Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Charalampos Proestos | -- | 2884 | 2023-10-06 13:17:35 | | | |
| 2 | Peter Tang | Meta information modification | 2884 | 2023-10-07 05:34:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kiokias, S.; Proestos, C.; Oreopoulou, V. Natural Food Antioxidants against LDL Damage/Atherosclerosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/49877 (accessed on 07 February 2026).
Kiokias S, Proestos C, Oreopoulou V. Natural Food Antioxidants against LDL Damage/Atherosclerosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/49877. Accessed February 07, 2026.
Kiokias, Sotirios, Charalampos Proestos, Vassilki Oreopoulou. "Natural Food Antioxidants against LDL Damage/Atherosclerosis" Encyclopedia, https://encyclopedia.pub/entry/49877 (accessed February 07, 2026).
Kiokias, S., Proestos, C., & Oreopoulou, V. (2023, October 06). Natural Food Antioxidants against LDL Damage/Atherosclerosis. In Encyclopedia. https://encyclopedia.pub/entry/49877
Kiokias, Sotirios, et al. "Natural Food Antioxidants against LDL Damage/Atherosclerosis." Encyclopedia. Web. 06 October, 2023.
Copy Citation
Radical oxygen species formed in human tissue cells by many endogenous and exogenous pathways cause extensive oxidative damage which has been linked to various human diseases.
LDL-oxidation
DNA-damage
antioxidant vitamins
oxidative stress
1. Introduction to Lipid Peroxidation and Antioxidants
Peroxidation of lipids, particularly of polyunsaturated fatty acids (PUFAs) is a process with marked implications: it shortens the shelf-life of food and drugs, it causes fragmentation of DNA, it damages cellular membranes and it promotes the genesis of many human diseases [1]. Lipid peroxidation is a complex biological process, initiated by free radicals, that results in the formation of conjugated dienes and lipid hydroperoxides [2]. These are usually degraded to a variety of products including alkanals, hydroxyalkenals, ketones, alkanes etc. [3]. Figure 1 reflects the lipid peroxidation process along with a few common oxidative biomarkers.
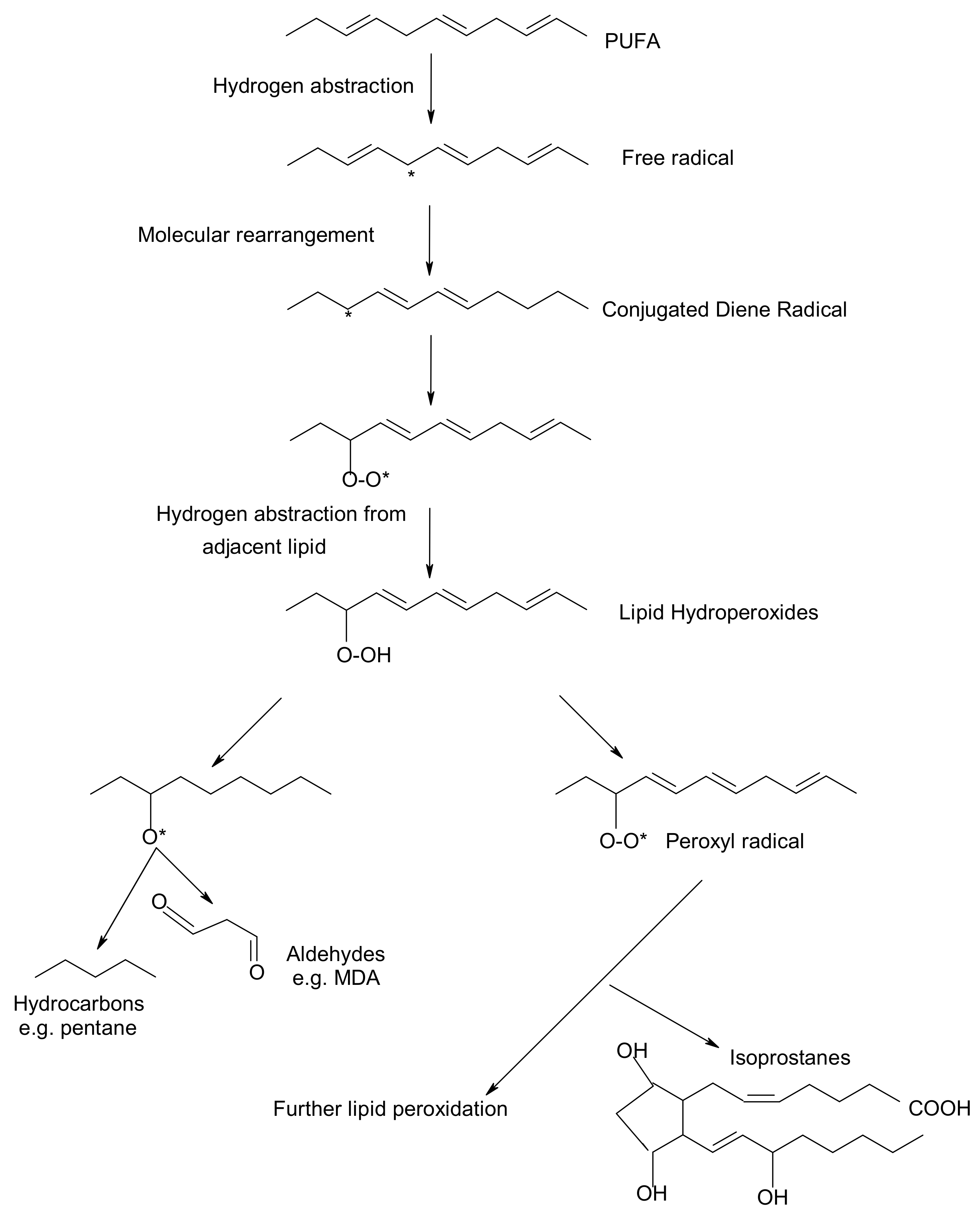
Figure 1. Overview of lipid peroxidation process and oxidative biomarkers (* indicates the presence of free radical).
Free radicals and oxidants play a dual role as both toxic and beneficial compounds, since they can be either harmful or helpful to the body [4][5]. They are formed in tissue cells by various endogenous and exogenous pathways [6]. The ability of free radicals to structurally modify cellular components, gene expression and protein production has led to the implication of their involvement in a variety of pathological conditions, including inflammation, aging, carcinogenesis and cardiovascular diseases [7][8]. Oxidative stress exerts an adverse impact on human health. Oxygen free radicals (such as hydroxyl radicals, superoxide radicals and other active oxygen species including also singlet oxygen) adversely alter lipids, proteins, and DNA [9]. A role of lipid peroxidation and oxidative stress in the association between thyroid diseases and breast cancer has been claimed by Dominguez and Castelao (2008) [10]. Actually, overproduction of free radicals in vivo and the consequent damage to biological molecules is increasingly regarded as an important event in the development of human diseases, including arthritis, thyroid, cancer, and atherosclerosis [11].
Bhattacharyya et al. (2014) [12] noted that reactive oxygen species (ROS) are produced within the gastrointestinal (GI) tract, since ingested materials and microbial pathogens can induce oxidative injury and GI inflammatory responses involving the epithelium and immune/inflammatory cells. Therefore, further investigation on how the ROS can contribute to diverse gastrointestinal dysfunction, or manifest dual roles in cancer promotion or cancer suppression would enhance understanding of inflammation-based GI diseases and facilitate the development of new therapies [13].
Certain oxidative biomarkers have linked oxidative stress and the development of health diseases. A substantial body of evidence indicates that measurement of prostaglandine (PG)-like compounds provides a direct and reliable approach to assess oxidative damage in vivo compared with other methods such as thiobarbituric acid reacting substances (TBARS) that have been also widely studied [14]. According to Barocas et al. (2011) [15] oxidative stress measured by urine F2-isoprostane level is associated with prostate cancer. In recent years, development of immunochemical detection of 4-Hydroxynonenal (HNE)-histidine adducts opened more advanced methodological possibilities for qualitative and quantitative detection of lipid peroxidation in various human and animal tissues [16].
To control and reduce in vivo oxidative damage, nature makes use of several types of antioxidants or radical trapping agents operating at different stages of the process [17][18]. These compounds, also known as biological antioxidants, react rapidly with free radicals and slow down the oxidative damage [19][20]. A body of evidence indicates that certain dietary compounds of plant origin can act as radical scavengers in model biological systems and in the human organism, thereby acting as dietary antioxidants [21][22]. Increased plasma total antioxidant capacity has been associated with a high consumption of fruits and vegetables rich in these vitamins, although limited information is available on whether this reflects the dietary intake of antioxidants [23]. The commonly used assays for ranking antioxidants share a common problem. Most estimates are based on methods conducted in solution and are, therefore, not necessarily relevant to processes that occur at the lipid–water interfaces in both membranes and micro emulsions, e.g., lipoproteins [24]. This research focuses on several natural compounds the levels of which in human body can be manipulated by supplements and dietary modifications. More specifically, the following ones have been reported to exert in vitro and in vivo antioxidant activities:
(i) Tocopherols and tocotrienols (vitamin E). Tocopherols and tocotrienols comprise a group of eight chromanol homologs extracted from natural sources (e.g., oils, nuts, leafy vegetables) that possess vitamin E activity in the diet [25]. They are natural monophenolic compounds with well-established antioxidant activities in food and biological systems [26]. The α-, β-, γ- and δ-tocopherols are characterized by a saturated side chain consisting of three isoprenoid units, whereas their corresponding tocotrienols have double bonds at the 3′, 7′ and 11′ position of the isoprenoid side chain [27] (structures are presented in Figure 2).
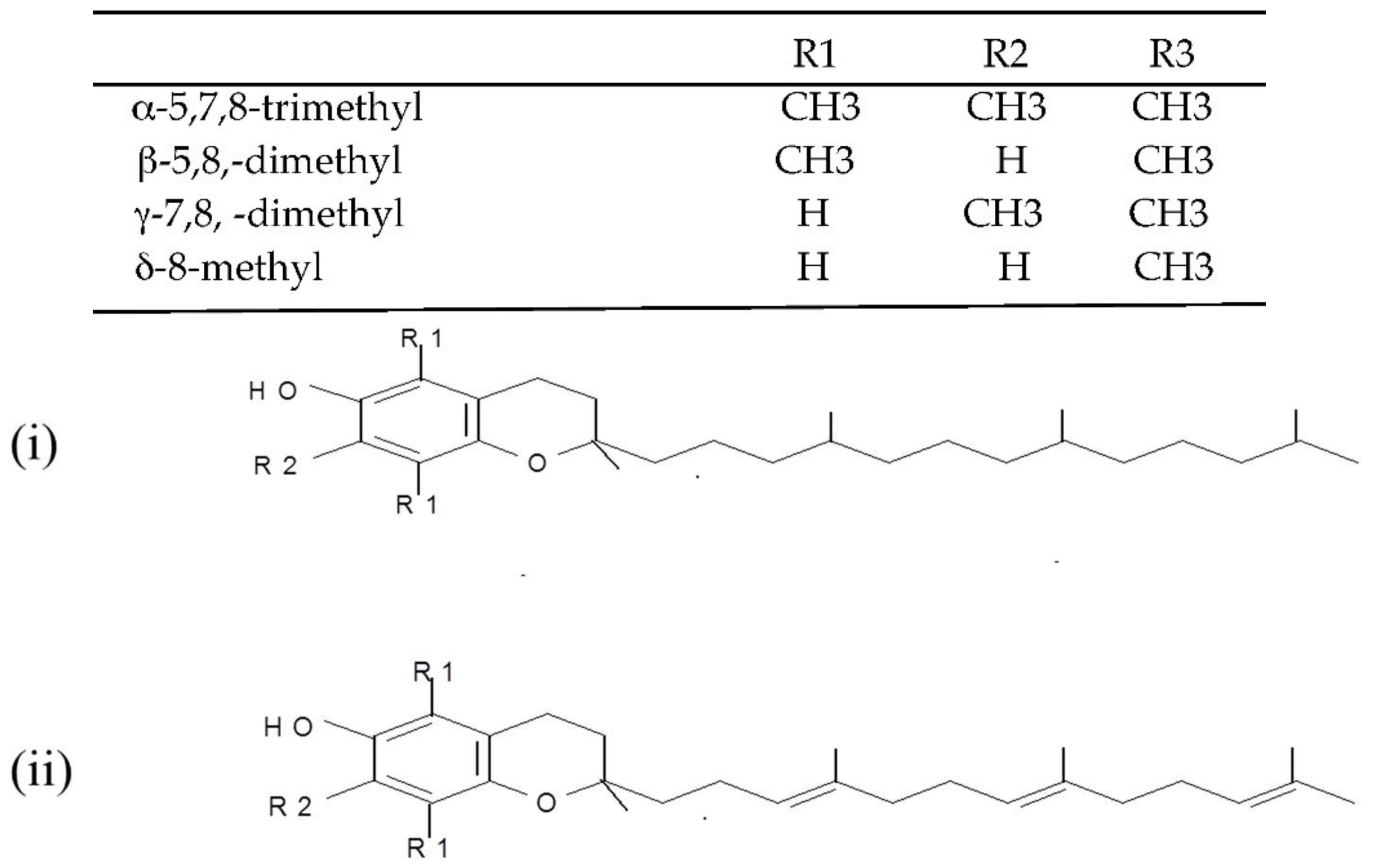
Figure 2. Chemical structures of the E vitamers (tocopherols-i and tocotrienols-ii).
(ii) L-Ascorbic acid (vitamin C). Vitamin C, also known as ascorbic acid, occurs in all tissues of living organisms where it is responsible for the normal functioning of important metabolic processes [28]. It is very widespread in nature (e.g., in oranges, green peppers, watermelon, grapefruit) and recognized as an antioxidant nutrient with multi-functional effects depending on the conditions of the food and biological systems [29]. L-Ascorbic acid is a six-carbon weak acid with a pKa of 4.2, which is reversibly oxidized due to its enediol structure with the loss of an electron to form the free radical semihydroascorbic acid [21].
(iii) Carotenoids (provitamins A). The carotenoids are natural pigments extracted from many sources (e.g., in carrots, plums, apricots, tomatoes, spinach) that are used for various food applications. Carotenoids have been increasingly studied in the last decade for their potential to act as in vitro and in vivo antioxidants [30][31]. Dietary supplementation with certain carotenoids possessing provitamin A activity (such as β-carotene and lycopene, Figure 3) has been associated in literature with a protective role against diseases (including aging, types of cancer, cardiovascular disease, cataracts, and age-related macular degeneration) [32].
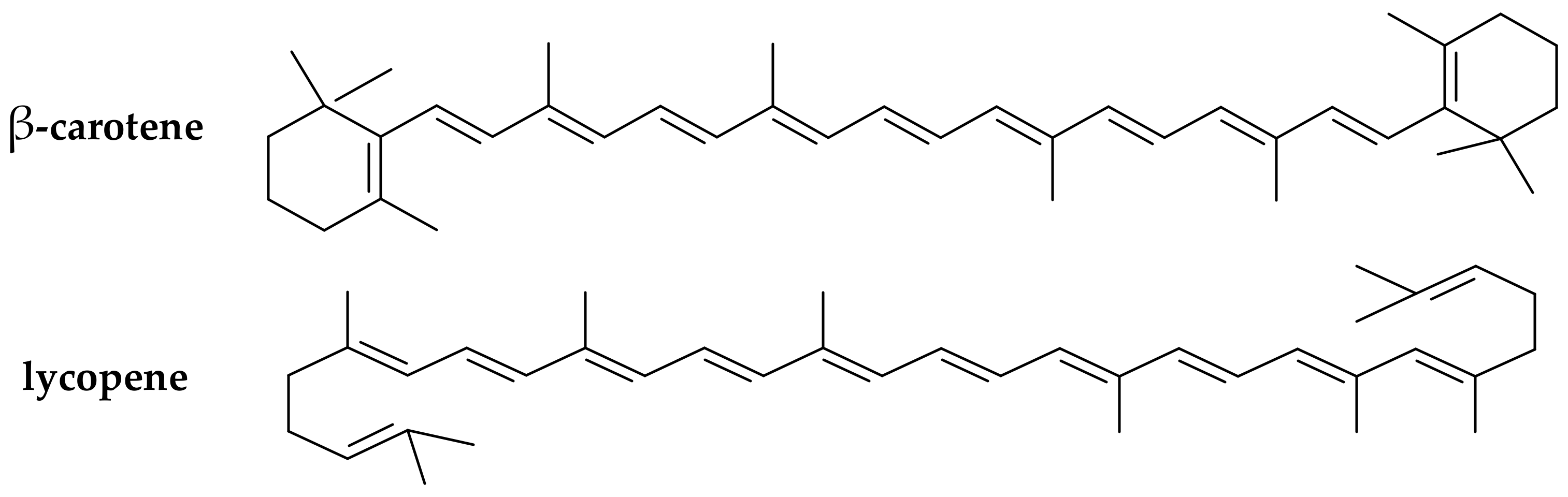
Figure 3. Chemical structure of the main provitamin A carotenes (β-carotene, lycopene).
(iv) Flavonoids and phenolic acids. The current work also reports the dietary antioxidant effects of various phenolic acids available in many natural sources (e.g., in olive oil, herbs, fruits) that have been widely explored in food systems [33][34]. Flavonoids, in particular, comprise a class of phenolic compounds with well established antioxidant properties strongly related to their structure [35].
2. Low-Density Lipoprotein (LDL) Oxidative Damage and Antioxidation
2.1. Link of LDL Oxidation to Atherogenesis and Common Monitoring Methods
Human low-density lipoprotein (LDL) is defined as the population of lipoproteins, which can be isolated from plasma by ultra centrifugation within a density gradient of 1019–1063 g/L [36]. Elevated plasma concentration of LDL is a risk factor for atherosclerosis and coronary artery disease [37]. A recent body of literature has reported that atherosclerosis develops following free radical processes that cause oxidative modification of LDL [38][39]. Atherosclerosis is a progressive disease of the arterial tree that involves deposition of cholesterols in the arterial intima leading finally to a thickening of the arterial wall and reduced luminal blood flow [40]. The lipid deposited is mainly LDL, derived from the circulation. Amarowicz and Pegg [41] claimed that the exact mechanism(s) of atherosclerosis in humans remains elusive, but one theory hypothesizes that this deleterious process results from the oxidative modification of LDL.
The oxidation of LDL is a complex process during which both the proteins and the lipids undergo oxidative changes and form complex products [42]. It is a lipid peroxidation reaction driven by free radicals. Reactive oxygen species, or thiols may be released and thus participate directly in the initiation of LDL oxidation [43]. Oxidation presumably begins when a reactive radical abstracts hydrogen from a PUFA of surface phospholipids or bulk lipids in the core of the LDL particle, a reaction which in the absence of sufficient concentrations of antioxidants results in the propagation of lipid peroxidation [44].
There are various ways to measure the effect of a diet on LDL oxidation, including: the level of thiobarbituric acid reactive substances (TBARS) and lipid hydroperoxides in plasma; anion exchange chromatography and electrophoresis motility of the LDL particles; the formation of conjugated dienes (CD) at 232 nm and fluorescence spectra on the oxidation of native LDL with a chemical inducer; and the uptake of oxidised LDL by macrophages [45][46]. Given their responsiveness to targeted nutritional interventions, markers of LDL oxidation have been employed in a rapidly growing number of clinical studies for more than two decades [47].
The evaluation of LDL oxidation in vivo is difficult, and most of the investigations deal with in vitro oxidised LDL, a process accompanied by characteristic changes of physicochemical and biological properties [38][48]. The most common method for the determination of antioxidant properties of natural phenolic compounds is the LDL oxidation assay. LDL is isolated from human plasma, and oxidation is induced by Cu2+ ions and is monitored spectrophotometrically via the change of CD absorption at 232 nm [41][45]. Subsequently, the initiators break down the existing lipid hydroperoxides and initiate the propagation stage according to the following reactions:
(a) Cu+ + LOOH → Cu2+ +OH− + LO·
(b) Cu+ + HOOH → Cu2+ +OH− + HO
The chronology of LDL oxidation by copper ions can be divided into three consecutive time phases: lag phase, propagation phase and decomposition phase. Secondary reactions of LDL oxidation leading to aldehydes (malondialdehyde, hexanal, 4-hydroxynonenal, etc.), are accelerated by transition metal ions, such as Fe2+, which may catalyse the decomposition of lipid hydroperoxides to alkoxyl radicals in a Fenton-type reaction [49].
3. Effect of Certain Natural Antioxidants against LDL Damage/Atherosclerosis
A body of research evidence has accumulated in the last 10–15 years demonstrating how bioactive food components can protect LDL from oxidation [50].
3.1. Effect of Carotenoids
Earlier studies in this research field have focused on the effect of individual synthetic carotenoids, relating the good packaging of supplemented via a diet β-carotene into the lipoprotein particles, with in vivo antioxidant activities [21][51]. Kiokias and Gordon [43] were among the first researchers to explore the effects of natural carotenoid extracts against ex vivo LDL oxidation. They set up a clinical trial with 30 healthy volunteers, who were supplemented for 3 weeks with a carotenoid mixture (palm oil carotenes, lycopene, paprika, lutein, bixin in a total amount of 30 mg active carotenoid/day) and reported an increased resistance of LDL to oxidation, compared with placebo (monitored by CD at 233 nm). In a recent study, Cocate et al. [39] reported that carotenoid consumption can strongly inhibit LDL oxidative damage in healthy middle-aged men. By conducting a cross-sectional study the authors concluded that the total daily carotenoid intake (β-cryptoxanthin, lycopene, lutein plus zeaxanthin, β-carotene and α-carotene) was inversely associated (p < 0.05) with the plasma oxidised-LDL concentrations. Similarly, Gammone et al. [52] reported a clear effect of marine-origin carotenoids against the LDL oxidative stress.
Choi et al. [53] concluded that supplementation with astaxanthin (a polar carotenoid classified to xanthophylls) has shown positive effects, by improving the LDL oxidative stress biomarkers in a placebo-controlled study performed on overweight and obese adults. In addition, astaxanthin exerted beneficial effects to the heart, both by reducing inflammation associated with atherosclerosis and by modifying blood levels of LDL-cholesterol and high-density lipoprotein (HDL)-cholesterol [54]. Ciccone et al. [55] noted that, despite some contradictions, there are many clinical and epidemiological data supporting the anti-inflammatory action and protective effect of carotenoids against cardiovascular events, with findings being more favourable for the natural carotenoids than for the synthetic ones.
Furthermore, a number of clinical trials concluded that a short term dietary supplementation of healthy volunteers with lycopene-rich products (providing ~30–50 mg lycopene/day) increases the resistance of LDL to oxidative deterioration [56][57]. On the contrary, other researchers have supported that relatively high doses of carotenoid supplements (e.g., 60–150 mg of active carotenoids/day) may result in excessively enriched LDL particles with carotenoid metabolites, thereby leading to even an increased susceptibility of LDL to oxidation rather than to any protective effect [21][45].
Overall, by analysing the existing evidence, it can be hypothesized that relatively low levels of carotenoid enrichment by dietary supplementation may be more effective at inhibiting oxidation of LDL ex vivo than larger in vitro enrichments that fails to produce any beneficial effect.
3.2. Effect of Vitamin C
Although earlier studies in smoking subjects, did not report any significant effect of dietary supplementation with vitamin C on LDL oxidation, more recent research results have shown a protective activity of ascorbic acid against LDL oxidation [58][59]. Hillstrom et al. [60] demonstrated that vitamin C inhibits lipid oxidation in HDL and preserves the antioxidant activity associated with this lipoprotein fraction. Shariat et al. [61] evaluated the in vitro antioxidant effects of various vitamins on LDL oxidation and concluded that vitamin C (50–200 mM) is able to inhibit LDL oxidation mediated by myeloperoxidase with a concentration dependent effect.
3.3. Effect of Vitamin E
Jacobson et al. [62] supplemented hyperlipedemic rabbits with 500 mg α-tocopherol/kg for 24 weeks, reporting an increased resistance to LDL oxidation (lag time of LDL oxidation in the treated group almost 2 times higher than in the placebo). Parameswari et al. [63] reported a beneficial effect of vitamin E, on the copper ion-induced oxidation of LDL, isolated from the serum of chronic renal failure and renal transplanted patients. Ghaffari and Ghiasvand [48] studied the effect of different concentrations of α-tocopherol on in vitro cupric ions induced oxidation of LDL. Their results revealed that α-tocopherol (0–100 μmol/L) may decrease free radicals in LDL and, thus, the rate of LDL oxidation by cupric ions. However other researchers did not observe any beneficial effects of tocopherols supplementation. Car et al. 2018 [64] supported that α-tocopherol can even act as pro-oxidant to facilitate lipid peroxidation in LDL, an adverse effect that can be prevented when ascorbate is acting as a coantioxidant. Dotan et al. 2009 [65] has further challenged the beneficial effects of tocopherols by claiming that indiscriminate, high doses of vitamin E supplementation results in increased mortality and should not be recommended to the general public.
Niki [66] supported that vitamin E and other antioxidants inhibit LDL oxidation efficiently in vitro; however, human clinical trials with vitamin E have not yielded positive results. An explanation for that could be that LDL oxidation proceeds by multiple pathways mediated not only by free radicals but also by other non-radical oxidants and vitamin E is effective only against free radical mediated oxidation. Furthermore, Niki (2011) [67] has provided an additional explanation for the non-protective effect of vitamin E against LDL deterioration in human clinical trials claiming that in contrast to animal experiments, vitamin E is given at the latter stage where oxidation is no more important. Free radicals must play a crucial role in the pathogenesis of atherosclerosis and vitamin E should be effective if given at right time to right subjects.
3.4. Effect of Flavonoids, Phenolic Acids and Antioxidant Mixtures
The functional groups of flavonoids attached to the three-ring system has been reported to trigger a positive impact against LDL oxidation [21]. In a study by Naderi et al. [68] the susceptibility of LDL to in vitro oxidation was monitored by the change in 234-absorbance in the presence and absence of several pure flavonoids at different concentrations. According to the results, flavonoids significantly protected against in vitro LDL oxidation, with genistein, morin and naringin exerting a stronger inhibitory activity than quercetin or apigenin.
Amarowicz and Pegg [41] reported that studies on LDL oxidation (monitored by measurement of the generation of conjugated dienes and trienes) confirmed (i) the antioxidant properties of several extracts obtained from plant materials (e.g., grapes, berries, orange, grapefruit, coffee, tea, chocolate, olives, nuts); and (ii) the in vitro protective effect of phenolic compounds (e.g., luteolinidin, apigenidin, caffeic acid, chlorogenic acid, catechin, quercetin, rutin) against LDL oxidation. Carmeli and Fogelman [69] conducted a study to determine the effect of a natural polyphenolic isoflavone (glabridin) on LDL oxidation, by measuring the formation of TBARS, and observed that after oral administration of a glabridrin-rich extract of licorice-root to healthy subjects for 6 months, their oxidative stress level as well as plasma LDL oxidation reduced by 20%. Lam et al. [70] examined the antioxidant effects of selected phenolic compounds from natural sources. According to their results, 6-gingerol and rhapontin were found to exhibit strong inhibition against in vitro lipid peroxidation in LDL induced by 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH), while barbaloin possessed weaker effects.
Costa-Mugica et al. [71] reported that lyophilized aqueous extracts and phenolic-rich fractions of seaweed (H. Incrassata) significantly inhibited LDL oxidation when evaluated by using heparin-precipitated LDL exposed to Cu2+ ions with AAPH as the free radical generator. The authors claimed that the observed effect could be related to the antioxidant potential of the polar phenolic fractions. In addition, Singh et al. [72] reported that LDL oxidative modification was significantly higher (p > 0.001) in diabetic patients as compared to control subjects. An explanation could be provided by the finding that the plasma antioxidant capacity had been decreased significantly (p > 0.001) in the unregulated diabetic group compared to the control group.
Aviram et al. [73] reported that dietary supplementation of polyphenol-rich pomegranate juice to atherosclerotic mice significantly inhibited the development of atherosclerotic lesions and this may be attributed to the protection of LDL against oxidation. On the contrary, Carru et al. [74] have recently reported that an extract of roasted coffee (rich in various phenolic compounds) acted as pro-oxidant accelerating the in vitro LDL oxidation triggered by copper sulphate.
Chu and Liu [75] developed a model based on peroxyl radical-initiated LDL oxidation, by use of the water-soluble free radical initiator AAPH, to assess the free radical scavenging capacity of antioxidants and extracts of natural products. The authors reported that all the tested concentrations of vitamin C and E and apple extract resulted in partial suppression and delay of LDL oxidation in terms of headspace hexanal, as a major decomposition product measured by a headspace gas chromatograph.
References
- Pinchuk, I.; Shoval, Y.; Lichtenberg, D. Evaluation of antioxidants: Scope, limitations and relevance of assays. Chem. Phys. Lipids 2012, 165, 638–647.
- Muller, F.-L.; Lustgarten, M.-S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503.
- Dimakou, C.; Kiokias, S.; Tsaprouni, I.; Oreopoulou, V. Effect of processing and storage parameters on oxidative deterioration of oil-in-water emulsions. Food Biophys. 2007, 2, 38–45.
- Lien, P.-H.; Hua, H.; Chuong, P.-H. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96.
- Preiser, J.-C. Oxidative stress. J. Parenter. Enter. Nutr. 2015, 36, 147–154.
- Yan, M.; Lo, C.-J.; Edwards, T.-J.; Baran, S.-P. Radicals: Reactive intermediates with translational potential. J. Am. Chem. Soc. 2016, 138, 12692–12714.
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 2012, 46, 382–419.
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199.
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126.
- Kovacic, P.; Somanathan, R. Nanoparticles: Toxicity, radicals, electron transfer, and antioxidants. Methods Mol. Biol. 2013, 1028, 15–35.
- Dominguez, M.-G.; Castelao, E.-J. Role of lipid peroxidation and oxidative stress in the association between thyroid diseases and breast cancer. Crit. Rev. Oncol. Hematol. 2008, 68, 107–114.
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Sheila, E.; Crowe, S.-E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354.
- Kim, Y.-J.; Kim, E.-H.; Hahn, K.-B. Oxidative stress in inflammation-based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012, 27, 1004–1010.
- Van’t Erve, T.-J.; Lih, F.B.; Jelsema, C.; Deterding, L.-J.; Eling, T.-E.; Mason, R.-P.; Kadiiska, M.-B. Reinterpreting the best biomarker of oxidative stress: The 8-iso-prostaglandin F2α/prostaglandin F2α ratio shows complex origins of lipid peroxidation biomarkers in animal models. Free Radic. Biol. Med. 2016, 95, 65–73.
- Barocas, D.-A.; Motel Motley, S.; Cookson, M.-S.; Chang, S.-S.; Penson, D.; Dai, Q.; Milne, G.; Roberts, L.-J.; Morrow, J.; Concepcion, R.-S.; et al. Oxidative stress measured by urine F2-isoprostane level is associated with prostate cancer. J. Urol. 2011, 85, 2102–2107.
- Weber, D.; Milkovic, L.; Bennett, S.-J.; Griffiths, H.-R.; Zarkovic, N.; Grune, T. Measurement of HNE-protein adducts in human plasma and serum by ELISA—Comparison of two primary antibodies. Redox Biol. 2013, 1, 226–233.
- Bennett, L.; Rojas, S.; Seefeldt, T. Role of antioxidants in the prevention of cancer. J. Exp. Clin. Med. 2012, 4, 215–222.
- Kumar, S. The Importance of Antioxidant and their role in Pharmaceutical science. Asian J. Res. Chem. Pharmac. Sci. 2014, 1, 27–44.
- Rizzo, A.-M.; Berselli, P.; Zava, S.; Montorfano, G.; Negroni, M.; Corsetto, P.; Berra, B. Endogenous antioxidants and radical scavengers. Adv. Exp. Med. Biol. 2010, 698, 52–67.
- Takashima, M.; Horie, M.; Shichirini, M.; Hagihara, Y.; Yoshida, Y.; Niki, E. Assessment of antioxidant capacity for scavenging free radicals in vitro: A rational basis and practical application. Free Radic. Biol. Med. 2012, 52, 1242–1252.
- Kiokias, S.; Varzakas, T.; Oreopoulou, V. In vitro activity of vitamins, flavonoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit. Rev. Food Sci. Nutr. 2008, 48, 78–93.
- Mamede, A.-C.; Tavares, S.-D.; Abrantes, A.-M.; Trindade, J.; Maia, J.-M.; Botelho, M.-F. The role of vitamins in cancer: A review. Nutr. Cancer 2011, 63, 479–494.
- Roginsky, V.; Lissi, A.-E. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254.
- Puertollano, M.-A.; Puertollano, E.; de Cienfugos, G.-A.; de Pablo, M.-A. Dietary antioxidants: Immunity and host defense. Curr. Top. Med. Chem. 2011, 11, 1752–1766.
- Traber, G.-M.; Jeffrey, A. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15.
- Kim, S.-K.; Im, G.-J.; An, Y.-S.; Lee, S.-H.; Jung, H.-H.; Park, S.-Y. The effects of the antioxidant α-tocopherol succinate on cisplatin-induced ototoxicity in HEI-OC1 auditory cells. Int. J. Pediatr. Otorhinolaryngol. 2016, 86, 9–14.
- Shahidi, F.; De Camargo, C.-A. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745.
- Bjelakovic, G.; Nikolova, D.; Gluud, L.-L. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. JAMA Clin. Evid. Syn. 2013, 310, 178–179.
- Narra, M.-R.; Rajendar, K.; Rudra, R.; Rao, J.-V.; Begum, G. The role of vitamin C as antioxidant in protection of biochemical and haematological stress induced by chlorpyrifos in freshwater fish Clarias batrachus. Chemosphere 2015, 132, 172–178.
- Kiokias, S.; Proestos, C.; Varzakas, T.-A. Review of the structure, biosynthesis, absorption of carotenoids-analysis and properties of their common natural extract. Curr. Res. Nutr. Food Sci. 2016, 4, 25–37.
- Meléndez-Martínez, A.-M.; Stinco, C.-M.; Brahm, P.-M.; Vicario, I.-M. Analysis of carotenoids and tocopherols in plant matrices and assessment of their in vitro antioxidant capacity. Methods Mol. Biol. 2014, 1153, 77–97.
- Pantavos, A.; Ruite, R.; Feskens, F.-E.; de Keyser, E.-C.; Hofman, A.; Stricker, H.-B.; Franco, O.-H.; Kiefte-de Jong, J.-C. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: The Rotterdam study. Int. J. Cancer 2015, 136, 2178–2186.
- Beker, B.-Y.; Bakir, T.; Sonmezoglu, F.-I.; Apak, R. Antioxidant protective effect of flavonoids on linoleic acid peroxidation induced by copper (II)/ascorbic acid. Chem. Phys. Lipids 2011, 164, 732–739.
- Mattia, C.-D.; Sacchetti, G.; Mastrocola, D.; Pittia, P. Effect of phenolic antioxidants on the dispersion state and chemical stability of olive oil ο/w emulsions. Food Res. Int. 2009, 42, 1163–1170.
- Tsimogiannis, D.; Oreopoulou, V. Defining the role of flavonoid structure on cottonseed oil stabilization: Study of A- and C-ring substitution. J. Am. Oil Chem. Soc. 2007, 84, 129–136.
- Dashti, M.; Kulik, W.; Hoek, F.; Veerman, E.-C.; Peppelenbosch, M.-P.; Rezaee, F. A phospholipidomic analysis of all defined human plasma lipoproteins. Sci. Rep. 2011, 1, 139–145.
- Mohammad, P.-I. Trans fatty acids—A risk factor for cardiovascular disease. Pak. J. Med. Sci. 2014, 30, 194–197.
- Winklhofer-Rooba, B.; Faustmanna, G.; Roob, J.-M. Low-density lipoprotein oxidation biomarkers in human health and disease and effects of bioactive compounds. Free Radic. Biol. Med. 2017, 111, 38–86.
- Cocate, P.-G.; Natali, A.-J.; Alfenas, R.-G.; de Oliveira, A. Carotenoid consumption is related to lower lipid oxidation and DNA damage in middle-aged men. Br. J. Nutr. 2015, 114, 257–264.
- Krauss, R.-M. Lipoprotein subfractions and cardiovascular disease risk. Curr. Opin. Lipidol. 2010, 21, 305–311.
- Amarowicz, R.; Pegg, R.-B. The potential protective effects of phenolic compounds against low-density lipoprotein oxidation. Curr. Pharm. Des. 2017, 23, 2754–2766.
- Parthasaranthy, S.; Raghavamenon, A.; Raghavamenon, S.; Garelnabi, O.-M.; Santanam, N. Oxidized low-density lipoprotein. Methods Mol. Biol. 2010, 610, 403–417.
- Kiokias, S.; Gordon, M. Dietary supplementation with a natural carotenoid mixture decreases oxidative stress. Eur. J. Clin. Nutr. 2003, 57, 1135–1140.
- Stocker, R.; Keaney, J.-F. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478.
- Kiokias, S.; Gordon, M. Properties of carotenoids in vitro and in vivo. Food Rev. Int. 2004, 20, 99–121.
- Kiokias, S. In vitro and in vivo antioxidant properties of natural carotenoid mixtures. Ph.D. Thesis, Faculty of Life Sciences, School of Food Biosciences, The University of Reading, Reading, UK, July 2002.
- Itabe, H. Oxidized low-density lipoprotein as a biomarker of in vivo oxidative stress: From atherosclerosis to periodontitis. J. Clin. Biochem. Nutr. 2012, 51, 1–8.
- Ghaffari, A.-M.; Ghiasvand, T. Kinetic study of low density lipoprotein oxidation by copper. Indian J. Clin. Biochem. 2010, 25, 29–36.
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazoni, E.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F.; et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 4, 242–247.
- Mahfouz, M.-M.; Zhou, O.; Kummerow, F.-A. Effect of curcumin on LDL oxidation in vitro, and lipid peroxidation and antioxidant enzymes in cholesterol fed rabbits. Int. J. Vitam. Nutr. Res. 2011, 81, 378–391.
- Fuhrman, B.; Volkova, N.; Rosenhalt, M.; Aviram, M. Lycopene synergistically inhibits LDL oxidation in combination with vitamin E, rosmarinic acid, carnosic acid, or garlic. Antioxid. Redox Signal. 2000, 2, 491–506.
- Gammone, M.-A.; Riccioni, G.; D’Orazio, N. Marine carotenoids against oxidative stress: Effects on human health. Mar. Drugs 2015, 13, 6226–6246.
- Choi, H.-D.; Youn, Y.-K.; Shin, W.-G. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum. Nutr. 2011, 66, 363–369.
- Li, C.-W.; Hellsten, A.; Jacobsson, L.-S.; Blomqvist, H.-M.; Olsson, A.-G.; Yuan, X. Alpha-tocopherol and astaxanthin decrease macrophage infiltration, apoptosis and vulnerability in atheroma of hyperlipidaemic rabbits. J. Mol. Cell. Cardiol. 2004, 37, 969–978.
- Ciccone, M.-M.; Cortese, M.-M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013, 1, 1–11.
- Bub, A.; Waltz, B.; Abrahamse, Z.; Adam, S.; Wever, J.; Muller, H.-S.; Rechenmmer, G. Moderate intervention with carotenoid rich vegetable products reduces lipid peroxidation in men. Am. Soc. Nutr. Sci. 2000, 135, 2200–2206.
- Upritchard, J.-E.; Sutherland, W.-H.; Mann, J.-I. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care 2000, 23, 733–738.
- Kiokias, S.; Varzakas, T.; Arvanitoyannis, I.; Labropoulos, A. Lipid oxidation. In Advances in Food Biochemistry; Yildiz, F., Ed.; CRC Press: New York, NY, USA, 2009; pp. 384–403.
- Wen, Y. The effect of pharmacological supplementation with Vit-C on LDL oxidation. Br. J. Clin. Pharmacol. 1997, 44, 94–97.
- Hillstrom, R.-J.; Yacapin-Ammons, A.-K.; Lynch, S.-M. Vitamin C inhibits lipid oxidation in human HDL. J. Nutr. 2003, 133, 3047–3051.
- Shariat, S.-Z.; Mostafavi, S.-A.; Khakpour, F. Antioxidant effects of vitamins C and E on the low-density lipoprotein oxidation mediated by myeloperoxidase. Iran. Biomed. J. 2013, 17, 22–28.
- Jacobsson, L.-S.; Yuan, X.-M.; Zieden, B.; Olsson, A.-G. Effects of α-tocopherol and astaxanthin on LDL oxidation and atherosclerosis in WHHL rabbits. Atherosclerosis 2004, 173, 231–237.
- Parameswari, C.-S.; Vijayageetha, B.; Vijayakumar, R.; Parameswari, C.-S.; Vijayageetha, B.; Vijayakumar, R. Effect of supplementation of vitamin E, vitamin C and reduced glutathione on copper ion induced lipoprotein oxidation in renal diseased patients—An in vitro study. Indian J. Clin. Biochem. 2006, 21, 131–140.
- Carr, C.-A.; Zhu, B.-Z.; Frei, B. Potential Antiatherogenic Mechanisms of Ascorbate (Vitamin C) and α-Tocopherol (Vitamin E). Circ. Res. 2018, 87, 349–354.
- Dotan, Y.; Lichtenberg, D.; Pinchuk, I. No evidence supports vitamin E indiscriminate supplementation. Biofactors 2009, 35, 469–473.
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515.
- Niki, E. Do free radicals play causal role in atherosclerosis? Low density lipoprotein oxidation and vitamin E. J. Clin. Biochem. Nutr. 2011, 48, 3–7.
- Naderi, G.-A.; Seddigheh, A.; Sarraf-Zadegan, N. Antioxidant effect of flavonoids on the susceptibility of LDL oxidation. Mol. Cell. Biochem. 2003, 246, 193–196.
- Carmeli, E.; Fogelman, Y. Antioxidant effect of polyphenolic glabridin on LDL oxidation. Toxicol. Ind. Health 2009, 25, 321–324.
- Lam, R.-Y.; Woo, A.-Y.; Leung, P.-S.; Cheng, C.-H. Antioxidant actions of phenolic compounds found in dietary plants on low-density lipoprotein and erythrocytes in vitro. J. Am. Coll. Nutr. 2007, 26, 233–242.
- Costa-Mugica, A.; Elsa Batista, A.; Diadelis Mondejar, G.; Soto-López, Y.; Brito-Navarro, V.; Maria Vázquez, A.; Brömme, D.; Zaldívar-Muñoz, C.; Vidal-Novoa, A.; de Oliveira, A.; et al. Inhibition of LDL-oxidation and antioxidant properties related to polyphenol content of hydrophilic fractions from seaweed Halimeda Incrassata. Braz. J. Pharm. Sci. 2012, 48.
- Singh, N.; Singh, S.-K.; Bhargava, V. Status of LDL oxidation and antioxidant potential of LDL in type II diabetes. Biomed. Res. 2010, 21, 416–418.
- Aviram, M.; Dornfel, M.; Kaplan, M.; Coleman, R.; Gaitini, D.; Nitecki, S.; Hofman, A.; Rosenblat, M.; Volkova, N.; Presser, D.; et al. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: Studies in atherosclerotic mice and in humans. Drugs Exp. Clin. Res. 2002, 28, 49–62.
- Carru, C.; Pasciu, V.; Sotgia, S.; Zinellu, A.; Nicoli, M.-C.; Deiana, L. The oxidative state of LDL is the major determinant of anti/prooxidant effect of coffee on Cu2+ catalysed peroxidation. Open Biochem. J. 2018, 12, 1–8.
- Chu, Y.-F.; Liu, R.-H. Novel low-density lipoprotein (LDL) oxidation model: Antioxidant capacity for the inhibition of LDL oxidation. J. Agric. Food Chem. 2004, 52, 6818–6823.
More
Information
Subjects:
Nutrition & Dietetics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
481
Revisions:
2 times
(View History)
Update Date:
07 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




