Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilyes Dammak | -- | 2203 | 2023-10-10 13:46:04 | | | |
| 2 | Jason Zhu | -1 word(s) | 2202 | 2023-10-11 04:05:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dammak, I.; Fersi, M.; Hachicha, R.; Abdelkafi, S. Municipal Wastewater Treatment Plant Effluents and Microalgae Growth. Encyclopedia. Available online: https://encyclopedia.pub/entry/50064 (accessed on 07 February 2026).
Dammak I, Fersi M, Hachicha R, Abdelkafi S. Municipal Wastewater Treatment Plant Effluents and Microalgae Growth. Encyclopedia. Available at: https://encyclopedia.pub/entry/50064. Accessed February 07, 2026.
Dammak, Ilyes, Mariem Fersi, Ridha Hachicha, Slim Abdelkafi. "Municipal Wastewater Treatment Plant Effluents and Microalgae Growth" Encyclopedia, https://encyclopedia.pub/entry/50064 (accessed February 07, 2026).
Dammak, I., Fersi, M., Hachicha, R., & Abdelkafi, S. (2023, October 10). Municipal Wastewater Treatment Plant Effluents and Microalgae Growth. In Encyclopedia. https://encyclopedia.pub/entry/50064
Dammak, Ilyes, et al. "Municipal Wastewater Treatment Plant Effluents and Microalgae Growth." Encyclopedia. Web. 10 October, 2023.
Copy Citation
Municipal wastewater (MWW) provides a promising platform for microalgae cultivation due to its rich content of essential nutrients. Research has showcased the multifaceted benefits of microalgae-based wastewater treatment, from the potent depollution capabilities of these organisms to their biomass potential for ecofriendly applications. A significant advantage lies in the ability of these systems to promote environmental sustainability without producing secondary pollutants, aligning with the circular economy model.
green biomass
municipal wastewater
microalgae
phytoremediation
1. Introduction
The use of microalgae in waste treatment began in the 1950s, with investigations into the ability of microalgae and bacteria to treat MWW [1]. With the advancement of studies, it was realized that the combination of microalgae and bacteria growth, together with MWW treatment, could be a solution to overcome the high costs of microalgae cultivation, achieve the expected removal of nutrients from the medium, and produce high added value products such as biodiesel [2].
The use of microalgae in MWW treatment depends on the interaction between trophic chains. Heterotrophic bacteria, including anaerobic microorganisms, consume organic material and release carbon dioxide (CO2), ammonium (NH4+), nitrate (NO3−), and phosphorus (PO43−), which are used by microalgae to generate new cells [3]. Microalgae, in turn, release photosynthetic oxygen, allowing aerobic and facultative anaerobic heterotrophic bacteria to degrade organic matter and reduce the BOD of the effluent [4].
Microalgae are versatile and can remove nitrogen and phosphorus from MWW to limit values below those required by norms and the World Health Organization Guidelines for MWW discharge into water bodies [5]. Microalgae cultures are capable of treating effluents with different properties, such as effluent after preliminary treatment, primary effluents after removal of suspended solids, secondary effluents after clarification obtained by the degradation of organic matter; concentrated effluents, that is, the liquid phase of the sludge from the anaerobic digester [6]; and even effluents from treatment by anaerobic systems. Depending on the effluent’s origin, the treatment system’s configuration will differ [5].
After treatment by aerobic systems, MWW typically exhibits a reduced organic load but maintains high nutrient concentrations. Conversely, anaerobically treated MWW may present different nutrient profiles, with specific processes possibly increasing the nutrient content of the effluent. Regardless of these distinctions, both postaerobic and postanaerobic effluents, due to their nutrient richness, are apt for microalgae treatment, acting as effective subsequent treatment options [7].
In work by Marchello et al. [8], the potential for microalgae growth in the effluent after anaerobic treatment and its effect on Coliforms bacteria was evaluated. The study showed increased treatment efficiency when aeration was added to the medium, showing a 4-log reduction in colony-forming units for total Coliforms and E. coli.
According to Slompo et al. [9], the use of microalgae to treat blackwater after anaerobic treatment in an up-flow anaerobic sludge blanket (UASB) reactor showed a removal log of 0.76 log for total Coliforms and 2.73 log for E. coli, representing an efficiency of 68.8% and 99.8%, respectively.
In the study conducted by Ruas et al. [10], the removal of pathogens, including Pseudomonas aeruginosa, E. coli, and Enterococcus faecalis, was assessed using a microalgae–bacteria consortium. The study considered multiple factors, such as the microalgae Chlorella vulgaris, activated sludge, aeration, CO2 supplementation, photoperiod, and the type of system (open and closed).
The findings revealed that the consortium significantly increased the removal of pathogens, exceeding 50%, and the removal efficiency reached an impressive 99.4% with the addition of aeration. Notably, the 24:0 photoperiod, characterized by a lack of solar radiation during the dark period, did not effectively remove P. aeruginosa and E. coli. This observation underscores the crucial role of solar radiation in promoting photosynthesis, which is essential for the microalgae’s metabolic activity. Enhanced photosynthesis, driven by solar radiation, likely contributes to the microalgae’s ability to generate oxygen and degrade organic matter, leading to improved pathogen removal in the presence of light.
Gutiérrez-Alfaro et al. [11] conducted a study to evaluate the ability to remove E. coli, Enterococcus sp., and Clostridium perfringens from different secondary processes and the behavior of these pathogens in solar disinfection. It was concluded that solar radiation significantly increased the removal of these pathogens in the effluent after secondary treatment (removal of 2.9 log of E. coli). It can thus be inferred that the application of treatment with microalgae using the appropriate photoperiod has a high potential for MWW treatment and disinfection [11]. Microalgae-based MWW treatment generates valuable byproducts that can be effectively repurposed. The microalgae biomass produced during treatment holds great potential as an agricultural fertilizer, enriching and rejuvenating depleted soils [12]. Additionally, after undergoing the microalgae-based treatment, the treated effluent is a water resource that can be safely reused for various nonpotable purposes [13]. This includes less restrictive activities where the stringent quality standards required for drinking water are unnecessary, offering an ecofriendly and sustainable approach to wastewater management.
2. Removal Mechanisms of Dominant Pollutants Using Microalgae
In the realm of MWW, microalgae-based technologies have emerged as a beacon of sustainability and efficiency. One of the primary mechanisms that microalgae employ is adsorption. Due to their expansive surface area and cell walls rich in lipids, proteins, and polysaccharides, microalgae can adsorb many pollutants commonly found in MWW [14]. Additionally, biosorption is a significant process wherein dead or living algal biomass passively uptakes and concentrates pollutants. The inherent functional groups, such as carboxyl, hydroxyl, and amine on microalgal cell surfaces, enhance their biosorption capacity, especially for heavy metals [15].
As microalgae proliferate in MWW, they assimilate and uptake, absorbing critical nutrients like nitrogen and phosphorus and integrating them into their biomass [16]. Another salient mechanism is biodegradation. Select strains of microalgae possess the capability to metabolize organic pollutants, converting them into benign or less harmful compounds. Certain strains can degrade intricate organic molecules in pharmaceutical residues frequently found in MWW [17].
The bioconversion ability of microalgae is another testament to their versatility. Through photosynthesis, they can process considerable amounts of carbon dioxide in wastewater, subsequently releasing oxygen—a process that mitigates greenhouse gas emissions and aids in wastewater oxygenation [18]. Some calcifying microalgae strains bring about coprecipitation, precipitating pollutants such as phosphates in the form of calcium phosphate [19].
Moreover, particular strains of microalgae bolster pathogen reduction in MWW by producing compounds toxic to bacteria and other pathogens [20]. Finally, the oxygen-production capability of microalgae, a byproduct of photosynthesis, is indispensable. It enhances the dissolved oxygen levels in wastewater, promoting the activity of aerobic bacteria and facilitating the breakdown of organic pollutants [21].
In summary, when applied to MWW treatment, microalgae present a multifaceted approach, targeting various pollutants through diverse mechanisms. The optimal application hinges on the judicious selection of microalgae strains and fine-tuning the treatment conditions [22].
3. Reuse of Treated Municipal Wastewater
The reuse of water makes it possible to alleviate the pressure on water resources caused by the high consumption of water for the development of human activities. Reuse after treatment and biomass recovery allows substituting water sources in less restrictive demands to release better quality water for more restrictive uses such as human supply [23].
Generally, treated MWW is used for:
-
Irrigation of parks and gardens located in public areas, centers, sports facilities, soccer fields, golf courses, school gardens and universities, lawns, decorative trees, and shrubs along avenues and highways;
-
Irrigation of garden areas around public and residential buildings;
-
Fire protection reserve;
-
Dust control in earth movements;
-
Aquatic decorative systems, such as fountains, mirrors, and waterfalls;
-
Toilet flushing in public restrooms and commercial and industrial buildings;
-
Washing of public trains and buses.
For the sake of the circular economy’s aims, various studies have been carried out on the reuse of MWW treated with microalgae and its potential for reuse [24].
4. Recovery of Microalgae Biomass
The post-treatment recovery of microalgae biomass is an integral step in wastewater treatment, offering potential applications in diverse domains. However, the inherent challenges associated with this separation make it a cost-intensive process.
Separating microalgae biomass can account for 20 to 60% of the total production cost [25]. As a result, the selected separation process must be efficient, swift, and cost-effective.
The most widely adopted separation method on a large scale is coagulation/flocculation, followed by sedimentation. This approach destabilizes particles, facilitating the separation of the solid–liquid phases [26]. Traditional coagulants, such as aluminum sulfate and ferric chloride, have been utilized in this context. However, a notable concern with these coagulants is their potential to induce biomass toxicity during the coagulation/flocculation process [27].
Given the challenges with traditional coagulants, the shift towards natural coagulants, especially those based on vegetable tannins, has gained traction. These tannin-based coagulants, derived from sources like quebracho, chestnut, or black wattle, have proven effective in microalgae separation without introducing harmful metals into the process [28].
Several studies have evaluated the efficacy of Tanfloc SG, a commercial coagulant/flocculant, in microalgae separation. For instance, Teixeira et al. [29] demonstrated that Tanfloc SG, at an optimal concentration of 100 mg·L−1, achieved over 90% removal efficiency in various parameters within a short sedimentation time. Ruggeri et al. [30] found that 35 mg·L−1 of Tanfloc SG led to 99% turbidity removal in treating microalgae Monoraphidium contortum.
The method chosen for recovering microalgae biomass post-treatment plays a pivotal role in determining the efficiency and safety of the process. As the shift towards natural coagulants gains momentum, it offers a promising avenue for sustainable and effective biomass recovery.
5. Potential Applications of Algal Biomass
The rapid growth of the global population has resulted in increased energy consumption, resource depletion, and escalating pollution levels. To combat these challenges, there is a pressing need for environmentally sustainable production and consumption systems that emphasize reuse and recycling. In this context, the integration of the biorefinery concept has emerged as a beacon of sustainable development. One such promising approach is the cultivation of microalgae in wastewater, which not only aids in wastewater treatment but also produces valuable microalgal biomass with many potential applications (Figure 2). Microalgae are renowned for their rich content of bioactive compounds, including carbohydrates, proteins, lipids, and pigments, which find uses across various sectors [31]. These applications span from their incorporation in animal feeds and soil biofertilization to their utility in cosmetics, pharmaceuticals, and energy production.
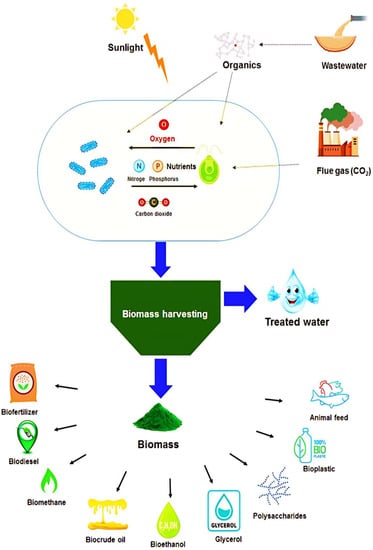
Figure 2. The potential applications of the generated biomass following microalgal treatment of wastewater.
Biomass for Animal Feed and Soil Fertilization
Microalgae’s high content of lipids, essential amino acids, and minerals positions them as a potent nutritional powerhouse, especially beneficial for mammals that cannot synthesize these components. This unique nutritional profile underscores their potential as a natural supplement in animal feeds, with particular significance for the booming aquaculture sector [32].
In the realm of agriculture, microalgae biomass demonstrates efficacy as a biofertilizer. Its application enriches the soil with organic matter and boosts levels of essential plant nutrients, including nitrogen, phosphorus, calcium, potassium, iron, and manganese, among others [33]. However, a caveat that warrants attention is the potential concentration of heavy metals in the harvested microalgal biomass. Elevated heavy metal concentrations can pose risks when the biomass is applied directly to the soil or used in animal feeds. It is imperative to employ pretreatment techniques or quality-control measures to ensure the safe application of this biomass. Recent research has delved into methods for reducing heavy metal concentrations in microalgal biomass, ensuring its safe use in various applications [34][35]. Emphasizing these precautions underscores the holistic approach toward harnessing the potential of microalgae while ensuring environmental and health safety.
Biomass for the Production of Bioenergy
The potential use of microalgae as renewable energy has been inspired due to their rapid growth rate and high lipid content. In the last decade, microalgae have become a third-generation biofuel feedstock due to their high triglyceride. Algal polysaccharides and triacylglycerol can also be starting materials for synthesizing bioethanol and biodiesel. Biomethane from wastewater-grown microalgae biomass was reported to depend on the biomass’s composition and the type of converted molecules (carbohydrates, proteins, or lipids). Towards the sustainability concept, the CO2 generated during anaerobic digestion and the conversion of methane to electricity could be integrated with microalgae cultivation in wastewater. Generally, energy recovered from wastewater-grown microalgal biomass is through different conversion techniques like hydrothermal liquefaction, anaerobic digestion and fermentation, transesterification, and pyrolysis. The choice of an adequate technique depends strictly on the strain used and the type of energy to produce [36].
Despite several scientific initiatives, the commercialization of microalgal biofuels has not yet been achieved since it is not economically feasible because of drying and extraction costs [37]. Therefore, microalgal biomass can also be used to produce other value-added compounds.
Other Valuable Compounds
Several bioactive compounds have been discovered and purified from marine microalgae. These valuable compounds, including polysaccharides, pigments, fatty acids, and proteins, demonstrate several biological activities [38]. For example, the peptide fraction isolated from pepsin-hydrolyzed microalgae protein waste had antigastric cancer properties, and the Angiotensin-converting enzyme isolated from microalgae C. vulgaris had a role in the regulation of hypertension. Polyunsaturated fatty acids, including ω-3, have also been shown to have antioxidant capacities, reduce hypertension, and have immune-regulating qualities. Microalgal pigments, like astaxanthin, β-carotene, phycocyanin, lutein, and violaxanthin, have been shown to have anticoagulant, antimutagenic, antibacterial, radioprotective, anticancer, and anti-inflammatory bioactivities [39]. Another emerging application of microalgal biomass is lactic acid and bioplastic production after converting carbohydrates. Glycerol could also be produced with a percentage of 10% as a byproduct during microalgal lipid biodiesel production. For cosmetic applications, different secondary metabolites and specific extracts from Tetraselmis sp. and Dunaliella sp. could be used as an alternative feedstock for producing various cosmetics, like antioxidants, UV-protectants, and antiaging, for skin care [36].
References
- Oswald, W.J.; Gotaas, H.B. Photosynthesis in sewage treatment. Trans. Am. Soc. Civ. Eng. 1957, 122, 73–105.
- Makut, B.B.; Das, D.; Goswami, G. Production of microbial biomass feedstock via co-cultivation of microalgae-bacteria consortium coupled with effective wastewater treatment: A sustainable approach. Algal Res. 2019, 37, 228–239.
- Lu, Y.; Zhang, X.; Gu, X.; Lin, H.; Melis, A. Engineering microalgae: Transition from empirical design to programmable cells. Crit. Rev. Biotechnol. 2021, 41, 1233–1256.
- Alazaiza, M.Y.; Albahnasawi, A.; Ahmad, Z.; Bashir, M.J.; Al-Wahaibi, T.; Abujazar, M.S.S.; Amr, S.S.A.; Nassani, D.E. Potential use of algae for the bioremediation of different types of wastewater and contaminants: Production of bioproducts and biofuel for green circular economy. J. Environ. Manag. 2022, 324, 116415.
- Gonzalez-Camejo, J.; Aparicio, S.; Jiménez-Benítez, A.; Pachés, M.; Ruano, M.; Borrás, L.; Barat, R.; Seco, A. Improving membrane photobioreactor performance by reducing light path: Operating conditions and key performance indicators. Water Res. 2020, 172, 115518.
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of nutrients from wastewaters using microalgae. Front. Sustain. Food Syst. 2018, 2, 59.
- Gonzalez-Camejo, J.; Jiménez-Benítez, A.; Ruano, M.; Robles, A.; Barat, R.; Ferrer, J. Optimising an outdoor membrane photobioreactor for tertiary sewage treatment. J. Environ. Manag. 2019, 245, 76–85.
- Marchello, A.E.; Lombardi, A.T.; Dellamano-Oliveira, M.J.; de Souza, C.W. Microalgae population dynamics in photobioreactors with secondary sewage effluent as culture medium. Braz. J. Microbiol. 2015, 46, 75–84.
- Slompo, N.D.M.; Quartaroli, L.; Fernandes, T.V.; da Silva, G.H.R.; Daniel, L.A. Nutrient and pathogen removal from anaerobically treated black water by microalgae. J. Environ. Manag. 2020, 268, 110693.
- Ruas, G.; Serejo, M.; Farias, S.; Scarcelli, P.; Boncz, M. Removal of pathogens from domestic wastewater by microalgal-bacterial systems under different cultivation conditions. Int. J. Environ. Sci. Technol. 2022, 19, 10177–10188.
- Gutiérrez-Alfaro, S.; Rueda-Márquez, J.J.; Perales, J.A.; Manzano, M.A. Combining sun-based technologies (microalgae and solar disinfection) for urban wastewater regeneration. Sci. Total Environ. 2018, 619, 1049–1057.
- Magwaza, S.T.; Magwaza, L.S.; Odindo, A.O.; Mditshwa, A. Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: A review. Sci. Total Environ. 2020, 698, 134154.
- Helmecke, M.; Fries, E.; Schulte, C. Regulating water reuse for agricultural irrigation: Risks related to organic micro-contaminants. Environ. Sci. Eur. 2020, 32, 4.
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2022, 13, 100205.
- Ramírez-García, R.; Gohil, N.; Singh, V. Recent advances, challenges, and opportunities in bioremediation of hazardous materials. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 517–568.
- Uggetti, E.; Sialve, B.; Trably, E.; Steyer, J.P. Integrating microalgae production with anaerobic digestion: A biorefinery approach. Biofuels Bioprod. Biorefining 2014, 8, 516–529.
- Moghaddam, A.; Khayatan, D.; Esmaeili Fard Barzegar, P.; Ranjbar, R.; Yazdanian, M.; Tahmasebi, E.; Alam, M.; Abbasi, K.; Esmaeili Gouvarchin Ghaleh, H.; Tebyaniyan, H. Biodegradation of pharmaceutical compounds in industrial wastewater using biological treatment: A comprehensive overview. Int. J. Environ. Sci. Technol. 2023, 20, 5659–5696.
- Viswanaathan, S.; Perumal, P.K.; Sundaram, S. Integrated approach for carbon sequestration and wastewater treatment using algal–bacterial consortia: Opportunities and challenges. Sustainability 2022, 14, 1075.
- Abdella, B.; Mahmoud, N.H.; Mohamed, J.H.; Moffit, S.M.; Elsherbiny, B.A.; El-Sheekh, M.M. Bioremediation of Organic and Heavy Metal Co-contaminated Environments. In Industrial Wastewater Reuse: Applications, Prospects and Challenges; Springer: Berlin/Heidelberg, Germany, 2023; pp. 393–420.
- Inuwa, A.; Pervez, A.; Nazir, R. Microalgae-based wastewater treatment system: Current state, antibiotic resistant bacteria and antibiotic resistance genes reduction potentials. Int. J. Environ. Sci. Technol. 2023, 1–20.
- Mathew, M.M.; Khatana, K.; Vats, V.; Dhanker, R.; Kumar, R.; Dahms, H.-U.; Hwang, J.-S. Biological approaches integrating algae and bacteria for the degradation of wastewater contaminants—A review. Front. Microbiol. 2022, 12, 801051.
- Leong, W.H.; Rawindran, H.; Ameen, F.; Alam, M.M.; Chai, Y.H.; Ho, Y.C.; Lam, M.K.; Lim, J.W.; Tong, W.-Y.; Bashir, M.J. Advancements of microalgal upstream technologies: Bioengineering and application aspects in the paradigm of circular bioeconomy. Chemosphere 2023, 339, 139699.
- Solé-Bundó, M.; Cucina, M.; Folch, M.; Tàpias, J.; Gigliotti, G.; Garfí, M.; Ferrer, I. Assessing the agricultural reuse of the digestate from microalgae anaerobic digestion and co-digestion with sewage sludge. Sci. Total Environ. 2017, 586, 1–9.
- Roy, M.; Mohanty, K. A comprehensive review on microalgal harvesting strategies: Current status and future prospects. Algal Res. 2019, 44, 101683.
- Ortiz, A.; García-Galán, M.J.; García, J.; Diez-Montero, R. Optimization and operation of a demonstrative full scale microalgae harvesting unit based on coagulation, flocculation and sedimentation. Sep. Purif. Technol. 2021, 259, 118171.
- Lv, J.; Liu, G.; Feng, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. Harvesting biomass of an oil-rich microalga Parachlorella kessleri TY02 by ferric chloride: Effects on harvesting efficiency, lipid production and municipal wastewater treatment. J. Environ. Manag. 2020, 273, 111128.
- Niemi, C.; Gentili, F.G. The use of natural organic flocculants for harvesting microalgae grown in municipal wastewater at different culture densities. Physiol. Plant. 2021, 173, 536–542.
- Urbina-Suarez, N.A.; Ayala-González, D.D.; Rivera-Amaya, J.D.; Barajas-Solano, A.F.; Machuca-Martínez, F. Evaluation of the Light/Dark Cycle and Concentration of Tannery Wastewater in the Production of Biomass and Metabolites of Industrial Interest from Microalgae and Cyanobacteria. Water 2022, 14, 346.
- Teixeira, M.S.; Speranza, L.G.; da Silva, I.C.; Moruzzi, R.B.; Silva, G.H.R. Tannin-based coagulant for harvesting microalgae cultivated in wastewater: Efficiency, floc morphology and products characterization. Sci. Total Environ. 2022, 807, 150776.
- Ruggeri, M.V.R.; Godoy, R.F.B.; Arroyo, P.A.; Trevisan, E. Evaluation of natural flocculant efficiency in the harvest of microalgae Monoraphidium contortum. SN Appl. Sci. 2021, 3, 1–9.
- Bhattacharya, M.; Goswami, S. Microalgae–A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580.
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal proteins: Production strategies and nutritional and functional properties. Bioresour. Technol. 2021, 332, 125125.
- Das, P.; Khan, S.; Chaudhary, A.K.; AbdulQuadir, M.; Thaher, M.I.; Al-Jabri, H. Potential applications of algae-based bio-fertilizer. Biofertil. Sustain. Agric. Environ. 2019, 41–65.
- Elleuch, J.; Hmani, R.; Drira, M.; Michaud, P.; Fendri, I.; Abdelkafi, S. Potential of three local marine microalgae from Tunisian coasts for cadmium, lead and chromium removals. Sci. Total Environ. 2021, 799, 149464.
- Dammak, M.; Hlima, H.B.; Tounsi, L.; Michaud, P.; Fendri, I.; Abdelkafi, S. Effect of heavy metals mixture on the growth and physiology of Tetraselmis sp.: Applications to lipid production and bioremediation. Bioresour. Technol. 2022, 360, 127584.
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of wastewaters by microalgae and the potential applications of the produced biomass—A review. Water 2020, 13, 27.
- Kim, J.Y.; Jung, J.-M.; Jung, S.; Park, Y.-K.; Tsang, Y.F.; Lin, K.-Y.A.; Choi, Y.-E.; Kwon, E.E. Biodiesel from microalgae: Recent progress and key challenges. Prog. Energy Combust. Sci. 2022, 93, 101020.
- Xiaogang, H.; Jalalah, M.; Jingyuan, W.; Zheng, Y.; Li, X.; Salama, E.-S. Microalgal growth coupled with wastewater treatment in open and closed systems for advanced biofuel generation. Biomass Convers. Biorefinery 2022, 12, 1939–1958.
- Srimongkol, P.; Sangtanoo, P.; Songserm, P.; Watsuntorn, W.; Karnchanatat, A. Microalgae-based wastewater treatment for developing economic and environmental sustainability: Current status and future prospects. Front. Bioeng. Biotechnol. 2022, 10, 904046.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Wastewater Treatment
Revisions:
2 times
(View History)
Update Date:
11 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




