
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Charlene Kay | -- | 3043 | 2023-10-16 11:57:33 | | | |
| 2 | Lindsay Dong | Meta information modification | 3043 | 2023-10-17 03:19:39 | | |
Video Upload Options
The majority of breast cancers are oestrogen receptor-positive (ER+). In ER+ cancers, oestrogen acts as a disease driver, so these tumours are likely to be susceptible to endocrine therapy (ET). ET works by blocking the hormone’s synthesis or effect. A significant number of patients diagnosed with breast cancer will have the spread of tumour cells into regional lymph nodes either at the time of diagnosis, or as a recurrence some years later. Patients with node-positive disease have a poorer prognosis and can respond less well to ET. The nodal metastases may be genomically similar or, as is becoming more evident, may differ from the primary tumour. However, nodal metastatic disease is often not assessed, and treatment decisions are almost always based on biomarkers evaluated in the primary tumour.
1. Background
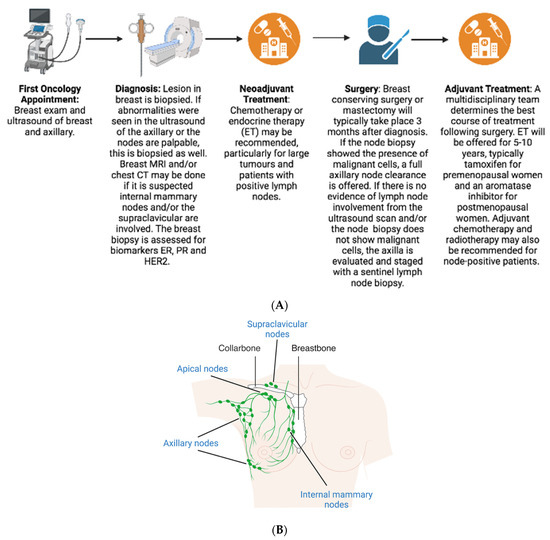
2. Diagnosis
3. Pathological Assessment
4. Treatment
4.1. Neoadjuvant
4.2. Surgery
4.3. Adjuvant
5. Prognosis
6. Prediction
6.1. Predict
6.2. Oncotype DX
6.3. MammaPrint
6.4. PAM50 (Prosigna)
6.5. EndoPredict
6.6. Breast Cancer Index
6.7. IHC4
7. Conclusions
References
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.M.; et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl. Cancer Inst. 2015, 107, djv048.
- Konecny, G.; Pauletti, G.; Pegram, M.; Untch, M.; Dandekar, S.; Aguilar, Z.; Wilson, C.; Rong, H.-M.; Bauerfeind, I.; Felber, M.; et al. Quantitative Association Between HER-2/neu and Steroid Hormone Receptors in Hormone Receptor-Positive Primary Breast Cancer. JNCI J. Natl. Cancer Inst. 2003, 95, 142–153.
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.G.; Cronin, K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 2014, 106, dju055.
- NIH National Cancer Institute. Cancer Stat Facts: Female Breast Cancer. 2022. Available online: http://seer.cancer.gov/statfacts/html/breast.html#survival (accessed on 20 March 2023).
- Kennecke, H.F.; Olivotto, I.A.; Speers, C.; Norris, B.; Chia, S.K.; Bryce, C.; Gelmon, K.A. Late risk of relapse and mortality among postmenopausal women with estrogen responsive early breast cancer after 5 years of tamoxifen. Ann. Oncol. 2007, 18, 45–51.
- NIH National Cancer Institute. Sentinel Lymph Node Biopsy Fact Sheet. 2019. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/staging/sentinel-node-biopsy-fact-sheet (accessed on 20 March 2023).
- Chang, J.M.; Leung, J.W.T.; Moy, L.; Ha, S.M.; Moon, W.K. Axillary nodal evaluation in breast cancer: State of the art. Radiology 2020, 295, 500–515.
- National Institute for Health Care Excellence. Early and Locally Advanced Breast Cancer: Diagnosis and Management ; NICE 2018; National Institute for Health Care Excellence: London, UK, 2018.
- Dixon, J.M.; Cartlidge, C.W.J. Twenty-five years of change in the management of the axilla in breast cancer. Breast J. 2020, 26, 22–26.
- Magnoni, F.; Galimberti, V.; Corso, G.; Intra, M.; Sacchini, V.; Veronesi, P. Axillary surgery in breast cancer: An updated historical perspective. Semin. Oncol. 2020, 47, 341–352.
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA J. Am. Med. Assoc. 2017, 318, 918–926.
- Dixon, J.M.; Grewar, J.; Twelves, D.; Graham, A.; Martinez-Perez, C.; Turnbull, A. Factors affecting the number of sentinel lymph nodes removed in patients having surgery for breast cancer. Breast Cancer Res. Treat. 2020, 184, 335–343.
- Choosing, W. Don’t Routinely Use Sentinel Node Biopsy in Clinically Node Negative Women ≥ 70 Years of Age with Early Stage Hormone Receptor Positive, HER2 Negative Invasive Breast Cancer. 2021. Available online: https://www.choosingwisely.org/clinician-lists/sso-sentinel-node-biopsy-in-node-negative-women-70-and-over/ (accessed on 21 March 2023).
- Matar, R.; Barrio, A.V.; Sevilimedu, V.; Le, T.; Heerdt, A.; Morrow, M.; Tadros, A. Can We Successfully De-Escalate Axillary Surgery in Women Aged ≥ 70 Years with Ductal Carcinoma in Situ or Early-Stage Breast Cancer Undergoing Mastectomy? Ann. Surg. Oncol. 2022, 29, 2263–2272.
- Chagpar, A.B.; McMasters, K.M.; Edwards, M.J. Can Sentinel Node Biopsy Be Avoided in Some Elderly Breast Cancer Patients? Ann. Surg. 2009, 249, 455–460.
- Lyman, G.H.; Temin, S.; Edge, S.B.; Newman, L.A.; Turner, R.R.; Weaver, D.L.; Benson, A.B.; Bosserman, L.D.; Burstein, H.J.; Cody, H.; et al. Sentinel Lymph Node Biopsy for Patients with Early-Stage Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2014, 32, 1365–1383.
- Nasser, I.A.; Lee, A.K.; Bosari, S.; Saganich, R.; Heatley, G.; Silverman, M.L. Occult axillary lymph node metastases in “node-negative” breast carcinoma. Hum. Pathol. 1993, 24, 950–957.
- Huvos, A.G.; Hutter, R.V.; Berg, J.W. Significance of axillary macrometastases and micrometastases in mammary cancer. Ann. Surg. 1971, 173, 44–46.
- Fisher, E.R.; Palekar, A.; Rockette, H.; Redmond, C.; Fisher, B. Pathologic findings from the National Surgical Adjuvant Breast Project (Protocol No. 4). V. Significance of axillary nodal micro- and macrometastases. Cancer 1978, 42, 2032–2038.
- Apple, S.K. Sentinel Lymph Node in Breast Cancer: Review Article from a Pathologist’s Point of View. J. Pathol. Transl. Med. 2016, 50, 83–95.
- Balla, A.; Weaver, D.L. Pathologic Evaluation of Lymph Nodes in Breast Cancer: Contemporary Approaches and Clinical Implications. Surg. Pathol. Clin. 2022, 15, 15–27.
- Houvenaeghel, G.; de Nonneville, A.; Cohen, M.; Chopin, N.; Coutant, C.; Reyal, F.; Mazouni, C.; Gimbergues, P.; Azuar, A.S.; Chauvet, M.P.; et al. Lack of prognostic impact of sentinel node micro-metastases in endocrine receptor-positive early breast cancer: Results from a large multicenter cohort. ESMO Open 2021, 6, 100151.
- Nicolini, A.; Ferrari, P.; Duffy, M.J. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin. Cancer Biol. 2018, 52, 56–73.
- Zardavas, D.; Irrthum, A.; Swanton, C.; Piccart, M. Clinical management of breast cancer heterogeneity. Nat. Rev. Clin. Oncol. 2015, 12, 381–394.
- Martínez-Pérez, C.; Turnbull, A.K.; Dixon, J.M. The evolving role of receptors as predictive biomarkers for metastatic breast cancer. Expert Rev. Anticancer Ther. 2019, 19, 121–138.
- Barrio, A.V.; Montagna, G.; Mamtani, A.; Sevilimedu, V.; Edelweiss, M.; Capko, D.; Cody Iii, H.S.; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.; et al. Nodal Recurrence in Patients with Node-Positive Breast Cancer Treated with Sentinel Node Biopsy Alone after Neoadjuvant Chemotherapy—A Rare Event. JAMA Oncol. 2021, 7, 1851–1855.
- Cao, L.; Sugumar, K.; Keller, E.; Li, P.; Rock, L.; Simpson, A.; Freyvogel, M.; Montero, A.J.; Shenk, R.; Miller, M.E. Neoadjuvant Endocrine Therapy as an Alternative to Neoadjuvant Chemotherapy among Hormone Receptor-Positive Breast Cancer Patients: Pathologic and Surgical Outcomes. Ann Surg Oncol 2021, 28, 5730–5741.
- Solá, M.; Alberro, J.A.; Fraile, M.; Santesteban, P.; Ramos, M.; Fabregas, R.; Moral, A.; Ballester, B.; Vidal, S. Complete Axillary Lymph Node Dissection Versus Clinical Follow-up in Breast Cancer Patients with Sentinel Node Micrometastasis: Final Results from the Multicenter Clinical Trial AATRM 048/13/2000. Ann. Surg. Oncol. 2013, 20, 120–127.
- Zhang, J.; Wang, C. Axillary radiotherapy: An alternative treatment option for adjuvant axillary management of breast cancer. Sci. Rep. 2016, 6, 26304.
- Li, L.; Chang, B.; Jiang, X.; Fan, X.; Li, Y.; Li, T.; Wu, S.; Zhang, J.; Kariminia, S.; Li, Q. Clinical outcomes comparison of 10 years versus 5 years of adjuvant endocrine therapy in patients with early breast cancer. BMC Cancer 2018, 18, 977.
- Lei, J.T.; Anurag, M.; Haricharan, S.; Gou, X.; Ellis, M.J. Endocrine therapy resistance: New insights. Breast 2019, 48 (Suppl. S1), S26–S30.
- Anurag, M.; Ellis, M.J.; Haricharan, S. DNA damage repair defects as a new class of endocrine treatment resistance driver. Oncotarget 2018, 9, 36252.
- Wu, X.; Baig, A.; Kasymjanova, G.; Kafi, K.; Holcroft, C.; Mekouar, H.; Carbonneau, A.; Bahoric, B.; Sultanem, K.; Muanza, T. Pattern of Local Recurrence and Distant Metastasis in Breast Cancer By Molecular Subtype. Cureus 2016, 8, e924.
- Jung, S.U.; Sohn, G.; Kim, J.; Chung, I.Y.; Lee, J.W.; Kim, H.J.; Ko, B.S.; Son, B.H.; Ahn, S.H.; Yang, S.W.; et al. Survival outcome of adjuvant endocrine therapy alone for patients with lymph node-positive, hormone-responsive, HER2-negative breast cancer. Asian J. Surg. 2019, 42, 914–921.
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017, 377, 1836–1846.
- Matikas, A.; Foukakis, T.; Swain, S.; Bergh, J. Avoiding over- and undertreatment in patients with resected node-positive breast cancer with the use of gene expression signatures: Are we there yet? Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1044–1050.
- Wishart, G.C.; Azzato, E.M.; Greenberg, D.C.; Rashbass, J.; Kearins, O.; Lawrence, G.; Caldas, C.; Pharoah, P.D. PREDICT: A new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res. 2010, 12, R1.
- UK National Health Service. Predict Tool v2.2 2020. Available online: https://breast.predict.nhs.uk/tool (accessed on 16 February 2023).
- Cao, L.; Stabellini, N.; Towe, C.W.; Miller, M.E.; Shenk, R.; Amin, A.L.; Montero, A.J. BPI22-014: Independent Validation of the PREDICT Prognostication Tool in U.S. Breast Cancer Patients Using the National Cancer Database (NCDB). J. Natl. Compr. Cancer Netw. 2022, 20, BPI22-014.
- Ahles, T.A.; Saykin, A.J. Breast Cancer Chemotherapy-Related Cognitive Dysfunction. Clin. Breast Cancer 2002, 3, S84–S90.
- Kim, G.M.; Kim, S.; Park, H.S.; Kim, J.Y.; Nam, S.; Park, S.; Kim, S.I.; Kim, D.Y.; Sohn, J. Chemotherapy-induced irreversible alopecia in early breast cancer patients. Breast Cancer Res. Treat. 2017, 163, 527–533.
- Di Nardo, P.; Lisanti, C.; Garutti, M.; Buriolla, S.; Alberti, M.; Mazzeo, R.; Puglisi, F. Chemotherapy in patients with early breast cancer: Clinical overview and management of long-term side effects. Expert Opin. Drug Saf. 2022, 21, 1341–1355.
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N. Engl. J. Med. 2004, 351, 2817–2826.
- van ‘t Veer, L.J.; Dai, H.; van de Vijver, M.J.; He, Y.D.; Hart, A.A.M.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536.
- van de Vijver, M.J.; He, Y.D.; van ‘t Veer, L.J.; Dai, H.; Hart, A.A.M.; Voskuil, D.W.; Schreiber, G.J.; Peterse, J.L.; Roberts, C.; Marton, M.J.; et al. A Gene-Expression Signature as a Predictor of Survival in Breast Cancer. N. Engl. J. Med. 2002, 347, 1999–2009.
- Drukker, C.A.; Bueno-De-Mesquita, J.M.; Retèl, V.P.; Van Harten, W.H.; Van Tinteren, H.; Wesseling, J.; Roumen, R.M.H.; Knauer, M.; Van ‘t Veer, L.J.; Sonke, G.S.; et al. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int. J. Cancer 2013, 133, 929–936.
- Cardoso, F.; van’t Veer, L.J.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.-Y.; Brain, E.; Causeret, S.; DeLorenzi, M.; et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375, 717–729.
- Bernard, P.S.; Parker, J.S.; Mullins, M.; Cheung, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167.
- Nielsen, T.O.; Parker, J.S.; Leung, S.; Voduc, D.; Ebbert, M.; Vickery, T.; Davies, S.R.; Snider, J.; Stijleman, I.J.; Reed, J.; et al. A Comparison of PAM50 Intrinsic Subtyping with Immunohistochemistry and Clinical Prognostic Factors in Tamoxifen-Treated Estrogen Receptor–Positive Breast Cancer. Clin. Cancer Res. 2010, 16, 5222–5232.
- Filipits, M.; Rudas, M.; Jakesz, R.; Dubsky, P.; Fitzal, F.; Singer, C.F.; Dietze, O.; Greil, R.; Jelen, A.; Sevelda, P.; et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin. Cancer Res. 2011, 17, 6012–6020.
- Dubsky, P.; Brase, J.C.; Jakesz, R.; Rudas, M.; Singer, C.F.; Greil, R.; Dietze, O.; Luisser, I.; Klug, E.; Sedivy, R.; et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br. J. Cancer 2013, 109, 2959–2964.
- Constantinidou, A.; Marcou, Y.; Toss, M.S.; Simmons, T.; Bernhisel, R.; Hughes, E.; Probst, B.; Meek, S.; Kakouri, E.; Georgiou, G.; et al. Clinical Validation of EndoPredict in Pre-Menopausal Women with ER-Positive, HER2-Negative Primary Breast Cancer. Clin. Cancer Res. 2022, 28, 4435–4443.
- Bartlett, J.M.S.; Sgroi, D.C.; Treuner, K.; Zhang, Y.; Ahmed, I.; Piper, T.; Salunga, R.; Brachtel, E.F.; Pirrie, S.J.; Schnabel, C.A.; et al. Breast Cancer Index and prediction of benefit from extended endocrine therapy in breast cancer patients treated in the Adjuvant Tamoxifen—To Offer More? (aTTom) trial. Ann. Oncol. 2019, 30, 1776–1783.
- Noordhoek, I.; Treuner, K.; Putter, H.; Zhang, Y.; Wong, J.; Meershoek-Klein Kranenbarg, E.; Kranenbarg, E.; Duijm-de Carpentier, M.; van de Velde, C.J.H.; Schnabel, C.A.; et al. Breast Cancer Index Predicts Extended Endocrine Benefit to Individualize Selection of Patients with HR+ Early-stage Breast Cancer for 10 Years of Endocrine Therapy. Clin. Cancer Res. 2021, 27, 311–319.
- Liefers, G.-J.; Noordhoek, I.; Zhang, Y.; Sgroi, D.C.; Putter, H.; Treuner, K.; Wong, J.; Meershoek-Klein Kranenbarg, E.; Duijm-de Carpentier, M.; van de Velde, C.; et al. An optimized Breast Cancer Index node-positive (BCIN+) prognostic model for late distant recurrence in patients with hormone receptor positive (HR+) node positive breast cancer. Ann. Oncol. 2021, 32, S23–S24.
- Shah, S.; Shaing, C.; Khatib, J.; Lodrigues, W.; Dreadin-Pulliam, J.; Anderson, B.B.; Unni, N.; Farr, D.; Li, H.-C.; Sadeghi, N.; et al. The Utility of Breast Cancer Index (BCI) Over Clinical Prognostic Tools for Predicting the Need for Extended Endocrine Therapy: A Safety Net Hospital Experience. Clin. Breast Cancer 2022, 22, 823–827.
- Cuzick, J.; Dowsett, M.; Pineda, S.; Wale, C.; Salter, J.; Quinn, E.; Zabaglo, L.; Mallon, E.; Green, A.R.; Ellis, I.O.; et al. Prognostic Value of a Combined Estrogen Receptor, Progesterone Receptor, Ki-67, and Human Epidermal Growth Factor Receptor 2 Immunohistochemical Score and Comparison with the Genomic Health Recurrence Score in Early Breast Cancer. J. Clin. Oncol. 2011, 29, 4273–4278.
- Yeo, B.; Zabaglo, L.; Hills, M.; Dodson, A.; Smith, I.; Dowsett, M. Clinical utility of the IHC4+C score in oestrogen receptor-positive early breast cancer: A prospective decision impact study. Br. J. Cancer 2015, 113, 390–395.
- Cheang, M.C.U.; Bliss, J.M.; Viale, G.; Speirs, V.; Palmieri, C.; Shaaban, A.; Lønning, P.E.; Morden, J.; Porta, N.; Jassem, J.; et al. Evaluation of applying IHC4 as a prognostic model in the translational study of Intergroup Exemestane Study (IES): PathIES. Breast Cancer Res. Treat. 2018, 168, 169–178.
- Abubakar, M.; Figueroa, J.; Ali, H.R.; Blows, F.; Lissowska, J.; Caldas, C.; Easton, D.F.; Sherman, M.E.; Garcia-Closas, M.; Dowsett, M.; et al. Combined quantitative measures of ER, PR, HER2, and KI67 provide more prognostic information than categorical combinations in luminal breast cancer. Mod. Pathol. 2019, 32, 1244–1256.
- Harel, N.; Cheema, S.; Williams, D.; Ireland-Jenkin, K.; Fancourt, T.; Dodson, A.; Yeo, B. The IHC4+C score: An affordable and reproducible non-molecular decision-aid in hormone receptor-positive breast cancer. Does it still hold value for patients in 2020? Asia-Pac. J. Clin. Oncol. 2021, 17, 368–376.
- Liu, M.; Tang, S.-X.; Tsang, J.Y.S.; Shi, Y.-J.; Ni, Y.-B.; Law, B.K.B.; Tse, G.M.K. Core needle biopsy as an alternative to whole section in IHC4 score assessment for breast cancer prognostication. J. Clin. Pathol. 2018, 71, 1084.
- Jin, L.; Chen, K.; Tan, C.; Li, J.; Luo, J.; Yang, Y.; Li, Y.; Li, S.; Zhu, L.; Hu, Y.; et al. Prognostic Value of Modified IHC4 Score in Patients with Estrogen Receptor-Positive Metastatic Breast Cancer. Oncologist 2020, 25, e1170–e1180.




