2. Bidirectional Relationship between Liver Disease and Nutritional Status
Liver disease negatively affects metabolism and the patient’s nutritional status and, simultaneously, a worsening of nutritional status could negatively affect the severity and progression of liver disease. Malnutrition is frequently caused by a variety of factors, including a decreased nutrient intake, gastrointestinal dysfunction leading to nutrient malabsorption, and increased protein catabolism leading to sarcopenia
[1][2][3][4][5][1,2,3,4,5]. In addition, some cirrhotic patients (≈30%) have an increased resting energy expenditure (>120%) that negatively affects nutritional status
[11]. It has been hypothesized that the altered hypermetabolism is caused by chronic inflammation (e.g., increased blood levels of interleukin-1 and interleukin-6 (IL-6))
[12]. Thus, the hypercatabolic status of critical illness together with this hypermetabolic status put the patient with liver disease at higher nutritional risk
[5].
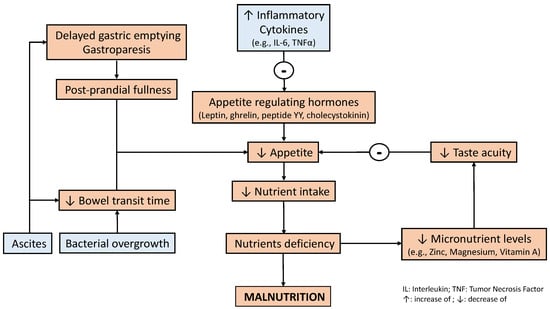
Patients with liver disease frequently experience symptoms that may contribute toward a reduced nutrient intake, such as gastrointestinal effects (e.g., increased gastric sensitivity to distension and delayed gut transit). Delayed gastric emptying has been reported in patients with liver disease and has been associated with post-prandial fullness and bloating
[13]. These patients suffer from alterations in taste acuity, which has been associated with deficiencies in trace elements including zinc, magnesium, and vitamin A
[14][15][14,15]. Appetite is also reduced due to increased inflammatory cytokines and alterations in appetite-regulating hormones (i.e., leptin, ghrelin, peptide YY, and cholecystokinin)
[16]. A decreased dietary intake in patients with liver disease enhances anorexia, which is also affected by micronutrient deficiency (i.e., low zinc levels) and metabolic alterations, such as hyperglycemia, that may contribute to increased inflammatory cytokine production (e.g., Tumor Necrosis Factor (TNF-α) and IL-6) and leptin
[17]. Functional problems such as gastroparesis and delayed bowel transit time, which may induce bacterial overgrowth, together with tense ascites, cause nausea and early satiety
[18].
In addition, hormonal alterations and dysfunction negatively influence both nutrient intake and metabolism. Increased insulin levels, caused by hyperinsulinemia and insulin resistance, induce satiety
[19]. Hyperglycemia may eventually contribute to the development of autonomic neuropathy, which modifies gustatory sensation and dietary intake and may also enhance the negative effect of liver disease on gastrointestinal functions, especially regarding motility (e.g., gastric emptying)
[20]. Another endocrine alteration is the lack of response to high levels of ghrelin, a peripherally derived orexigenic hormone that normally increases appetite and food intake. Despite the presence of high ghrelin levels in cirrhotic patients, appetite is not increased
[21].
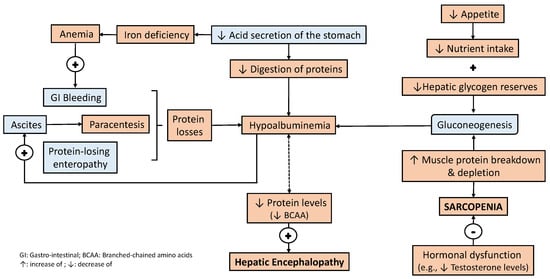
In a patient with liver disease, the digestion, absorption, and metabolism of nutrients are all also negatively affected. Fat and fat-soluble vitamin malabsorption due to impaired bile acid metabolism is common
[22]. A reduction in acid secretion in the stomach, or even achlorhydria, may be present in patients with liver cirrhosis, regardless of Helicobacter pylori infection, with a variable prevalence
[18][23][18,23]. This may contribute to the impaired digestion of macronutrients (e.g., proteins) and some micronutrients (e.g., vitamin B12 and iron), which may ultimately worsen their absorption and increase their deficit. Consequently, this may also contribute to worsening clinical manifestations of nutrient deficiency (e.g., anemia)
[24].
Alcohol abuse, which is the most frequent cause of liver disease, may contribute to the poor nutritional intake caused by the development of chronic pancreatitis, which is per se associated with fat and micronutrient malabsorption and metabolic alterations (e.g., hyperglycemia), which may produce liver damage
[25]. Alcohol inhibits fatty acid oxidation, leading to triglyceride accumulation in the liver and the development of fatty liver disease or NAFLD
[26].
The liver plays a central role in amino acid and protein metabolism, and a reduction in blood levels is not surprising (e.g., hypoalbuminemia). Indeed, a reduction in branched chained amino acid (BCAA) serum levels is associated with the occurrence of hepatic encephalopathy
[27]. Protein losses are caused by gastrointestinal bleeding and frequent paracentesis, which may be further worsened by protein-losing enteropathy, contributing to the development of hypoalbuminemia
[28]. It is important to highlight that alterations in carbohydrate metabolism also contribute to lower amino acid levels and protein deficit. A decreased hepatic glucose production and lower hepatic glycogen reserves increase gluconeogenesis from amino acids and even secondary protein breakdown from muscle
[29]. All these effects are enhanced by hypermetabolism accompanied by a reduced food intake, leading to a negative caloric and protein balance, which is a vicious circle that worsens the malnutrition and clinical manifestations of liver disease
[30].
Some data suggest that preservation of the body’s lean mass is important during the evolution of patients with liver disease since it is associated with fewer complications
[31][32][31,32]. Muscle wasting or sarcopenia is the most objective feature of chronic protein malnutrition in cirrhosis and is also an important predictor of survival in decompensated liver disease, quality of life, and the ability to respond to stressors, such as surgery, prolonged ventilator support, longer ICU and hospital stays, higher risk of infections, mortality, and even lower survival chances during the perioperative course of liver transplantation
[31][32][33][34][35][31,32,33,34,35]. Testosterone, which plays an important role in protein synthesis, is highly reduced (≈90% approximately) in cirrhosis and protein breakdown
[36]. Despite malnutrition not being a formal contraindication for liver transplantation, it is well known that in candidates for liver transplantation, malnutrition adversely affects the perioperative course, and nutritional status should be improved before surgery.
Some metabolic and nutritional factors related to the occurrence of malnutrition in liver disease should be better elucidated; nevertheless, it is obvious that the metabolic and nutritional status strongly impacts liver disease and vice versa. These pathophysiological factors are common to all degrees of liver disease in patients admitted to the ICU and vary depending on the severity of the liver failure. However, a reduced nutritional intake (
Figure 1) and protein depletion (
Figure 2) both seem to play a key role in the occurrence of malnutrition and sarcopenia in these patients, which ultimately strongly influence outcomes.
Figure 1. Factors associated with reduced nutrient intake in liver disease. Inflammatory factors negatively influence appetite-regulating hormones. Simultaneously, both a lower taste acuity and some liver-related complications also negatively influence appetite. The latter is related to reduced motility (i.e., gastric emptying and bowel transit time), which may impact appetite and contribute to a reduced nutrient intake
[13][14][15][16][17][18][19][20][21][13,14,15,16,17,18,19,20,21].
Figure 2. Factors related to protein depletion in liver disease. Protein depletion and the development of sarcopenia are closely related to a reduced nutrient intake and the activation of mechanisms to guarantee the metabolic demands of carbohydrates. Liver-related insensitive protein losses and impaired protein digestion both negatively affect protein depletion, which also enhances the development of liver-related complications (e.g., hepatic encephalopathy)
[31][32][33][34][35][36][31,32,33,34,35,36].
3. Nutrition Management in Patients with Liver Disease
Evidence for malnutrition as a prognostic indicator in patients with decompensated liver disease has mostly been derived from the liver transplantation population, in which severe malnutrition has been associated with higher perioperative mortality
[13]. However, common models for determining the prognosis and degree of liver disease (i.e., model of end-stage liver disease and the Child–Pugh score) do not evaluate nutritional status. Identifying the degree of malnutrition in patients with liver disease is the first step toward addressing nutrition therapy in these patients.
3.1. Evaluation of Nutritional Status in Patients with Liver Disease
Evaluating nutritional status and the presence of malnutrition involves different parameters, such as physical examination, anthropometric measurements, laboratory tests, and instrumental examination, along with the determination of the patient’s functional capacity
[9]. These parameters may be modified due to the inherent alterations associated with critical illness and liver disease (e.g., fluid overload and alterations in protein metabolism) which make it difficult to determine the nutritional status.
As there is no gold standard,
thwe re
searchers reccommend using a combination of different methods to evaluate and determine the extent of malnutrition in patients with liver disease. Simultaneous evaluation of BMI and ascites/fluid retention together with the degree of liver disease (i.e., the Child–Turcotte–Pugh score and Model for End-Stage Liver Disease score) may be helpful for this purpose. The SGA, LDUST, and the RFH–NPT seem to be the most suitable tools for screening malnutrition in these patients. Evaluating sarcopenia and physical activity should be mandatory since these are considered surrogate markers of nutritional and functional status irrespective of the degree of liver disease. Despite there not being any gold standard or ideal tool in these patients, the presence of sarcopenia needs to be evaluated (e.g., by CT scans, MRI, BIA, etc.).
3.2. What Do Clinical Practice Guidelines Recommend?
It is important to remark that most recommendations for nutrition therapy in patients with liver disease arise from a pathophysiological basis and expert opinion or consensus; the degree of evidence is moderate due to the lack of nutrition studies in this specific population of ICU patients. As a result, there are few main contemporary international guidelines available specifically addressing nutrition therapy in liver disease
[5].
The EASL has published clinical practice guidelines on nutrition in advanced liver disease, including how to recognize and assess nutritional problems and what the consequences of malnutrition are and its correction, in addition to addressing different clinical scenarios, such as hepatic encephalopathy and before/after transplantation
[37][52]. The ESPEN guidelines on clinical nutrition in liver disease include recommendations on different issues of nutritional management in various forms of liver disease (e.g., ALF, ACLF, cirrhosis, major liver surgery, or transplantation)
[38][68]. Indeed, a recent ESPEN review underlined the importance of sarcopenia in cirrhosis and made some recommendations for clinical practice
[39][69].
It is important to note that none of these guidelines specifically address the population admitted to the ICU with liver disease; the recommendations are selected and adapted based on the characteristics of liver disease patients with critical illness. In consequence, there are some concerns related to nutrition therapy and metabolic status in these patients that are not emphasized in the guidelines and require careful attention. First, these patients have major vitamin and trace element deficits, and identifying these deficits (if possible) may be of crucial importance
[24]. The routine administration of vitamins and trace element supplementation may be considered when these patients are admitted to an ICU.
Second, some patients develop a hypercatabolic status based on the metabolic characteristics that liver disease entails; however, they are unable to tolerate full enteral nutrition (EN) since they are critically ill (e.g., severe or prolonged shock)
[10]. The hypercatabolic status combined with the stress response of their critical status rapidly leads to malnutrition because of the difficulty in achieving nutritional and metabolic requirements. Thus, nutrition therapy should be individualized based on these considerations; nutrition therapy should follow the recommendations for ICU patients (i.e., early and progressive administration) and the specifications described above (e.g., reduce protein administration when hepatic encephalopathy is present).
Third, enteral is the main administration route to maintain the integrity of the gastric mucosa and gut barrier, yet patients frequently experience difficulties in achieving the entire nutrition therapy volume because liver disease and ICU admission both entail gastrointestinal dysfunction (e.g., delayed gastric emptying)
[40][70]. We should follow prompt strategies to optimize the enteral route during the first 72 h of ICU admission, such as early use of prokinetics, and consider post-pyloric administration, especially if we suspect previous malnutrition or chronic liver disease. In addition, the use of PN should be considered early on (i.e., day 3–4 of ICU admission) to achieve nutritional targets and avoid the occurrence or development of malnutrition
[24]. Older patients (i.e., >50 years old) under PN more frequently have ascites and a positive fluid balance; however, these patients usually exhibit a higher risk of malnutrition compared with patients on EN
[41][71]. Physicians should be aware of the detrimental effects that a positive fluid balance caused by PN may have on gastrointestinal function, which ultimately are related to worse outcomes
[42][72]. EN, even with minimal delivery, should be considered in combination with PN to maintain gut structure and minimize the adverse effects (i.e., induced gut and liver inflammation) that PN may entail
[43][73]. It is important to remark that PN with a higher proportion of omega-3 polyunsaturated fatty acids (ω3-PUFA) may help minimize this adverse effect
[44][74].
Finally, appropriate glycemic management may be an important metabolic target to control. Glycemia may be monitored every 2 h with several objectives: to maintain glucose levels between 150 and 180 mg/dL, avoid hypoglycemia, and detect hyperglycemia or high glycemic variability during admission. It is important to remember that hyperglycemia may exacerbate intracranial pressure when patients develop intracranial hypertension in the setting of hepatic encephalopathy
[45][75]. All these concerns should be considered for a practical approach to nutrition therapy in these patients.
3.3. A Practical Approach to Nutrition Therapy in Patients with Liver Disease
The researchers previously underlined that patients with cirrhosis may have a higher energy requirement due to increased protein catabolism; however, we must balance this phenomenon with nutrition tolerance, which is lower in the setting of critical illness and worsening liver disease
[5][33][37][38][5,33,52,68]. It is clear that an adequate protein intake is important to prevent protein–calorie malnutrition and avoid sarcopenia
[39][69]. The energy supply should be mixed (i.e., carbohydrates and lipids) but limiting the lipid intake is recommended due to the increased risk of hepatic steatosis or worsening of hypertriglyceridemia. The higher risk of vitamin deficiencies due to intestinal malabsorption, higher energy demands, and previous deficits, mostly involving vitamins A, D, E, and K, as well as iron and zinc, should make their routine administration necessary.
Some of the aforementioned recommendations may apply to patients with liver disease who are not admitted to an ICU because the pathophysiological basis of liver disease applies to all patients. However, the nutrition guidance provided cannot apply to all patients with liver disease as ICU patients suffer from alterations that prevent adequate nutrition therapy.
3.4. Considerations in the Perioperative Patient
Nutrition therapy improves outcomes (i.e., mortality, length of hospital stays, and postoperative complications) in all types of perioperative scenarios in patients with liver disease (i.e., liver transplantation, liver resections, and hepatocellular carcinoma)
[40][70]. The application of nutrition therapy in patients with severe nutritional risk (i.e., SGA C) is recommended for 10–14 days before major surgery to optimize their nutritional status, even if surgery must be delayed because of the presence of malnutrition
[46][78]. This would definitively positively affect the outcome of the patient, especially in terms of a lower incidence of postoperative complications.
After surgery, especially after liver transplantation, enteral nutrient intake should be started early in the postoperative period, even though transpyloric access and the composition of the nutritional formula should be adapted to the patient’s metabolic stress. The prevalence of sarcopenia does not seem to decrease, and although patients gain weight, sarcopenic obesity may co-exist
[47][79]. A greater food intake and physical inactivity are responsible for the positive energy balance, which is observed in up to 88% of patients after liver transplantation
[48][80]. Several metabolic complications related to weight gain and immunosuppression are developed in the long-term post-transplant. The risk of arterial hypertension, dyslipidemia, and diabetes mellitus increases after surgery and impacts outcomes as well as survival
[49][81]. This set of metabolic disorders yields an increased risk of metabolic syndrome, described in approximately half of liver transplant recipients
[50][82]. Despite weight gain seeming positive for nutritional status after liver transplantation, this is an additional risk factor for developing metabolic syndrome, which makes a nutritional follow-up necessary.