
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Diego Franco | -- | 1541 | 2023-10-11 11:01:59 | | | |
| 2 | Lindsay Dong | Meta information modification | 1541 | 2023-10-13 02:54:18 | | |
Video Upload Options
Cardiac regeneration is an ancestral trait in vertebrates, a general capacity that seems to be inversely correlated with evolutionary complexity across the animal kingdom. To better understand the biology of MI, as well as to develop different therapeutic strategies, in vitro, ex vivo and in vivo models have been developed.
1. Introduction
2. Experimental Models of Cardiac Regeneration
3. Injury Models to Study Cardiac Healing
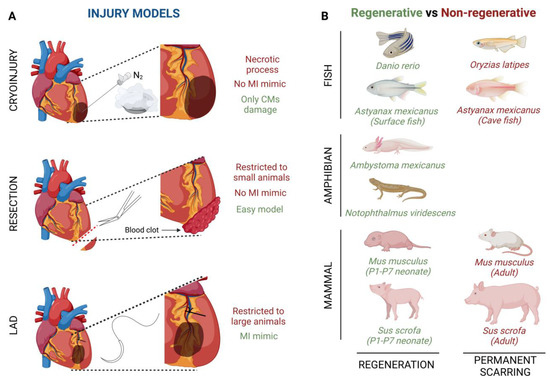
4. Heart Regeneration after Injury in Different Animal Models
4.1. Invertebrate Models
4.2. Vertebrate Models
Fish models
Several fish species have shown the ability to repair an injured heart through the induction of CM proliferation (Figure 1B). For example, zebrafish (Danio rerio) have the ability of total heart regeneration 60 days after ~20% apical resection of the ventricle, by activating the proliferation rate of the CMs present in the surrounding injured area [16][42]. Something similar happens when ~25% of the zebrafish’s ventricle is damaged by using a cryoprobe. In this type of heart injury model, although the regeneration process is much slower, the heart achieves restoration in approximately ~180 days [43][44][45]. Finally, Poss’ lab developed a tamoxifen-inducible genetic ablation model that allowed the promotion of 60% of CM cell death. In this case, 30-day post tamoxifen induction they observed a complete re-muscularized ventricle [46]. Moreover, a few years ago, Gonzalez-Rosas et al. (2018) [47] reported that most of the zebrafish’s CMs contain only two sets of homologous chromosomes. These diploid CMs have the ability to proliferate, inducing heart regeneration [48]. Finally, considering that zebrafish resides in a hypoxic environment, this restrictive O2 condition enables CM dedifferentiation and proliferation leading to heart regeneration [42].
Amphibian models
Similar to fishes, some urodele amphibians have the ability to regenerate several tissues and organs [49][50] (Figure 1B). For example, axolotls (Ambystoma mexicanus), which are neotenic aquatic urodele amphibians, are capable of regenerating the heart after injury, resection and cryo-injury, but only in the larval phase [49][50][51]. However, when axolotls undergo metamorphosis and get into the adulthood stage, regeneration, in general terms, is reduced and some morphological defects are still observed [52]. This animal group loses its overall regenerative capacity as it undergoes metamorphosis, concomitantly with maturation of the immune system, which is comparable to that of mammals [53][54]. There are no studies about heart regeneration in adult axolotls, however it is important to highlight that thyroid hormone, which is necessary in this species to undergo metamorphosis into a land-dwelling adult, impairs heart regeneration in zebrafish [51][55]. In these terms, newts as Notophthalmus viridescens, bear heart regeneration capacity dependent on the type of injury [17][56][57][58][59] (Figure 1B).
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus. 23 July 2020. Available online: https://www.cureus.com/articles/36728-global-epidemiology-of-ischemic-heart-disease-results-from-the-global-burden-of-disease-study (accessed on 20 July 2023).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021.
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264.
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. In International Review of Cell and Molecular Biology, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 298, 229p.
- Hausenloy, D.J.; Barrabes, J.A.; Bøtker, H.E.; Davidson, S.M.; Di Lisa, F.; Downey, J.; Engstrom, T.; Ferdinandy, P.; Carbrera-Fuentes, H.A.; Heusch, G.; et al. Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic Res. Cardiol. 2016, 111, 70.
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551.
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/reperfusion injury following acute myocardial infarction: A critical issue for clinicians and forensic pathologists. Mediat. Inflamm. 2017, 2017, 7018393.
- Jennings, R.B.; Sommers, H.M.; Smyth, G.A.; Flack, H.A.; Linn, H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch. Pathol. 1960, 70, 68–78.
- Kathiresan, S.; Srivastava, D. Genetics of human cardiovascular disease. Cell 2012, 148, 1242–1257.
- Lu, B.; Yu, H.; Zwartbol, M.; Ruifrok, W.P.; van Gilst, W.H.; de Boer, R.A.; Silljé, H.H.W. Identification of hypertrophy- and heart failure-associated genes by combining in vitro and in vivo models. Physiol. Genom. 2012, 44, 443–454.
- Sarkar, K.; Cai, Z.; Gupta, R.; Parajuli, N.; Fox-Talbot, K.; Darshan, M.S.; Gonzalez, F.J.; Semenza, G.L. Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning. Proc. Natl. Acad. Sci. USA 2012, 109, 10504–10509.
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Natl. Inst. Health 2009, 324, 98–102.
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436.
- Yun, M.H. Changes in Regenerative Capacity through Lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432.
- Vivien, C.J.; Hudson, J.E.; Porrello, E.R. Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regen. Med. 2016, 1, 16012.
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190.
- Becker, R.O.; Chapin, S.; Sherry, R. Regeneration of the ventricular myocardium in amphibians. Nature 1974, 248, 145–147.
- Soonpaa, M.H.; Field, L.J. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am. J. Physiol. Circ. Physiol. 1997, 272, H220–H226.
- Yadav, V.; Chong, N.; Ellis, B.; Ren, X.; Senapati, S.; Chang, H.C.; Zorlutuna, P. Constant-potential environment for activating and synchronizing cardiomyocyte colonies with on-chip ion-depleting perm-selective membranes. Lab A Chip 2020, 20, 4273–4284.
- Ellis, B.W.; Dmitry, O.; Traktuev; Merfeld-Clauss, S.; Can, U.I.; Wang, M.; Bergeron, R.; Zorlutuna, P.; March, K.L. Adipose Stem Cell Secretome Markedly Improves Rodent Heart and hiPSC-derived Cardiomyocyte Recovery from Cardioplegic Transport Solution Exposure. Stem Cells 2021, 39, 170–182.
- He, L.; Zhou, B. Cardiomyocyte proliferation: Remove brakes and push accelerators. Cell Res. 2017, 27, 959–960.
- Zhao, M.T.; Ye, S.; Su, J.; Garg, V. Cardiomyocyte Proliferation and Maturation: Two Sides of the Same Coin for Heart Regeneration. Front. Cell Dev. Biol. 2020, 8, 594226.
- Peter, A.K.; Bjerke, M.A.; Leinwand, L.A. Biology of the cardiac myocyte in heart disease. Mol. Biol. Cell 2016, 27, 2149–2160.
- Watanabe, M.; Horie, H.; Kurata, Y.; Inoue, Y.; Notsu, T.; Wakimizu, T.; Adachi, M.; Yamamoto, K.; Morikawa, K.; Kuwabara, M.; et al. Esm1 and Stc1 as angiogenic factors responsible for protective actions of adipose-derived stem cell sheets on chronic heart failure after rat myocardial infarction. Circ. J. 2021, 85, 657–666.
- Choi, S.-C.; Seo, H.-R.; Cui, L.-H.; Song, M.-H.; Noh, J.-M.; Kim, K.-S.; Choi, J.-H.; Kim, J.-H.; Park, C.-Y.; Joo, H.J.; et al. Modeling hypoxic stress in vitro using human embryonic stem cells derived cardiomyocytes matured by fgf4 and ascorbic acid treatment. Cells 2021, 10, 2741.
- Ellis, B.W.; Acun, A.; Isik Can, U.; Zorlutuna, P. Human IPSC-derived myocardium-on-chip with capillary-like flow for personalized medicine. Biomicrofluidics 2017, 11, 024105.
- Basara, G.; Gulberk Ozcebe, S.; Ellis, B.W.; Zorlutuna, P. Tunable human myocardium derived decellularized extracellular matrix for 3d bioprinting and cardiac tissue engineering. Gels 2021, 7, 70.
- Ren, X.; Ellis, B.W.; Ronan, G.; Blood, S.R.; DeShetler, C.; Senapati, S.; March, K.L.; Handberg, E.; Anderson, D.; Pepine, C.; et al. A multiplexed ion-exchange membrane-based miRNA (MIX·miR) detection platform for rapid diagnosis of myocardial infarction. Lab A Chip 2021, 21, 3876–3887.
- Zhang, H.; Dvornikov, A.V.; Huttner, I.G.; Ma, X.; Santiago, C.F.; Fatkin, D.; Xu, X. A Langendorff-like system to quantify cardiac pump function in adult zebrafish. DMM Dis. Model. Mech. 2018, 11, dmm034819.
- Li, H.; Liu, C.; Bao, M.; Liu, W.; Nie, Y.; Lian, H.; Hu, S. Optimized Langendorff perfusion system for cardiomyocyte isolation in adult mouse heart. J. Cell. Mol. Med. 2020, 24, 14619–14625.
- Rossello, X.; Hall, A.R.; Bell, R.M.; Yellon, D.M. Characterization of the Langendorff Perfused Isolated Mouse Heart Model of Global Ischemia-Reperfusion Injury: Impact of Ischemia and Reperfusion Length on Infarct Size and LDH Release. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 286–295.
- Lindsey, M.L.; Bolli, R.; Canty, J.M., Jr.; Du, X.-J.; Frangogiannis, N.G.; Frantz, S.; Gourdie, R.G.; Holmes, J.W.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H812–H838.
- Alqarni, F.; Alsaadi, M.; Karem, F. MR image analysis of ex-vivo mouse model of heart ischemia. Saudi J. Biol. Sci. 2021, 28, 1990–1998.
- Montero-Bullon, J.F.; Aveiro, S.S.; Melo, T.; Martins-Marques, T.; Lopes, D.; Neves, B.; Girão, H.; Rosário MDomingues, M.; Domingues, P. Cardiac phospholipidome is altered during ischemia and reperfusion in an ex vivo rat model. Biochem. Biophys. Rep. 2021, 27, 101037.
- Haubner, B.J.; Adamowicz-Brice, M.; Khadayate, S.; Tiefenthaler, V.; Metzler, B.; Aitman, T.; Penninger, J.M. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging 2012, 4, 966–977.
- Darehzereshki, A.; Rubin, N.; Gamba, L.; Kim, J.; Fraser, J.; Huang, Y.; Billings, J.; Mohammadzadeh, R.; Wood, J.; Warburton, D.; et al. Differential regenerative capacity of neonatal mouse hearts after cryoinjury. Dev. Biol. 2015, 399, 91–99.
- Mahmoud, A.I.; Porrello, E.R.; Kimura, W.; Olson, E.N.; Sadek, H.A. Surgical models for cardiac regeneration in neonatal mice. Nat. Protoc. 2014, 9, 305–311.
- Bei, Y.; Chen, C.; Hua, X.; Yin, M.; Meng, X.; Huang, Z.; Qi, W.; Su, Z.; Liu, C.; Lehmann, H.I.; et al. A modified apical resection model with high accuracy and reproducibility in neonatal mouse and rat hearts. Npj Regen. Med. 2023, 8, 9.
- Ye, L.; D’agostino, G.; Loo, S.J.; Wang, C.X.; Su, L.P.; Tan, S.H.; Tee, G.Z.; Pua, C.J.; Pena, E.M.; Cheng, R.B.; et al. Early regenerative capacity in the porcine heart. Circulation 2018, 138, 2798–2808.
- Garbern, J.C.; Mummery, C.L.; Lee, R.T. Model Systems for Cardiovascular Regenerative Biology. Cold Spring Harb. Perspect. Med. 2013, 3, a014019.
- Reddy, P.C.; Gungi, A.; Unni, M. Cellular and Molecular Mechanisms of Hydra Regeneration. In Evo-Devo: Non-Model Species in Cell and Developmental Biology; Results and Problems in Cell Differentiation; Tworzydlo, W., Bilinski, S.M., Eds.; Springer International Publishing: Cham, Germany, 2019; Volume 68, pp. 259–290. Available online: http://link.springer.com/10.1007/978-3-030-23459-1_12 (accessed on 28 July 2023).
- Jopling, C.; Sleep, E.; Raya, M.; Martí, M.; Raya, A.; Izpisúa Belmonte, J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609.
- Chablais, F.; Veit, J.; Rainer, G.; Jaźwińska, A. The zebrafish heart regenerates after cryoinjury- induced myocardial infarction. BMC Dev. Biol. 2011, 11, 21.
- González-Rosa, J.M.; Martín, V.; Peralta, M.; Torres, M.; Mercader, N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 2011, 138, 1663–1674.
- Hein, S.J.; Lehmann, L.H.; Kossack, M.; Juergensen, L.; Fuchs, D.; Katus, H.A.; Hassel, D. Advanced Echocardiography in Adult Zebrafish Reveals Delayed Recovery of Heart Function after Myocardial Cryoinjury. PLoS ONE 2015, 10, e0122665.
- Wang, J.; Panáková, D.; Kikuchi, K.; Holdway, J.E.; Gemberling, M.; Burris, J.S.; Singh, S.P.; Dickson, A.L.; Lin, Y.-F.; Sabeh, M.K.; et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 2011, 138, 3421–3430.
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J. Am. Coll. Cardiol. 2018, 71, 1696–1706.
- Manuel, G.-R.J.; Michka, S.; Dorothy, F.; Mark, H.S.; Loren, J.F.; Burns, C.E.; Geoffrey, C. Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish Article Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Dev. Cell 2018, 44, 433–446.
- Cano-Martínez, A.; Vargas-González, A.; Guarner-Lans, V.; Prado-Zayago, E.; León-Oleda, M.; Nieto-Lima, B. Functional and structural regeneration in the axolotl heart (Ambystoma mexicanum) after partial ventricular amputation. Arch. Cardiol. Mex. 2010, 80, 21147570.
- Lauridsen, H.; Pedersen, M. Circulating cells contribute to cardiac regeneration in the axolotl. FASEB J. 2015, 29, 1029.14.
- Jacobs, G.F.M.; Michielsen, R.P.A.; Kühn, E.R. Thyroxine and Triiodothyronine in Plasma and Thyroids of the Neotenic and Metamorphosed Axolotl Ambystoma mexicanurn: Influence of TRH Injections. Gen. Comp. Endocrinol. 1998, 70, 145–151.
- Monaghan, J.R.; Stier, A.C.; Michonneau, F.; Smith, M.D.; Pasch, B.; Maden, M.; Seifert, A.W. Experimentally induced metamorphosis in axolotls reduces regenerative rate and fidelity. Regeneration 2014, 1, 2–14.
- Rollins-Smith, L.A. Metamorphosis and the amphibian immune system. Immunol. Rev. 1998, 166, 221–230.
- Godwin, J.W.; Rosenthal, N. Scar-free wound healing and regeneration in amphibians: Immunological influences on regenerative success. Differentiation 2014, 87, 66–75.
- Hirose, K.; Payumo, A.Y.; Cutie, S.; Hoang, A.; Zhang, H.; Guyot, R.; Lunn, D.; Bigley, R.B.; Yu, H.; Wang, J.; et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 2019, 364, 184–188.
- Laube, F.; Heister, M.; Scholz, C.; Borchardt, T.; Braun, T. Re-programming of newt cardiomyocytes is induced by tissue regeneration. J. Cell Sci. 2006, 119, 4719–4729.
- Oberpriller, J.O.; Oberpriller, J.C. Response of the adult newt ventricle to injury. J. Exp. Zool. 1974, 187, 249–253.
- Witman, N.; Murtuza, B.; Davis, B.; Arner, A.; Morrison, J.I. Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev. Biol. 2011, 354, 67–76.
- Piatkowski, T.; Mühlfeld, C.; Borchardt, T.; Braun, T.; Chong, J.J.; Reinecke, H.; Iwata, M.; Torok-Storb, B.; Stempien-Otero, A.; Murry, C.E.; et al. Reconstitution of the Myocardium in Regenerating Newt Hearts is Preceded by Transient Deposition of Extracellular Matrix Components. Stem Cells Dev. 2013, 22, 1921–1931.
- Rumyantsev, P.P. Growth and hyperplasia of cardiac muscle cells. Sov. Med. Rev. 1991. Available online: https://www.taylorfrancis.com/books/mono/10.4324/9781315076652/growth-hyperplasia-cardiac-muscle-cells-rumyantsev (accessed on 20 July 2023).
- Novikov, A.I.; Khloponin, P.A. O reparativnykh protsessakh v émbrional’nom i postémbrional’nom miokardiogeneze Gallus domesticus L.
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080.
- Gunadasa-Rohling, M.; Masters, M.; Maguire, M.L.; Smart, S.C.; Schneider, J.E.; Riley, P.R. Magnetic Resonance Imaging of the Regenerating Neonatal Mouse Heart. Circulation 2018, 138, 2439–2441.
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Johnson, B.A.; Grinsfelder, D.; Canseco, D.; Mammen, P.P.; Rothermel, B.A.; Olson, E.N.; Sadek, H.A. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. USA 2013, 110, 187–192.
- Strungs, E.G.; Ongstad, E.L.; O’Quinn, M.P.; Palatinus, J.A.; Jourdan, L.J.; Gourdie, R.G. Cryoinjury models of the adult and neonatal mouse heart for studies of scarring and regeneration. Methods Mol. Biol. 2013, 1037, 343–353.
- Sophy, A.J.; Michele, A.S.; Frank, K.L.; Martin, B.; Michael, H.; Shaun, R.; Jane, C.L.; Robert, M.D.; Alexander, Y.; Bernd, F.; et al. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. USA 2012, 109, 13380–13385.




