Historically, the vast majority of patients have had all their axillary lymph nodes removed via ALND at the time of breast surgery
[10][13], often removing 10–20 lymph nodes. However, the results of the sentinel node biopsy trials and the American College of Surgeons Oncology Group’s (ACOSOG) Z0011 trial changed this. This randomised phase 3 clinical trial included 856 women with T1 or T2 invasive cancer, of whom 446 had a SLNB alone and 446 had a SLNB followed by an ALND. The results showed no significant difference in 10-year overall survival between the two groups of patients with no palpable lymph nodes and 1 or 2 metastatic lymph nodes on SLNB, thus discouraging routine use of ALND
[11][14]. ALND can cause side effects and complications, including lymphedema, numbness and infection so for many this was a welcome change.
The sentinel lymph node(s) are defined as the first lymph nodes cancer cells are most likely to spread to from the primary lesion
[6][8]. The identification and removal of several sentinel nodes is routine but is surgeon-dependent
[12][15]. In the majority of cases there is more than a single sentinel node. There are a variety of methods that can be used to identify sentinel nodes. These include the use of isotope and blue dye. Intradermal injection of isotope appears to produce the greatest uptake of radioactivity in the nodes. Blue dye has the complication of anaphylaxis manifested by oedema, erythema, tachycardia, “blue” hives, bronchospasms, dysrhythmias, vasodilation, and less commonly cardiovascular collapse. Skin staining with blue dye is another issue for some patients. Blue dye in addition to isotope is now often limited to patients who have had neoadjuvant chemotherapy. Indocyanine green and magtrace are other agents that can be used for SLNB. Both require special detection systems. Issues with magtrace includes cost, brown skin staining and problems using MRI for a period after injection. To circumvent these problems, the magtrace is injected deep in the breast and around the tumour. Much of the magtrace is then removed at surgery.
The use of SLNB in the over-70 population has been debated, and there is acceptance that SLNB may not be necessary if the patient has clinically and ultrasound-negative axilla and HR+ early BC
[13][14][18,19]. Patients of this age often have comorbidities and are at higher risk of adverse events following SLNB, and findings from a SLNB are unlikely to change the recommended adjuvant therapy
[15][20].
If there is no cancer present on pathological assessment, then the patient has lymph node-negative disease and no further axillary treatment is recommended
[16][22]. If only isolated tumour cells (ITC) or micrometastases are present, the patient is also considered to have node-negative BC, and no further axillary treatment is suggested
[8][10]. Disease in lymph nodes smaller than or equal to 0.2 mm are considered ITC deposits and do not influence prognosis
[17][23]. A micrometastasis is defined as a metastatic lesion between 0.2 mm and 2 mm in diameter, whilst a macrometastasis is a metastatic lesion larger than 2 mm in diameter
[18][19][20][21][24,25,26,27].
Evidence that micrometastases have less impact prognostically is supported by a recent multi-centre study of sentinel node micrometastases in ER+ early BC
[22][30], although other studies have suggested otherwise
[21][27]. ITCs and micrometastases do not have the power to predict recurrence for the individual patient; however, when grouped into large analysis cohorts, metastatic tumour burden has been shown to be a continuous prognostic variable and thus their significance should not be overlooked
[21][27].
3. Pathological Assessment
While a number of different biomarkers have proven useful in prognostic and predictive gene signatures, the receptors ER, PR and HER2 remain key clinical biological markers despite it being several decades since their first adoption
[23][42]. Ki67, a measure of proliferation, is also often assessed. Treatment decisions are made based on the levels of these biomarkers in the primary lesion at diagnosis and they are not typically re-evaluated at the time of surgery when the tumour is removed, or in the nodal tissue if positive nodes are also removed. It should also be noted that biopsies are a snapshot of the tumour only, and whilst they can reveal the presence or absence of cancer, it should not be assumed the entire tumour is biologically identical. BC can be incredibly heterogenous—not just between different patients but within a single tumour, making treatment decisions difficult at times
[24][25][43,44].
The presence or absence of the hormone receptors ER and PR is assessed by IHC in the diagnostic biopsy and assigned an Allred score of 0–8 by a pathologist. A score of 0 indicates no staining, and a score of 8 describes strongly positive staining in two thirds to 100% of cells throughout the section. A score for HER2 is also determined, first by IHC (0–3+) and then by fluorescence in situ hybridization (FISH) if the IHC score was intermediate (2+). If the FISH score is above a threshold of 2.2, the HER2 gene is considered amplified, and the cancer deemed HER2+. Assessment of the primary tumour’s receptor status as described above is a well-established standard of practice, and scores for these biomarkers are instrumental in guiding treatment selection.
4. Treatment
4.1. Neoadjuvant
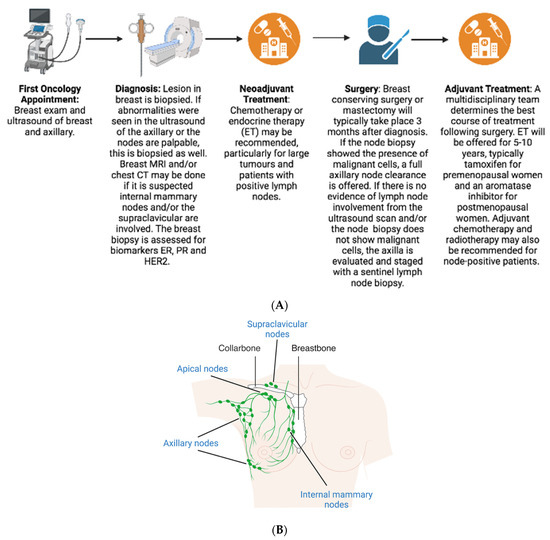
Treatment decisions for BC are made by a multidisciplinary team, especially for higher risk, node-positive patients. The patient’s age, health, comorbidities, and BC-specific factors, including how many positive nodes are present, help determine these treatment decisions. Treatment guidelines vary depending on the BC subtype (HR+/HER2-, HR+/HER2+, HR-/HER2+, or triple-negative) and patients with positive nodes are more likely to be candidates for neoadjuvant and adjuvant chemotherapy.
Neoadjuvant chemotherapy (NAC) can downsize and downstage both the primary tumour and the axilla, and greatly reduce the need for ALND in clinically N1 patients
[26][46]. Neoadjuvant endocrine therapy (NET) is also an option for node-positive patients
[27][47], although less utilised globally than NAC.
4.2. Surgery
In the UK, NICE guidelines recommend performing SLNB rather than axillary clearance if there is no evidence of axillary involvement via ultrasound or the axillary biopsy was negative
[8][10]. There has been some debate on the number of nodes that need to be removed in a SLNB to definitively say the axilla is negative.
The Z0011 trial was instrumental in bringing about change in axillary clearance rates, as it showed no significant difference in survival between patients with no palpable lymph nodes and 1 or 2 metastatic lymph nodes after sentinel node assessment, granting these patients a reprieve from further surgery
[11][14]. Those with micrometastases or isolated tumour cells in their sentinel nodes are treated as node-negative and also do not gain benefit from further axillary treatment, as supported by data from the AATRM trial
[8][28][10,49].
Full axillary clearance, or axillary radiotherapy if the patient is to undergo a mastectomy, are suitable options for patients with positive lymph nodes following SLNB. Despite equivalent disease-free survival and overall survival, axillary radiotherapy is underused compared to ALND yet remains a suitable option for some
[9][29][11,50].
4.3. Adjuvant
Following surgery, adjuvant therapy options are discussed by a multidisciplinary team and the NHS Predict tool can be used to estimate prognosis and predict benefit from adjuvant therapies. Typically, men and premenopausal women with ER+ BC are first offered adjuvant tamoxifen, while postmenopausal women receive an AI. Treatment for ER+ breast cancers is now frequently offered for longer than the standard 5 years of adjuvant treatment, in particular for those who are deemed higher risk for recurrence, such as those with node-positive disease
[30][51]. This may be either extended (longer than 5 years) tamoxifen therapy or switching to an AI after 5 years of tamoxifen.
While the majority of patients with ER+ BC typically respond well to ET initially, resistance to endocrine therapies is a significant problem. Approximately 10% of ER+ cancers will be intrinsically resistant, and in 30–40% of patients, their tumours acquire resistance over time
[31][32][52,53]. When these patients acquire resistance and the cancer recurs, it may recur locally in the breast, regionally in the axillary lymph nodes, or in other distant sites, emphasising the need for a better understanding of endocrine resistance
[33][54].
The patient may also be offered adjuvant chemotherapy if the risk of recurrence is deemed high enough based on the tumour profile, the extent of any axillary nodal involvement, risk assessment using Predict, or tumour profiling using tests like Oncotype DX. Some node-positive patients may be able to avoid chemotherapy in the adjuvant setting. A recent study in South Korea reported similar survival statistics between patients who received only ET and patients who received both ET and chemotherapy
[34][55].
5. Prognosis
The 5-year survival rate for localised invasive BC, defined as disease in the breast only, is over 99%; however, only 63.5% of patients are confirmed as having disease solely in the breast at the time of diagnosis. Overall, 29% of patients have cancer present in their regional lymph nodes when diagnosed, and 6% have distant metastases. The 5-year survival rate drops to 86.1% and 30.0% for regional and distant disease, respectively
[4][1].
Node-positive BC comes with a 31% risk of distant metastasis within 20 years of primary diagnosis if initially diagnosed with 1–3 positive nodes and jumps to 52% in those with 4–9 positive nodes. The 20-year risk of death is 15%, 28% and 49% for patients with node-negative (N0), 1–3 (N1) and 4–9 positive nodes (N2), respectively
[35][37]. These significant differences in survival highlight the crucial importance of being able to accurately predict response and recurrence in those with node-positive BC.
6. Prediction
Predicting treatment response in patients with node-positive BC aids in decision making conversations with their oncologist, facilitates more personalised care, and ultimately improves survival outcomes. There are a number of predictive tools used in BC care, some more accurate than others in node-positive disease. Because patients with node-positive BC at diagnosis make up a minority of cases and have been studied less, predicting who within this group will respond well to ET and other adjuvant treatments has been difficult, resulting in both over and under-treatment of different subgroups
[36][58].
6.1. Predict
The online Predict tool
[37][60], endorsed by the AJCC, is often used in treatment decision making in early invasive BC to predict the survival outcome if one or several treatments are given
[38][61]. It was originally developed using data from 5000 women with BC and later validated with data from 23,000 women. The tool is used routinely in the UK and has now been validated in BC patients in the United States
[39][62].
6.2. Oncotype DX
Oncotype DX is another prediction tool often used in BC care. This predictive gene test is specifically for use in ER+/HER2- disease to predict adjuvant chemotherapy benefit. Given the well-known side effects from chemotherapy
[40][41][42][65,66,67], this toxic treatment should be avoided for some patients when it is safe to do so.
Oncotype DX, also a prognostic indicator, uses real-time reverse-transcription polymerase chain reaction (RT-PCR) to evaluate gene expression levels of 21 genes (16 cancer-related genes and 5 reference) in RNA extracted from formalin-fixed, paraffin-embedded (FFPE) breast tumour tissue. From this data, a recurrence score (RS) between 0 and 100 is generated and predicted chemotherapy benefit and risk of distant recurrence determined. An RS score < 18 is deemed low-risk, RS 18–30 intermediate-risk and RS > 31 high-risk
[43][68].
6.3. MammaPrint
The MammaPrint test uses a 70-gene signature related to early disease invasion and metastasis to predict disease outcome and, more specifically, those patients most likely to develop a BC recurrence or metastasis and who therefore require chemotherapy
[44][45][75,76]. The test is carried out on DNA microarrays. MammaPrint was first evaluated in the RASTER (microarRAy-prognoSTics-in-breast-cancER) study
[46][77], then validated by the prospective randomised MINDACT trial (Microarray In Node-negative Disease may Avoid ChemoTherapy)
[47][78] and, similarly to Oncotype DX, is established as appropriate for node-positive patients with a maximum of three positive nodes.
6.4. PAM50 (Prosigna)
The Prediction Analysis of Microarray 50 (PAM50) test, an FFPE RNA-based quantitative RT-PCR assay, was designed to distinguish prognostic significance in known biological subtypes of BC and determine those who would benefit from NAC
[48][81]. In fact, the test can determine which breast cancers are more likely to metastasise as well as identify a low-risk subset of patients unlikely to need chemotherapy
[49][82].
6.5. EndoPredict
EndoPredict (EP) is a multigene test that has been proven prognostically successful in establishing both early and late metastatic risk in postmenopausal patients with ER+/HER2- BC
[50][51][91,92]. The test, which includes a proliferative and oestrogen signalling gene signature with a total of 12 genes, is completed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). It has also recently been validated in premenopausal women, including those with up to three positive nodes
[52][93]. EP can predict a patient’s risk of distant recurrence in the 10 years following surgery, perhaps more notably the risk of late recurrence in years (up to 15 following surgery) and whether a patient will benefit from chemotherapy or not. EPclin (which combines EP plus two clinical variables—tumour size and nodal status) also generates an individual risk score for each patient.
6.6. Breast Cancer Index
The Breast Cancer Index (BCI) is a predictive test that can predict recurrence as well as indicate benefit from extended adjuvant ET
[53][54][55][56][96,97,98,99]. The test incorporates 11 genes and can predict a patient’s risk of distant recurrence up to 10 years after surgery. As with most of the predictive gene tests, BCI is validated for node-negative BC patients and node-positive patients with a maximum of three positive nodes.
6.7. IHC4
The IHC4 test uses the results of four IHC markers—ER, PR, HER2 and Ki67—to predict 10-year distant recurrence free survival and benefit from chemotherapy in women who have had 5 years of adjuvant ET
[57][58][59][60][61][100,101,102,103,104]. Clinical and pathological features including the patient’s age, tumour size, tumour grade, nodal status, and type of ET administered for 5 years (tamoxifen vs. AI) are also included in a revised version of the test (IHC4+C)
[61][104]. If adequately sampled, a core biopsy can be used for the test if a whole FFPE section is not available
[62][105]. A modified IHC4 test has also recently been proven prognostically useful for those with metastatic ER+/HER2- BC
[63][106].
7. Conclusions
In conclusion, node-positive breast disease is complex and has a higher risk of recurrence than node-negative cancer. There is an unmet need for more studies in patients with more than three positive nodes (i.e., N2 or N3). While this cohort is already established as higher risk due to their nodal status, better stratification and more accurate prediction could enable a more refined treatment selection, such as some patients being able to avoid chemotherapy and/or overtreatment with other adjuvant therapies. Crucially, this will necessitate that predictive tools perform biological assessment of the actual nodal disease, as no predictive gene test currently considers the nodal tissue. Characterisation, biomarker and mutation studies in cancerous nodal tissue is likely to shed light on the mechanisms of metastasis and reveal treatable targets, enabling a move toward better management and improved outcomes in patients with ER+, node-positive BC.