
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan Carlos Lopez-Delgado | -- | 3180 | 2023-10-14 10:14:09 | | | |
| 2 | Peter Tang | + 1 word(s) | 3181 | 2023-10-16 04:38:26 | | |
Video Upload Options
Nutrition therapy in critically ill patients with liver disease represents a challenge for Intensive Care Units (ICUs). Nutritional status is correlated with the degree of hepatic dysfunction and the presence of malnutrition worsens outcomes in these patients. The nutritional risk that critically ill patients represent, together with the pathophysiological alterations of liver disease, especially in terms of nutrition intake and protein depletion, leads to malnutrition and sarcopenia. Nutrition therapy improves the survival of these patients; however, this is challenging since they more frequently experience difficulties with nutrition delivery. In consequence, both evaluation of nutritional status and an individualized approach seem mandatory for achieving nutrition objectives.
1. Introduction
2. Bidirectional Relationship between Liver Disease and Nutritional Status
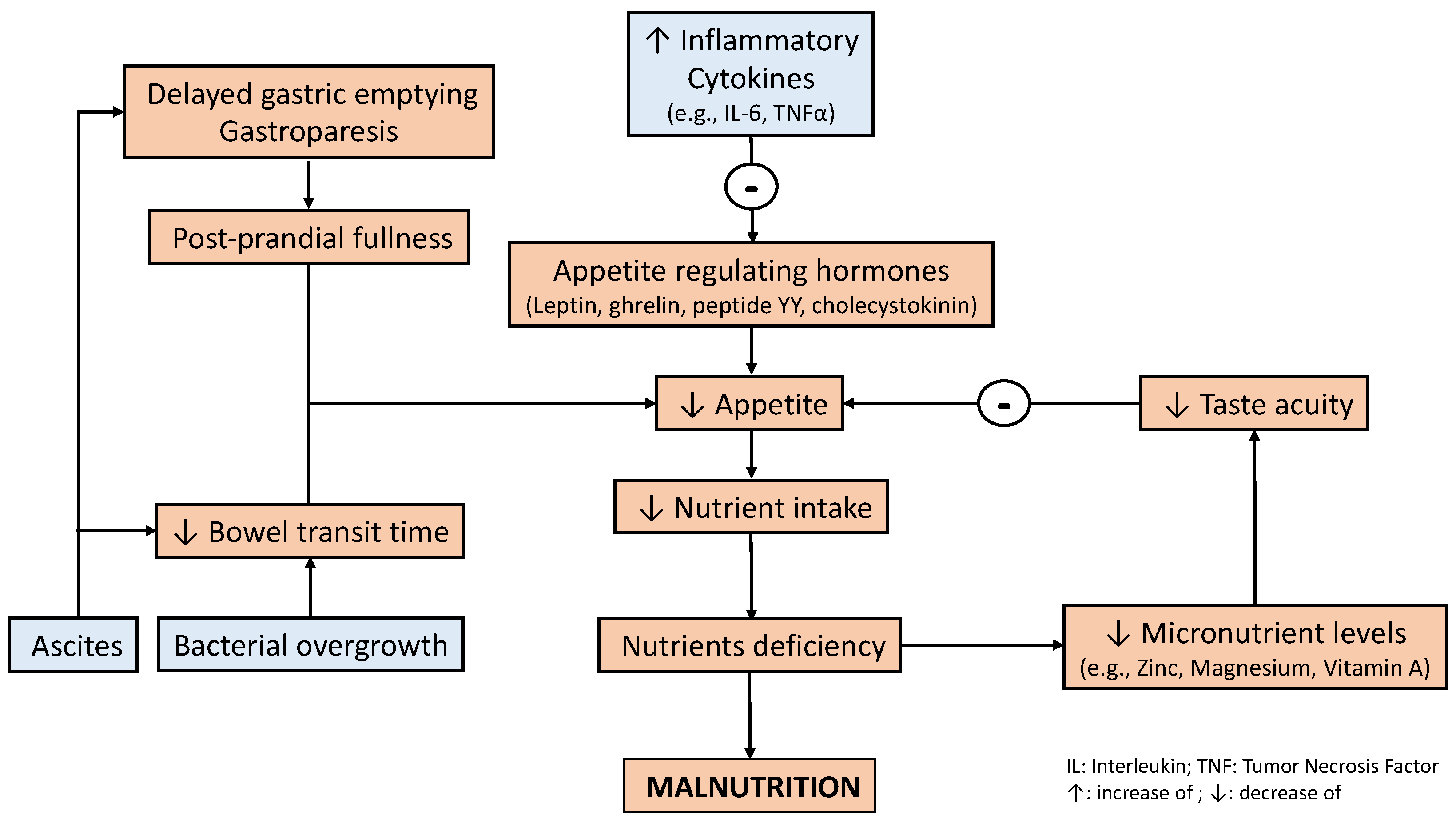
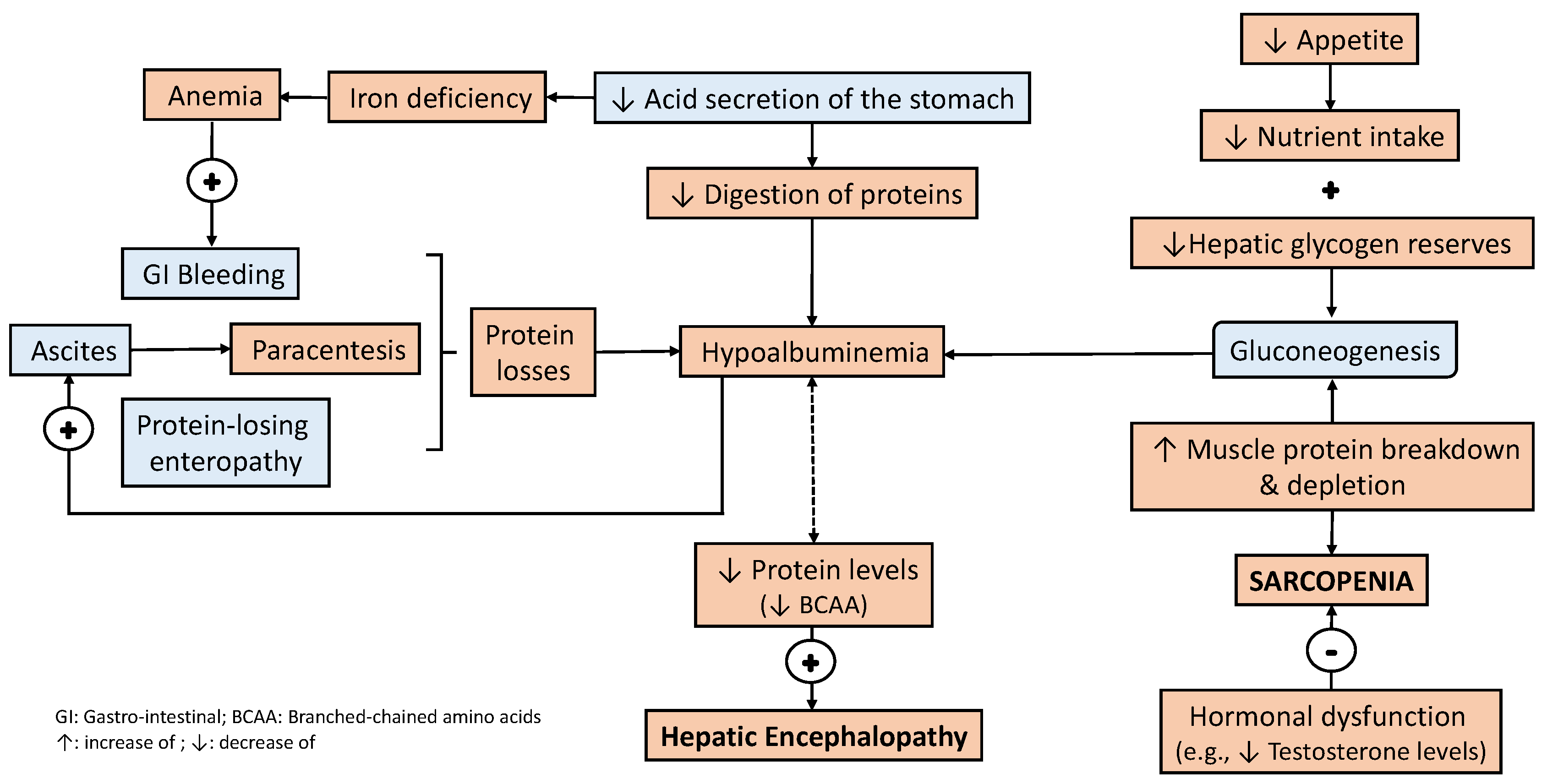
3. Nutrition Management in Patients with Liver Disease
3.1. Evaluation of Nutritional Status in Patients with Liver Disease
3.2. What Do Clinical Practice Guidelines Recommend?
3.3. A Practical Approach to Nutrition Therapy in Patients with Liver Disease
3.4. Considerations in the Perioperative Patient
References
- Patton, H.M. Nutritional assessment of patients with chronic liver disease. Gastroenterol. Hepatol. 2012, 8, 687–690.
- Sevastianos, V.A.; Dourakis, S.P. Malnutrition and Sarcopenia in Advanced Liver Disease. J. Nutr. Food Sci. 2016, 6, 487.
- Huynh, D.K.; Selvanderan, S.P.; Harley, H.A.; Holloway, R.H.; Nguyen, N.Q. Nutritional care in hospitalized patients with chronic liver disease. World J. Gastroenterol. 2015, 21, 12835–12842.
- Periyalwar, P.; Dasarathy, S. Malnutrition in cirrhosis: Contribution and consequences of sarcopenia on metabolic and clinical responses. Clin. Liver Dis. 2012, 16, 95–131.
- Lorencio, C.; Bonet Sarís, A.; Navas Moya, E. Recommendations for specialized nutritional-metabolic treatment of the critical patient: Nonsurgical abdominal disease. Metabolism and Nutrition Working Group of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC). Med. Intensiva. 2020, 44 (Suppl. S1), 60–64.
- Perez Ruiz de Garibay, A.; Kortgen, A.; Leonhardt, J.; Zipprich, A.; Bauer, M. Critical care hepatology: Definitions, incidence, prognosis and role of liver failure in critically ill patients. Crit. Care 2022, 26, 289.
- Shergill, R.; Syed, W.; Rizvi, S.A.; Singh, I. Nutritional support in chronic liver disease and cirrhotics. World J. Hepatol. 2018, 10, 685–694.
- Saunders, J.; Brian, A.; Wright, M.; Stroud, M. Malnutrition and nutrition support in patients with liver disease. Frontline Gastroenterol. 2010, 1, 105–111.
- Hammad, A.; Kaido, T.; Aliyev, V.; Mandato, C.; Uemoto, S. Nutritional Therapy in liver transplantation. Nutrients 2017, 9, 1126.
- Chang, Y.; Liu, Q.Y.; Zhang, Q.; Rong, Y.M.; Lu, C.Z.; Li, H. Role of nutritional status and nutritional support in outcome of hepatitis B virus-associated acute-on-chronic liver failure. World J. Gastroenterol. 2020, 26, 4288–4301.
- Prieto-Frias, C.; Conchillo, M.; Payeras, M.; Inarrairaegui, M.; Davola, D.; Fruhbeck, G.; Salvador, J.; Rodriguez, M.; Richter, J.A.; Mugueta, C.; et al. Factors related to increased resting energy expenditure in men with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 139–145.
- Lin, S.Y.; Wang, Y.Y.; Sheu, W.H. Increased serum leptin concentrations correlate with soluble tumour necrosis factor receptor levels in patients with cirrhosis. Clin. Endocrinol. 2002, 57, 805–811.
- Kalaitzakis, E. Gastrointestinal dysfunction in liver cirrhosis. World J. Gastroenterol. 2014, 20, 14686–14695.
- Nicoll, R.; Gerasimidis, K.; Forrest, E. The Role of Micronutrients in the Pathogenesis of Alcohol-Related Liver Disease. Alcohol Alcohol. 2022, 57, 275–282.
- Saeed, A.; Dullaart, R.P.F.; Schreuder, T.C.M.A.; Blokzijl, H.; Faber, K.N. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2017, 10, 29.
- Perry, B.; Wang, Y. Appetite regulation and weight control: The role of gut hormones. Nutr. Diabetes 2012, 2, e26.
- Thuluvath, P.J.; Triger, D.R. Autonomic neuropathy and chronic liver disease. Q. J. Med. 1989, 72, 737–747.
- Shindo, K.; Machida, M.; Miyakawa, K.; Fukumura, M. A syndrome of cirrhosis, achlorhydria, small intestinal bacterial overgrowth, and fat malabsorption. Am. J. Gastroenterol. 1993, 88, 2084–2091.
- Lee, J.H.; Kwon, Y.J.; Park, K.; Lee, H.S.; Park, H.K.; Han, J.H.; Ahn, S.B. Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 3039.
- Liu, K.; Yang, L.; Wang, G.; Liu, J.; Zhao, X.; Wang, Y.; Li, J.; Yang, J. Metabolic stress drives sympathetic neuropathy within the liver. Cell Metab. 2021, 33, 666–675.e4.
- Marchesini, G.; Bianchi, G.; Lucidi, P.; Villanova, N.; Zoli, M.; De Feo, P. Plasma ghrelin concentrations, food intake, and anorexia in liver failure. J. Clin. Endocrinol. Metab. 2004, 89, 2136–2141.
- Hofmann, A.F. The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 1999, 159, 2647–2658.
- Lodato, F.; Azzaroli, F.; Di Girolamo, M.; Feletti, V.; Cecinato, P.; Lisotti, A.; Festi, D.; Roda, E.; Mazzella, G. Proton pump inhibitors in cirrhosis: Tradition or evidence-based practice? World J. Gastroenterol. 2008, 14, 2980–2985.
- Kappus, M.R. Acute Hepatic Failure and Nutrition. Nutr. Clin. Pract. 2020, 35, 30–35.
- Li, B.R.; Pan, J.; Du, T.T.; Liao, Z.; Ye, B.; Zou, W.B.; Chen, H.; Ji, J.T.; Zheng, Z.H.; Wang, D.; et al. Risk Factors for Steatorrhea in Chronic Pancreatitis: A Cohort of 2153 Patients. Sci. Rep. 2016, 6, 21381.
- Correnti, J.M.; Gottshall, L.; Lin, A.; Williams, B.; Oranu, A.; Beck, J.; Chen, J.; Bennett, M.J.; Carr, R.M. Ethanol and C2 ceramide activate fatty acid oxidation in human hepatoma cells. Sci. Rep. 2018, 8, 12923.
- Kinny-Koster, B.; Bartels, M.; Becker, S.; Scholz, M.; Thiery, J.; Ceglarek, U.; Kaiser, T. Plasma Amino Acid Concentrations Predict Mortality in Patients with End-Stage Liver Disease. PLoS ONE 2016, 11, e0159205.
- Anastácio, L.R.; Davisson Correia, M.I. Nutrition therapy: Integral part of liver transplant care. World J. Gastroenterol. 2016, 22, 1513–1522.
- Petersen, K.F.; Krssak, M.; Navarro, V.; Chandramouli, V.; Hundal, R.; Schumann, W.C.; Landau, B.R.; Shulman, G.I. Contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production in cirrhosis. Am. J. Physiol. 1999, 276, E529–E535.
- Ferreira, L.G.; Ferreira Martins, A.I.; Cunha, C.E.; Anastácio, L.R.; Lima, A.S.; Correia, M.I. Negative energy balance secondary to inadequate dietary intake of patients on the waiting list for liver transplantation. Nutrition 2013, 29, 1252–1258.
- Ridola, L.; Gioia, S.; Faccioli, J.; Riggio, O.; Nardelli, S. Gut liver muscle brain axis: A comprehensive viewpoint on prognosis in cirrhosis. J. Hepatol. 2022, 77, 262–263.
- Tantai, X.; Liu, Y.; Yeo, Y.H.; Praktiknjo, M.; Mauro, E.; Hamaguchi, Y.; Engelmann, C.; Zhang, P.; Jeong, J.Y.; van Vugt, J.L.A.; et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 76, 588–599.
- Martin, P.; DiMartini, A.; Feng, S.; Brown, R.; Fallon, M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014, 59, 1144–1165.
- Preiser, J.C.; van Zanten, A.R.; Berger, M.M.; Biolo, G.; Casaer, M.P.; Doig, G.S.; Griffiths, R.D.; Heyland, D.K.; Hiesmayr, M.; Iapichino, G.; et al. Metabolic and nutritional support of critically ill patients: Consensus and controversies. Crit. Care 2015, 19, 35.
- Ebadi, M.; Bhanji, R.A.; Mazurak, V.C.; Montano-Loza, A.J. Sarcopenia in cirrhosis: From pathogenesis to interventions. J. Gastroenterol. 2019, 54, 845–859.
- Grossmann, M.; Hoermann, R.; Gani, L.; Chan, I.; Cheung, A.; Gow, P.J.; Li, A.; Zajac, J.D.; Angus, P. Low testosterone levels as an independent predictor of mortality in men with chronic liver disease. Clin. Endocrinol. 2012, 77, 323–328.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193.
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M.; Burgos Peláez, R.; Rivera Irigoin, R. ESPEN Practical Guideline: Clinical nutrition in liver disease. Nutr. Hosp. 2022, 39, 434–472.
- Vasques, J.; Guerreiro, C.S.; Sousa, J.; Pinto, M.; Cortez-Pinto, H. Nutritional support in cirrhotic patients with sarcopenia. Clin. Nutr. ESPEN 2019, 33, 12–17.
- Koretz, R.L. Nutritional support in liver disease—An updated systematic review. Curr. Opin. Gastroenterol. 2023, 39, 115–124.
- Aslam, M.; Farooq, S.; Rizwan, B.; Asghar, A. Assessment of nutritional status of the cirrhotic patients on enteral and parenteral feeding. Nutr. Health 2022, 28, 69–76.
- Besen, B.A.; Gobatto, A.L.; Melro, L.M.; Maciel, A.T.; Park, M. Fluid and electrolyte overload in critically ill patients: An overview. World J. Crit. Care. Med. 2015, 4, 116–129.
- Lucchinetti, E.; Lou, P.H.; Wawrzyniak, P.; Wawrzyniak, M.; Scharl, M.; Holtzhauer, G.A.; Krämer, S.D.; Hersberger, M.; Rogler, G.; Zaugg, M. Novel Strategies to Prevent Total Parenteral Nutrition-Induced Gut and Liver Inflammation, and Adverse Metabolic Outcomes. Mol. Nutr. Food Res. 2021, 65, e1901270.
- Videla, L.A.; Hernandez-Rodas, M.C.; Metherel, A.H.; Valenzuela, R. Influence of the nutritional status and oxidative stress in the desaturation and elongation of n-3 and n-6 polyunsaturated fatty acids: Impact on non-alcoholic fatty liver disease. Prostaglandins Leukot. Essent Fatty Acids 2022, 181, 102441.
- Fallahzadeh, M.A.; Rahimi, R.S. Hepatic Encephalopathy and Nutrition Influences: A Narrative Review. Nutr. Clin. Pract. 2020, 35, 36–48.
- Plauth, M.; Cabré, E.; Riggio, O.; Assis-Camilo, M.; Pirlich, M.; Kondrup, J.; DGEM (German Society for Nutritional Medicine); Ferenci, P.; Holm, E.; Vom Dahl, S.; et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin. Nutr. 2006, 25, 285–294.
- Kouz, J.; Vincent, C.; Leong, A.; Dorais, M.; Räkel, A. Weight gain after orthotopic liver transplantation: Is nonalcoholic fatty liver disease cirrhosis a risk factor for greater weight gain? Liver Transpl. 2014, 20, 1266–1274.
- Choudhary, N.S.; Saigal, S.; Saraf, N.; Mohanka, R.; Rastogi, A.; Goja, S.; Menon, P.B.; Mishra, S.; Mittal, A.; Soin, A.S. Sarcopenic obesity with metabolic syndrome: A newly recognized entity following living donor liver transplantation. Clin. Transpl. 2015, 29, 211–215.
- Parekh, J.; Corley, D.A.; Feng, S. Diabetes, hypertension and hyperlipidemia: Prevalence over time and impact on long-term survival after liver transplantation. Am. J. Transpl. 2012, 12, 2181–2187.
- Anastácio, L.R.; Ferreira, L.G.; de Sena Ribeiro, H.; Liboredo, J.C.; Lima, A.S.; Correia, M.I.T.D. Metabolic syndrome after liver transplantation: Prevalence and predictive factors. Nutrition 2011, 27, 931–937.




