. Finally, this article focuses on the potential of prebiotics as a target therapy to manipulate the GM and presents the gaps in the literature and research.
- gut–organ axis
- gut microbiota dysbiosis
- prebiotics
1. Introduction
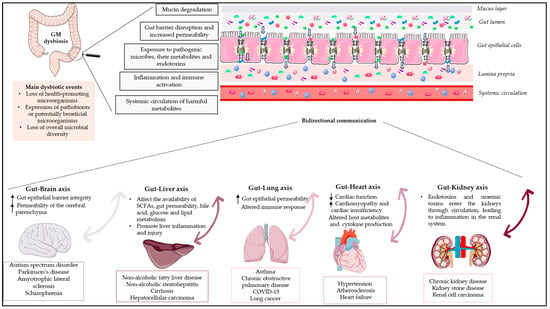
2. The Gut–Brain Axis
The gut–brain axis comprises a complex physiological system that enables bidirectional communication between the gut and the host nervous system. [16]. This bidirectional communication within the gut–brain axis elucidates how messages from the GM influence brain function and how signals from the brain impact gastrointestinal physiology and gut microbial activity [17]. These bidirectional communications involve the central nervous system (CNS), intrinsic branches of the enteric nervous system (ENS), extrinsic parasympathetic and sympathetic branches of the autonomic nervous system (ANS), the hypothalamic–pituitary–adrenal axis (HPA), neuroimmune pathways (neurotransmitters, hormones, and neuropeptides), and the gut microenvironment [15][18][15,18]. The HPA axis, a component of the limbic system, is considered the central stress efferent axis that coordinates the organism’s adaptive responses to all stressors. Environmental stress and elevated systemic pro-inflammatory cytokines activate this system, which, via the secretion of the corticotropin-releasing factor from the hypothalamus, stimulates adrenocorticotropic hormone secretion from the pituitary gland, which ultimately results in cortisol release from the adrenal glands [6]. Thus, the combination of neural and hormonal lines of communication allows the brain to influence the activities of gut functional effector cells, including immune cells, epithelial cells, and enteric neurons [19]. On the other hand, these same cells are influenced by the GM, which may influence these central processes directly and indirectly via immune system activation, the production of neurotransmitters, and the production of short-chain fatty acids (SCFAs) and key dietary amino acids such as tryptophan and its metabolites [20]. Furthermore, the GM can act through the permeability of the gut barrier, with an increase in circulating lipopolysaccharide (LPS), modulating the levels of brain-derived neurotrophic factor and altering neuroendocrine and neural pathways. In addition, the brain affects gut peristalsis, and sensory and secretion function, mainly via the vagus nerve. The vagus nerve, which transmits information from the luminal environment to the CNS, is the major nerve of the parasympathetic system of the ANS and a crucial modulatory constitutive direct communication pathway between the GM and the brain [21]. The vagus nerve consists of sensory and motor neurons and has been extensively studied for its involvement in hunger, satiety, and stress response but also for its major role in the regulation of inflammation via neuronal motor efferents [22]. The gut–brain axis is expected to have many effects on mood, motivation, and higher cognitive functions, in addition to ensuring that gastrointestinal homeostasis is properly maintained [6]. Disruption of the delicate balance between host and gut bacteria could be a contributing factor behind various diseases. The dysregulation of the gut–brain axis has been linked by numerous researchers to various immunologic, neurologic, and psychiatric disorders.2.1. Gut Dysbiosis in Neurologic Diseases
GM dysbiosis interferes with the development of local and systemic inflammatory states, resulting in altered gut epithelial barrier integrity, allowing the release of hormones, microbial metabolites, and components by the GM that reach the brain via the vagus nerve, crossing the blood–brain barrier, and inducing neurodegenerative processes [23]. Moreover, dysbiosis increases the permeability of the cerebral parenchyma, which may result in neuroinflammation and dysfunctional neuronal cells. Emerging research indicates that gut dysbiosis may influence the onset and progression of a variety of neurological disorders, such as autism spectrum disorder (ASD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and schizophrenia. Table 1 provides a summary of the main dysbiotic events on the GM composition identified in neurological disorders. Subsequent sections will delve into the analysis of representative diseases and their associated dysbiotic events.2.1.1. Dysbiosis in Autism Spectrum Disorder
Autism spectrum disorder (ASD) is a complex group of neurodevelopmental disorders characterised by aberrant social interactions and communication, repetitive and stereotyped patterns of behaviour, and abnormal sensory responses. [24]. According to a recent systematic literature review, the prevalence of ASD in US children ranked 1.70 and 1.85% in children aged 4 and 8 years, respectively, while the prevalence in Europe ranged between 0.38 and 1.55% [25]. Although genetic and environmental factors have been linked to the development of ASD, the precise etiology remains unknown. Recent research has highlighted the role of the gut–brain axis in various neuropsychiatric disorders, including autism spectrum disorder. In addition, individuals with ASD frequently experience gastrointestinal disturbances, such as constipation, diarrhoea, flatulence, increased gut permeability, and abdominal pain [26][27][26,27]. Several studies have highlighted differences in the GM composition between ASD and neurotypical children [28]. It should be noted, however, that among studies related to ASD, no specific microbial species has been found to be significantly different, as various factors such as diet, age, sex, population, and severity of autism should be taken into account [28]. Although changes in the GM composition of autistic children are not always consistent across studies, patients frequently exhibit microbial imbalances of multiple types, including higher abundances of Bacteroides, Parabacteroides, Clostridium, Faecalibacterium, and Phascolarctobacterium and a lower relative abundance of Streptococcus and Bifidobacterium [26][29][26,29]. The gastrointestinal symptoms of individuals with ASD seem to be significantly correlated with the degree of behavioural and cognitive impairment. For example, in individuals with ASD, irritability, aggressiveness, sleep disturbances, and self-injury are strongly associated with GI symptoms [26][30][26,31]. This evidence suggests that gastrointestinal abnormalities, perhaps linked to gut dysbiosis, may be associated with ASD [31][32]. Consistent with this hypothesis, a meta-analysis by Iglesias-Vázquez et al. [29] suggests that there is a dysbiosis in ASD children that may influence the development and severity of ASD symptomatology. More specifically, this study concluded that the microbiota of ASD individuals was mainly composed of the phyla Bacteroidetes, Firmicutes, and Actinobacteria and also showed a significantly higher abundance of the genera Bacteroides, Parabacteroides, Clostridium, Faecalibacterium, and Phascolarctobacterium and a lower percentage of Coprococcus and Bifidobacterium. Taken together, all these alterations in the GM could be associated with increased GI disturbances in individuals with ASD.2.1.2. Dysbiosis in Parkinson’s Disease
Parkinson’s disease (PD) is the second most common degenerative disorder of the brain, affecting seven to ten million people worldwide [32][33]. PD is mainly characterised by multifactorial motor and non-motor symptoms, including resting tremor, muscular rigidity, slowness of movement, and gait abnormality, as well as cognitive disturbances, depression, mood deflection, sensory alternations, and sleep alternations [32][33][33,34]. The principal pathology of PD is characterised by the loss of dopamine-producing neurons present in a specific region of the brain, known as the substantia nigra, accompanied by the accumulation of alfa-synuclein (alfa-syn) in the form of Lewy bodies and Lewy neurites, a condition known as synucleinopathy [34][35]. Complex genetic and environmental factors are involved in the etiology of PD; however, the cause of PD remains unknown. Gastrointestinal symptoms are observed in most PD patients, including hypersalivation, dysphagia, constipation, nausea, altered bowel habits, and defecatory dysfunction [32][33]. Several studies have demonstrated GM abnormalities in patients with PD [35][36][37][36,37,38]. A meta-analysis conducted by Romano et al. [38][39] re-analysing the ten currently available 16S microbiome datasets found significant alterations in the PD-associated microbiome. More specifically, the authors concluded that enrichment of the genera Lactobacillus, Akkermansia, and Bifidobacterium and depletion of bacteria belonging to the Lachnospiraceae family and the Faecalibacterium genus, emerged as the most consistent PD gut microbiome changes, suggesting that the observed dysbiosis may be a result of pro-inflammation, which could be linked to the GI symptom manifestation in PD patients [38][39]. In another study, consistent increases were principally shown in the family Verrucomicrobiaceae, genus Akkermansia, and species Akkermansia muciniphila, while health-promoting genera and butyrate producers Roseburia and Faecalibaterium were reported to decrease in PD patients [39][40]. Emerging studies have shown the correlations between GM alterations and the phenotypes of PD, including both motor and non-motor symptoms [40][41][42][41,42,43]. These alterations in the GM of patients may reveal a mechanism, as this observed dysbiosis has been associated with increased intestinal barrier permeability and subsequent gut inflammation. This hypothesis is supported by a number of studies that demonstrate that GM dysbiosis in PD is shown to be associated with the disrupted intestinal barrier, which is closely associated with gut inflammation, an established symptom in PD patients [43][44][44,45].2.1.3. Dysbiosis in Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease defined by progressive loss of cortical, brain stem, and spinal motor neurons, resulting in weakness and wasting of the musculature [45][46][46,47]. In addition, ALS presents extra-motor features, including cognitive and behavioural disturbances [47][48]. Over 90% of ALS cases are sporadic (sALS) and of unknown cause, while the remaining 10% are familial (fALS) since they carry a mutation in one of the disease-related genes [47][48]. Mutations of superoxide dismutase 1 (SOD1), FUS RNA binding protein (FUS/TLS), C9orf72-SMCR8 complex subunit (C9orf72), and TAR DNA binding protein (TARDBP/TDP-43) are more commonly associated with ALS [48][49]. ALS etiology and pathophysiology require further elucidation, and in spite of massive efforts having been invested, there is no cure available at present, leading to death by respiratory failure within 2–5 years from symptom onset [49][50]. Recent studies demonstrate a strong pathophysiological crosstalk between the GM and ALS [50][51]. ALS pathogenesis has been linked to alterations in GM composition, impaired metabolism, an altered innate immune response, and the production of gut-derived neurotoxins by Clostridia species that induce brain damage [50][51]. Due to a number of factors, such as the small sample size, the observed heterogeneity within the study population, the various experimental procedures and data analysis, and the heterogeneity of the GM regardless of health status, the results of human studies conducted to determine the potential role of the GM in ALS patients are frequently inconclusive. Despite the contradictory results among the studies, some important findings could be observed, which include the following: (1) Differences in the GM populations between ALS patients and healthy individuals. For example, in the study of Fang et al. [51][52], which examined six ALS patients and five healthy people without ALS, the authors demonstrated significant differences in GM composition between the two groups. More specifically, in the gut of ALS patients, a reduced ratio of Firmicutes/Bacteroidetes was accompanied by a decreased abundance of butyrate-producing Oscillibacter, Anaerostipes, and Lachnospira counts and an increased abundance of glucose-metabolizing Dorea. More recently, comparing the GM of 10 ALS patients and their spouses (n = 10), it was found that the populations of the ALS patients’ GM were more diverse and deficient in Prevotella spp., suggesting that modifying the gut microbiome, such as via amelioration of Prevotella spp. deficiency, and/or altering butyrate metabolism, may have translational value for ALS treatment [52][53]. (2) GM composition alters during the course of the ALS. Gioia and colleagues [53][54] studied the GM of 50 ALS patients and 50 matched controls and demonstrated that the GM of ALS patients differed from that of controls. Also, the composition of the intestinal microbiota changed as the disease progressed, as indicated by a significant decrease in the number of operational taxonomy units observed during the follow-up. Intriguingly, an imbalance between potentially protective microbial groups, such as Bacteroidetes, and those with potential neurotoxic or pro-inflammatory activity, such as Cyanobacteria, has been observed. Overall, these findings indicate the implication of the GM in ALS disease; however, it has been difficult to ascertain whether these changes in the GM are the cause of ALS, an aggravating factor for the disease, or the result of the disease. Additional human clinical research evidence is required in order to establish the exact role of the GM in the pathogenesis of ALS.2.1.4. Dysbiosis in Schizophrenia
Schizophrenia is a complex, heterogeneous, neurodevelopmental disorder with deficits across many dimensions [54][55]. The expression of the underlying genetic vulnerability is shaped by a multifaceted combination of prenatal and early postnatal environmental factors [55][56][56,57]. These factors may sensitise a developing brain and its information processing ability to the subsequent accumulation of additional environmental insults, which may overwhelm compensatory capacities during adolescence and emerge as psychotic symptoms [57][58]. Subtle deficits in cognition, social communication, and functioning are often evident prior to the onset of overt psychotic symptoms [58][59], and the majority of people experience recurring psychotic relapses with variable degrees of functional impairment [59][60]. A precise integrative mechanistic understanding of the interaction of genetic and environmental processes across the neurodevelopmental trajectory in this condition remains elusive. The link between schizophrenia and the GM has garnered increasing attention in recent years. The main findings of existing studies examining the link between the GM and schizophrenia include the following: (a) Patients with schizophrenia have a deviant GM compared to healthy controls. The diversity and composition of the GM were substantially altered in schizophrenia patients, according to these findings [60][61][61,62]. Zheng et al. [60][61] found significant alterations in beta diversity but not alpha diversity between the GM of patients and controls. In the schizophrenia group, an enhanced count of bacterial families like Prevotellaceae, Veillonellaceae, Bacteroidaceae, and Coriobacteriaceae was observed compared to healthy controls, while Ruminococcus and Roseburia abundances were significantly lower in patients with schizophrenia. (b) Specific bacteria may function as biomarkers to differentiate patients with schizophrenia from healthy individuals [62][63][63,64]. Shen et al. identified 12 biomarkers that could be used as diagnostic factors to differentiate the schizophrenia cohort from the control cohort, including Gammaproteobacteria (at class level), Enterobacteriales (at order level), Alcaligenaceae, Enterobacteriaceae, and Lachnospiraceae (at family level), Acidaminococcus, Phascolarctobacterium, Blautia, Desulfovibrio, and Megasphaera (at genus level), and Plebeius fragilis (at species level). (c) Differences in the GM between remission and acute schizophrenia. Pan et al. [63][64] demonstrated differences between acute and remission patients, indicating that alterations in the intestinal microbiota may influence the prognosis of the disease and suggesting the GM’s potential as a non-invasive diagnostic tool. (d) Differences in the GM between first-episode drug-naïve and chronically medicated schizophrenia patients [64][65]. Chronically antipsychotic-treated schizophrenia patients showed lower microbial richness and diversity as compared to first-episode drug-naïve schizophrenia patients and healthy controls, suggesting that the gut microbiome may be implicated in the pathophysiology of schizophrenia via modulation of specific brain structures [64][65]. (e) The role of the gut–brain axis. The GM was found to be associated with schizophrenia via processes involved in the gut–brain axis, including immune-regulating pathways, neurotransmitter synthesis, the production of bioactive microbial metabolites, and tryptophan metabolism [65][66]. Schizophrenia-related behaviour has been observed in mice by Zheng et al. [60][61], who demonstrated that transplantation of the GM from schizophrenia patients induces schizophrenia-like behaviours in germ-free recipient rodents, suggesting that the GM can affect the brain neurochemistry associated with the onset of schizophrenia.2.2. The Role of Prebiotics in Neurological Diseases
In recent years, different studies, including mostly in vitro and in vivo studies, and only a few human studies, have shown the beneficial effects of prebiotics on brain function [66][67][67,68]. The proposed mechanisms for prebiotic-based modulation of the GM–brain axis include the following [68][69][70][69,70,71]: (i) decreased inflammation in gut inflammatory disorders, preventing the presence of inflammatory compounds in the brain; (ii) improvement of GM composition and modulation of brain function, enhancing the composition of the GM; and (iii) influence on the production of neurochemicals. In addition, it has been suggested that, compared to probiotics, prebiotics could be advantageous due to probiotics’ inability to survive in the GI tract [68][69]. Numerous clinical studies examine the impact of probiotics and symbiotics on neurological conditions [71][72][73][72,73,74]. On the other hand, the supplementation of prebiotics to manipulate the GM as a novel treatment for neurological diseases has not been investigated, and there are only a few human studies that examine the effectiveness of prebiotics, while in ALS there have been no clinical studies (Table 2). The first study to examine the effects of prebiotics on ASD was conducted by Grimaldi et al. [74][75]. More particularly, the authors assessed the impact of a prebiotic (B-GOS® mixture, Clasado Biosciences Ltd., Reading, UK) on GM composition and metabolic activity in 30 autistic children. According to the results, the administration of B-GOS led to modulation of the GM composition in autistic children following unrestricted diets. This modulation primarily affected bifidobacterial populations and also affected other bacterial groups, including members of the Lachnospiraceae family such as Coprococcus spp., Dorea formicigenerans, and Oribacterium spp. [74][75]. Furthermore, another study noted an amelioration of GM dysbiosis in children with ASD [75][76]. Dietary supplementation with partially hydrolysed guar gum (PHGG) in ASD children increased the relative prevalence of Acidaminococcus and Blautia, whereas the relative prevalence of Streptococcus, Odoribacter, and Eubacterium decreased. Also, prebiotic intervention decreased the behavioural irritability of ASD children [75][76]. Two studies have been conducted examining the effect of prebiotic supplementation with a simultaneous effect on GM modulation in Parkinson’s disease [76][77][77,78]. In the study of Becker et al. [77][78], an 8-week prebiotic intervention with resistant starch (RS) was conducted, enrolling 87 subjects distributed across three study arms: 32 PD patients who received RS, 30 control subjects who also received RS, and 25 PD patients who were provided with dietary instructions only. According to the results, a reduction in non-motor symptom load and a stable gut microbiome in PD patients after RS intervention were observed. In the study of Hall et al. [76][77], an open-label, non-randomised study was conducted in 10 newly diagnosed and 10 non-medicated and treated PD participants, wherein the impact of 10 days of prebiotic (bar containing resistant starch and rice brain) intervention was evaluated. The prebiotic supplementation resulted in a reduction in the relative abundance of potentially pro-inflammatory bacteria, such as Proteobacteria and Escherichia coli, while increasing the relative abundance of SCFA-producing bacteria, including Faecalibacterium prausnitzii. In addition, the unified Parkinson’s disease rating scale improved with prebiotic treatment [76][77]. The effects of prebiotic supplementation on schizophrenia were studied by Ido et al. [78][79]. More specifically, a female subject with schizophrenia was administered a prebiotic preparation of lactosucrose while keeping her medication unchanged. According to the results, after three months of lactosucrose administration, there was an improvement in psychotic symptoms, a significant decrease in the abundance of Clostridium, and an increased Bifidobacterium-to-Clostridium ratio [78][79]. More research is required to determine the effects of prebiotics in the management of neurological diseases. While there have been promising studies suggesting potential benefits, more comprehensive and long-term human research is needed to establish conclusive evidence.| Neurodegenerative Disease |
|---|
| Disease | Main Dysbiotic Events in GM | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study Design | Population | Prebiotic | Compound |
Effects on the Disease | Beneficial Effects on GM |
Reference | |||
| Autism spectrum disorder (ASD) |
|
||||||||
| Neurological diseases | Randomised, double-blind, | [15][26][15[29],26,29] | |||||||
| placebo-controlled study |
30 children diagnosed with ASD were categorised into two groups, A and B, based on their dietary habits. Group A consisted of children with unrestricted diets (n = 18), while Group B comprised those following an exclusion diet (n = 12). Subsequently, within each of these groups, children were assigned randomly to two feeding subgroups using a random number system. Group I received a placebo, while Group II was administered B-GOS® | B-GOS | [ | 75 | ] | Parkinson’s disease (PD) |
|
[ | |
| Cohort study | 13 ASD children aged 4–9 years | 38][39][39,40] | |||||||
| Partially hydrolysed guar gum (6 g/day) for two months or longer | Decrease the behavioural irritability | The relative prevalence of | Acidaminococcus | and Blautia increased, whereas the relative prevalence of Streptococcus, Odoribacter, and Eubacterium decreased | [75][76] | Amyotrophic lateral sclerosis (ALS) |
| ||
| Open-label, non-randomised study |
|
20 participants with PD, consisting of 10 newly diagnosed, non-medicated individuals with PD and 10 individuals who were already receiving treatment for PD[51][53][52,54] | |||||||
| Prebiotics in the form of a bar containing resistant starch, rice bran, | resistant maltodextrin, and inulin for 10 days (one bar = 10 g fibre) | Unified Parkinson’s Disease Rating Scale improved with treatment | The consumption of prebiotics resulted in a reduction in the relative abundance of potentially pro-inflammatory bacteria, such as Proteobacteria and Escherichia coli, while increasing the relative abundance of bacteria known to produce SCFAs, including Faecalibacterium prausnitzii | [76][77] | Schizophrenia |
|
[60][62][63][61[,63,64,65] |
| ® | ||||||
| mixture (Bimuno | ® | ; Clasado Biosciences Ltd., Reading, UK) 1.8 g: 80% GOS content for a 6-week feeding period | Improvement in social behaviour scores | The administration of B-GOS led to modulation of the GM composition in autistic children following unrestricted diets. This modulation primarily affected bifidobacterial populations and also influenced other bacterial groups, including members of the | Lachnospiraceae family such as Coprococcus spp., Dorea formicigenerans, and Oribacterium spp. | [74] |
| Monocentric, | ||||||
prospective, open-label clinical trial | 64 | The study included 87 subjects distributed across three study arms: 32 PD patients who received resistant starch, 30 control subjects who also received resistant starch, and 25 PD patients who were provided with dietary instructions only] | 5 g of resistant starch twice per day orally over a period of 8 weeks | Reduction in non-motor symptom load in the PD patients who received resistant starch | Stabilised faecal microbial diversity | [77][78] |
| 1 female subject with schizophrenia | A prebiotic preparation of lactosucrose (OligoOne®) 3.0 g/day was administered, with the medication unchanged | Improvement of psychotic symptoms | After three months of lactosucrose administration, there was a significant decrease in the abundance of Clostridium and an increased Bifidobacterium to Clostridium ratio. Additionally, improvements were observed in bowel movements, and there was a reduction in constipation | [78][79] | ||
| Liver diseases | Placebo-controlled, randomised pilot trial | 14 individuals with liver-biopsy-confirmed NASH | The subjects were randomised to receive oligofructose (8 g/day for 12 weeks followed by 16 g/day for 24 weeks) or isocaloric placebo for 9 months | Prebiotic improved liver steatosis relative to placebo and improved overall NAS score | Oligofructose supplementation led to an increase in Bifidobacterium levels, while it resulted in a reduction of bacteria belonging to Clostridium cluster XI and I | [79][80] |
| Small cohort single-centre study | Twenty-four subjects with histologically confirmed liver cirrhosis and a body mass index (BMI) of 25.78 kg/m2 were compared to 29 healthy controls | In the patient group, lactitol was administered orally at a dosage of 5 g three times daily, and samples were collected after four weeks of treatment | All clinical parameters, including MELD, showed no difference between pre- and post-lactitol treatment groups | After the lactitol intervention, there was an increase in the levels of health-promoting lactic acid bacteria, such as Bifidobacterium longum, B. pseudo-catenulatum, and Lactobacillus salivarius. Additionally, there was a significant decrease in the pathogen Klebsiella pneumonia and the associated antibiotic-resistant genes and virulence factors | [80][81] | |
| Heart diseases | Randomised, placebo-controlled, double-blind cross-over trial | Untreated individuals with hypertension, being of either sex, 18–70 years of age, and having a BMI of 18.5–35 kg/m2 | Participants were initially assigned to either Diet A or Diet B for a duration of 3 weeks. Diet A included HAMSAB (prebiotic acetylated and butyrylated high amylose maize starch) administered at a daily dosage of 40 g, while Diet B consisted of a daily intake of 40 g of a placebo over the same 3-week period. After a 3-week washout period, participants switched to the opposite diet arm for another 3 weeks | Reduction in ambulatory systolic blood pressure | HAMSAB intervention promoted the growth of the commensal bacteria P. distasonis and R. gauvreauii and supported the restoration of local production of SCFAs by these microbes | [81][82] |
| Kidney diseases | Double-blind, parallel, randomised, placebo-controlled trial | 20 patients with end-stage CKD undergoing haemodialysis | The participants were randomised to two groups: one received biscuits containing 20 g/d of high-amylose maize-resistant starch type 2 (HAM-RS2), an insoluble, fermentable fibre, while the other received regular wheat flour (placebo) for the first month and 25 g/d during the second month | Decrease in in systemic inflammation (serum urea, IL-6, TNFα, and malondialdehyde) | Supplementation of amylose-resistant starch, HAM-RS2, in patients with CKD led to an increase in Faecalibacterium | [82][83] |
| Randomised controlled clinical trial | 32 patients with CKD in stages 3 and 4 were recruited and randomly assigned to intervention (n = 16) and control (n = 16) groups |
Patients in intervention group received 30 mm lactulose syrup three timesa day for an 8-week period. Control group received placebo 30 mm three times a day | Creatinine significantly decreased in intervention group | Lactulose administration increase faecal Bifidobacteria and Lactobacillus counts in CKD patients |
[83][84] | |
| Randomised, double-blind, placebo-controlled, crossover study | 12 patients undergoing haemodialysis | Patients were randomised to consume inulin (10 g/d for females; 15 g/d for males) or maltodextrin (6 g/d for females; 9 g/d for males) for 4 weeks, with a 4-week washout period | Inulin did not reduce faecal p-cresol or indoles, or plasma concentrations of p-cresyl sulphate or indoxyl sulphate | Inulin increased the relative abundance of the phylum Verrucomicrobia and its genus Akkermansia. In addition, inulin and maltodextrin resulted in an increased relative abundance of the phylum Bacteroidetes and its genus Bacteroides | [84][85] | |
| Randomised single-centre, single-blinded control trial | 59 predialysis participants with CKD in stages 3 to 5 were randomised | 59 participants were randomised to either the β-glucan prebiotic intervention group (13.5 g of β-glucan prebiotic fibre supplement containing 6 g of fibre, of which 3 g was β-glucan per serving) daily (n = 30) or the control group (n = 29) for 14 weeks |
Supplementation of β-glucan fibre resulted in reduced plasma levels of the free fraction of colon-derived uremic toxins, without a change in kidney function over the 14-week study period |
High prevalence of Bacteroides 2 in the CKD population | [85][86] |
