
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hiromi Yanagisawa | -- | 2733 | 2023-10-06 05:10:39 | | | |
| 2 | Catherine Yang | -3 word(s) | 2730 | 2023-10-07 05:00:51 | | |
Video Upload Options
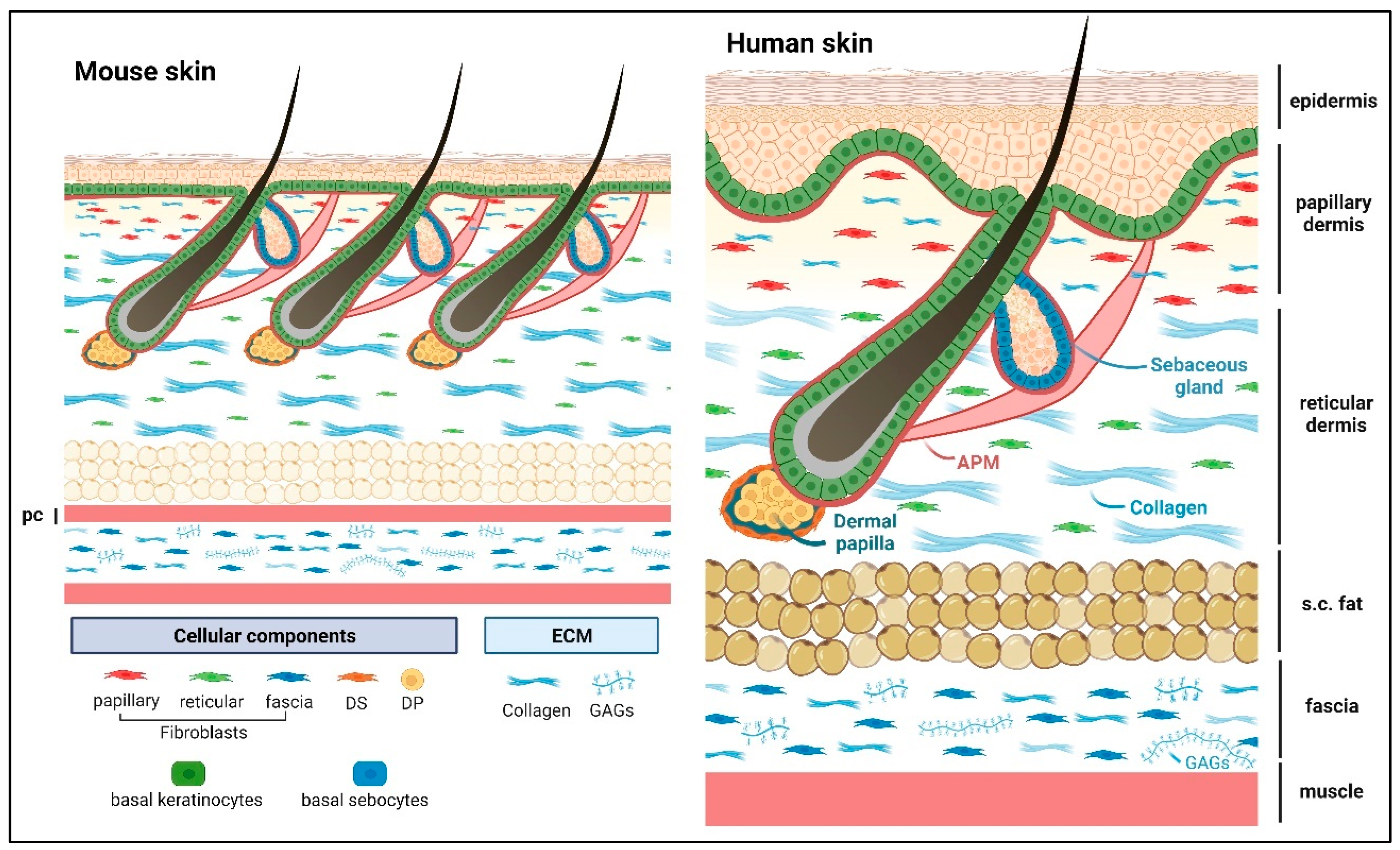
Matricellular proteins are nonstructural, modular, extracellular proteins that exert their effects by binding to cell surface receptors, extracellular matrix (ECM) proteins, soluble signaling molecules, and proteases, thereby modulating cellular responses to changes in their microenvironment, particularly during tissue remodeling. The skin is the largest organ of the body and protects us against environmental insults. It shields the body from mechanical abrasion, pathological infections, dehydration, and fluctuations in body temperature, while the nerves in the skin also provide us with sensations of touch. The skin needs to act as a resilient mechanical barrier, yet provide structural flexibility. The functional unit of skin consists of the stratified epidermis and dermis (including dermal adipose and skin appendages such as hair follicles, sweat, and sebaceous glands) as well as the panniculus carnosus (PC) muscle and the subcutaneous fascia. Notably, the human skin has a thicker epidermis and dermis compared with mouse skin, and the epidermis exhibits undulations forming the rete ridge and inter-ridge (also known as dermal papillae) structures that are absent in mouse skin.
1. Epidermis
2. Dermis
| Skin Compartment |
Matricellular Protein |
Function | References & Study Model |
|---|---|---|---|
| Epidermis | Fibulin 2 | binds to laminin 332 for BM Stability | [4] (mouse) |
| Fibulin 7 | BM localization, binds to collagen IV in vitro | [6] (mouse) | |
| SPARC | binds to collagen IV in vitro and induces expression of collagen IV and VII | [7] (human skin & 3D culture), [8] (molecular structure), [13] (summary of works in mouse) | |
| Hemicentin 1 (Fibulin 6) |
BM stability | [9] (mouse), [10] (mouse & zebrafish) | |
| Thrombospondin 1 | inhibits angiogenesis | [11] (human skin), [14] (summary of works in mouse), [15] (mouse xenograft) | |
| CCN3 | promotes DDR1 binding to collagen IV and inhibits melanocytes proliferation in UV-mediated stress | [24] (human skin reconstructs), [25] (summary of works in human skin & cell culture) | |
| Dermis | Tenascin C | growth factors sequestration, regulates cell proliferation |
[36] (summary), [37] (mouse NR6 cells), [38] (human skin), [39] (mouse), [40][41] (molecular interactions) |
| Fibronectin * | growth factor sequestration, supports cell attachment and migration |
[30] (summary), [31] (mouse) | |
| Fibrillin 1 * | supports elastic fiber formation and homeostasis | [34] (human & bovine tissue) | |
| Fibulin 2 | [35] (mouse), [34] (human & bovine tissue) | ||
| [45] (summary in human & mouse), [46][47] (human genetic mutations), [48] (mouse) | |||
| Fibulin 4 | |||
| Fibulin 5 | |||
| Periostin | modulates collagen structure and stability, regulates keratinocyte proliferation | [43] (human skin), [44] (mouse skin 3D culture) | |
| Thrombospondin 1 | dermal vascularization balance, interacts with collagen I |
[14] (summary), [15] (mouse xenograft), [49] (in vivo mouse & human dermal fibroblasts) | |
| Thrombospondin 2 | collagen structural arrangement and abundance | [50] (mouse) | |
| SPARC | [51] (mouse) | ||
| Dermal adipose tissue | SPARC | wound healing, modulates adipogenesis | [52][53] (mouse) |
| CCDC80/URB/DRO1 | modulates adipogenesis | [54] (mouse tissue and 3T3-L1 cells), [55] (human & mouse tissue), [56] (mouse) | |
| Hair follicle | Fibulin 1 | BM homeostasis | [57] (mouse), [58] [summary] |
| Periostin | tendon-related genes, may provide stability for arrector pili muscle and hair follicle connection | [58][59] (mouse), [60][61] (rat) | |
| Tenascin C | |||
| SPARC | |||
| Sebaceous glands | Fibronectin* | regulates cell differentiation | [62] (mouse) |
| Tenascin C | growth factor sequestration | [63] (human & mouse eyelids) | |
| Panniculus carnosus muscle |
Fibulin 4 | Panniculus carnosus muscle homeostasis | [64] (mouse) |
3. Dermal Adipose Tissue
4. The Hair Follicle
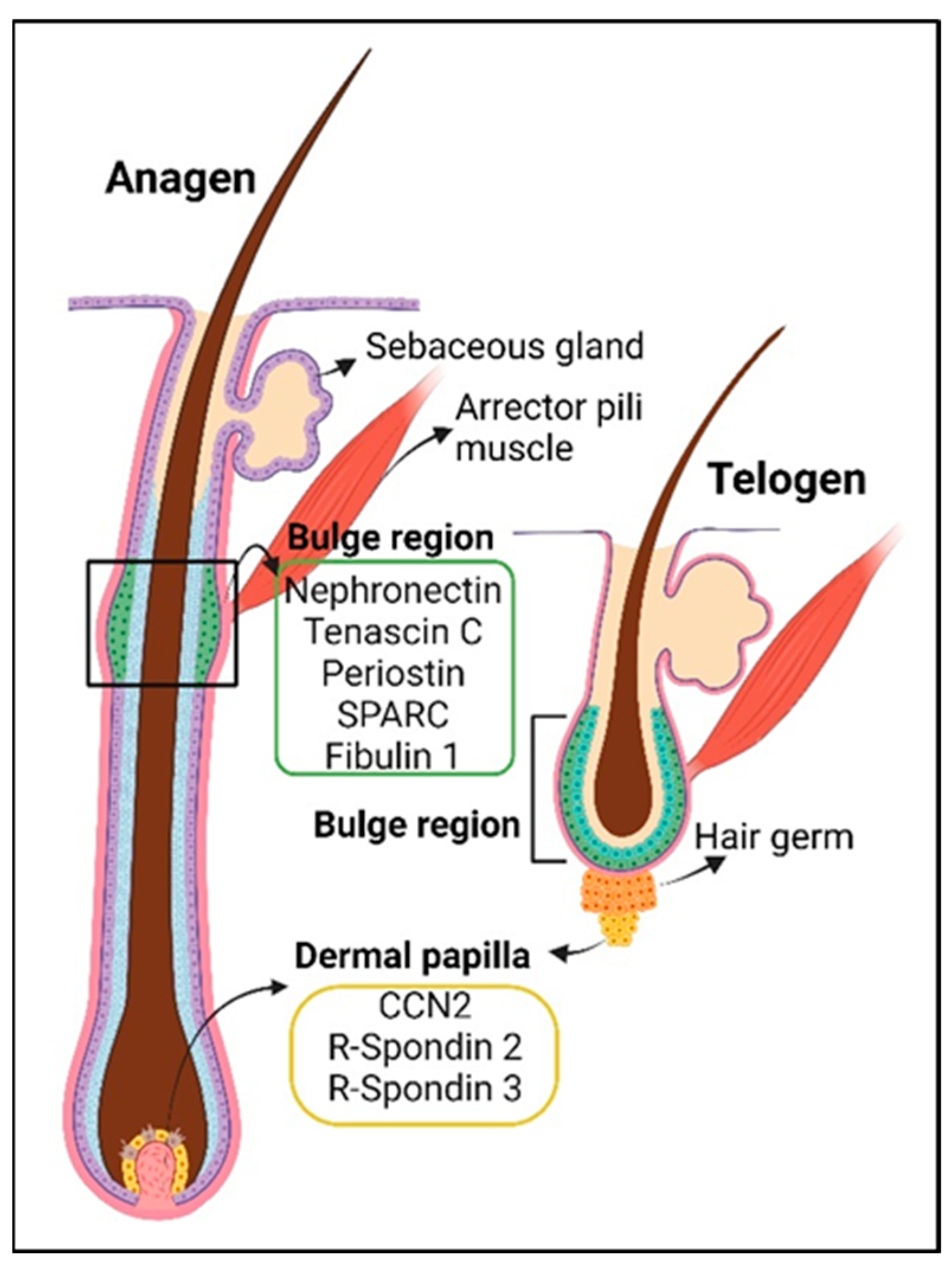
5. The Sebaceous Gland
| Process | Matricellular Protein |
Function | References & Study Model |
|---|---|---|---|
| Hair follicle stem cells homeostasis | Tenascin C | promotes WNT signaling and maintains hair follicle stem cells | [39] (mouse) |
| Periostin | pro-cell proliferation in the hair follicles post-wounding | [93] (mouse) | |
| CCN2 | maintenance of hair follicle stem cell quiescence | [94] (mouse) | |
| R-spondin 2 | activates hair follicle stem cells, cell proliferation of dermal papilla and dermal sheath | [95] (human hair follicles & mouse), [96] (human scalp & mouse skin, [81] (mouse) | |
| R-spondin 3 | cell proliferation of dermal papilla and dermal sheath | [81] (mouse), [96] (human scalp & mouse skin) | |
| Mindin (Spon2) | gene expression is decreased in aging hair follicle stem cells, role unknown in the hair follicle | [97] (mouse) | |
| Thrombospondin 1 | gene expression is increased in aging hair follicle stem cells, hair follicle and vasculature regression in catagen | [97] (mouse skin), [98] (mouse) | |
| Sebaceous gland stem cells | Fibronectin * | maintenance of basal sebocytes | [62] (mouse) |
6. The Panniculus Carnosus Muscle
7. Subcutaneous Fascia
References
- Lippens, S.; Denecker, G.; Ovaere, P.; Vandenabeele, P.; Declercq, W. Death penalty for keratinocytes: Apoptosis versus cornification. Cell Death Differ. 2005, 12 (Suppl. 2), 1497–1508.
- Nystrom, A.; Bruckner-Tuderman, L. Matrix molecules and skin biology. Semin. Cell Dev. Biol. 2019, 89, 136–146.
- Fleischmajer, R.; Timpl, R. Ultrastructural localization of fibronectin to different anatomic structures of human skin. J. Histochem. Cytochem. 1984, 32, 315–321.
- Longmate, W.M.; Monichan, R.; Chu, M.L.; Tsuda, T.; Mahoney, M.G.; DiPersio, C.M. Reduced fibulin-2 contributes to loss of basement membrane integrity and skin blistering in mice lacking integrin alpha3beta1 in the epidermis. J. Investig. Dermatol. 2014, 134, 1609–1617.
- Utani, A.; Nomizu, M.; Yamada, Y. Fibulin-2 binds to the short arms of laminin-5 and laminin-1 via conserved amino acid sequences. J. Biol. Chem. 1997, 272, 2814–2820.
- Raja, E.; Changarathil, G.; Oinam, L.; Tsunezumi, J.; Ngo, Y.X.; Ishii, R.; Sasaki, T.; Imanaka-Yoshida, K.; Yanagisawa, H.; Sada, A. The extracellular matrix fibulin 7 maintains epidermal stem cell heterogeneity during skin aging. EMBO Rep. 2022, 23, e55478.
- Nakamura, T.; Yoshida, H.; Ota, Y.; Endo, Y.; Sayo, T.; Hanai, U.; Imagawa, K.; Sasaki, M.; Takahashi, Y. SPARC promotes production of type IV and VII collagen and their skin basement membrane accumulation. J. Dermatol. Sci. 2022, 107, 109–112.
- Sasaki, T.; Hohenester, E.; Gohring, W.; Timpl, R. Crystal structure and mapping by site-directed mutagenesis of the collagen-binding epitope of an activated form of BM-40/SPARC/osteonectin. EMBO J. 1998, 17, 1625–1634.
- Welcker, D.; Stein, C.; Feitosa, N.M.; Armistead, J.; Zhang, J.L.; Lutke, S.; Kleinridders, A.; Bruning, J.C.; Eming, S.A.; Sengle, G.; et al. Hemicentin-1 is an essential extracellular matrix component of the dermal-epidermal and myotendinous junctions. Sci. Rep. 2021, 11, 17926.
- Zhang, J.L.; Richetti, S.; Ramezani, T.; Welcker, D.; Lutke, S.; Pogoda, H.M.; Hatzold, J.; Zaucke, F.; Keene, D.R.; Bloch, W.; et al. Vertebrate extracellular matrix protein hemicentin-1 interacts physically and genetically with basement membrane protein nidogen-2. Matrix Biol. 2022, 112, 132–154.
- Wight, T.N.; Raugi, G.J.; Mumby, S.M.; Bornstein, P. Light microscopic immunolocation of thrombospondin in human tissues. J. Histochem. Cytochem. 1985, 33, 295–302.
- Bozo, R.; Szel, E.; Danis, J.; Guban, B.; Bata-Csorgo, Z.; Szabo, K.; Kemeny, L.; Groma, G. Cartilage Oligomeric Matrix Protein Negatively Influences Keratinocyte Proliferation via alpha5beta1-Integrin: Potential Relevance of Altered Cartilage Oligomeric Matrix Protein Expression in Psoriasis. J. Investig. Dermatol. 2020, 140, 1733–1742.e7.
- Bradshaw, A.D. The role of SPARC in extracellular matrix assembly. J. Cell Commun. Signal. 2009, 3, 239–246.
- Detmar, M. The role of VEGF and thrombospondins in skin angiogenesis. J. Dermatol. Sci. 2000, 24 (Suppl. 1), S78–S84.
- Streit, M.; Velasco, P.; Brown, L.F.; Skobe, M.; Richard, L.; Riccardi, L.; Lawler, J.; Detmar, M. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am. J. Pathol. 1999, 155, 441–452.
- Ghetti, M.; Topouzi, H.; Theocharidis, G.; Papa, V.; Williams, G.; Bondioli, E.; Cenacchi, G.; Connelly, J.T.; Higgins, C.A. Subpopulations of dermal skin fibroblasts secrete distinct extracellular matrix: Implications for using skin substitutes in the clinic. Br. J. Dermatol. 2018, 179, 381–393.
- Hunzelmann, N.; Hafner, M.; Anders, S.; Krieg, T.; Nischt, R. BM-40 (osteonectin, SPARC) is expressed both in the epidermal and in the dermal compartment of adult human skin. J. Investig. Dermatol. 1998, 110, 122–126.
- Wrana, J.L.; Overall, C.M.; Sodek, J. Regulation of the expression of a secreted acidic protein rich in cysteine (SPARC) in human fibroblasts by transforming growth factor beta. Comparison of transcriptional and post-transcriptional control with fibronectin and type I collagen. Eur. J. Biochem. 1991, 197, 519–528.
- Ford, R.; Wang, G.; Jannati, P.; Adler, D.; Racanelli, P.; Higgins, P.J.; Staiano-Coico, L. Modulation of SPARC expression during butyrate-induced terminal differentiation of cultured human keratinocytes: Regulation via a TGF-beta-dependent pathway. Exp. Cell Res. 1993, 206, 261–275.
- Agarwal, P.; Zwolanek, D.; Keene, D.R.; Schulz, J.N.; Blumbach, K.; Heinegard, D.; Zaucke, F.; Paulsson, M.; Krieg, T.; Koch, M.; et al. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J. Biol. Chem. 2012, 287, 22549–22559.
- Siriwach, R.; Ngo, A.Q.; Higuchi, M.; Arima, K.; Sakamoto, S.; Watanabe, A.; Narumiya, S.; Thumkeo, D. Single-cell RNA sequencing identifies a migratory keratinocyte subpopulation expressing THBS1 in epidermal wound healing. iScience 2022, 25, 104130.
- Hsu, Y.C.; Fuchs, E. Building and Maintaining the Skin. Cold Spring Harb. Perspect. Biol. 2022, 14, a040840.
- Domingues, L.; Hurbain, I.; Gilles-Marsens, F.; Sires-Campos, J.; Andre, N.; Dewulf, M.; Romao, M.; Viaris de Lesegno, C.; Mace, A.S.; Blouin, C.; et al. Coupling of melanocyte signaling and mechanics by caveolae is required for human skin pigmentation. Nat. Commun. 2020, 11, 2988.
- Fukunaga-Kalabis, M.; Martinez, G.; Liu, Z.J.; Kalabis, J.; Mrass, P.; Weninger, W.; Firth, S.M.; Planque, N.; Perbal, B.; Herlyn, M. CCN3 controls 3D spatial localization of melanocytes in the human skin through DDR1. J. Cell Biol. 2006, 175, 563–569.
- Fukunaga-Kalabis, M.; Santiago-Walker, A.; Herlyn, M. Matricellular proteins produced by melanocytes and melanomas: In search for functions. Cancer Microenviron. 2008, 1, 93–102.
- Rognoni, E.; Watt, F.M. Skin Cell Heterogeneity in Development, Wound Healing, and Cancer. Trends Cell Biol. 2018, 28, 709–722.
- Sabatelli, P.; Gara, S.K.; Grumati, P.; Urciuolo, A.; Gualandi, F.; Curci, R.; Squarzoni, S.; Zamparelli, A.; Martoni, E.; Merlini, L.; et al. Expression of the collagen VI alpha5 and alpha6 chains in normal human skin and in skin of patients with collagen VI-related myopathies. J. Investig. Dermatol. 2011, 131, 99–107.
- Watt, F.M.; Fujiwara, H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 2011, 3, a005124.
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 682414.
- Huang, J.; Heng, S.; Zhang, W.; Liu, Y.; Xia, T.; Ji, C.; Zhang, L.J. Dermal extracellular matrix molecules in skin development, homeostasis, wound regeneration and diseases. Semin. Cell Dev. Biol. 2022, 128, 137–144.
- Martino, M.M.; Tortelli, F.; Mochizuki, M.; Traub, S.; Ben-David, D.; Kuhn, G.A.; Muller, R.; Livne, E.; Eming, S.A.; Hubbell, J.A. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl. Med. 2011, 3, 100ra189.
- Cescon, M.; Gattazzo, F.; Chen, P.; Bonaldo, P. Collagen VI at a glance. J. Cell Sci. 2015, 128, 3525–3531.
- Lettmann, S.; Bloch, W.; Maass, T.; Niehoff, A.; Schulz, J.N.; Eckes, B.; Eming, S.A.; Bonaldo, P.; Paulsson, M.; Wagener, R. Col6a1 null mice as a model to study skin phenotypes in patients with collagen VI related myopathies: Expression of classical and novel collagen VI variants during wound healing. PLoS ONE 2014, 9, e105686.
- Reinhardt, D.P.; Sasaki, T.; Dzamba, B.J.; Keene, D.R.; Chu, M.L.; Gohring, W.; Timpl, R.; Sakai, L.Y. Fibrillin-1 and fibulin-2 interact and are colocalized in some tissues. J. Biol. Chem. 1996, 271, 19489–19496.
- Lemaire, R.; Korn, J.H.; Schiemann, W.P.; Lafyatis, R. Fibulin-2 and fibulin-5 alterations in tsk mice associated with disorganized hypodermal elastic fibers and skin tethering. J. Investig. Dermatol. 2004, 123, 1063–1069.
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327.
- Swindle, C.S.; Tran, K.T.; Johnson, T.D.; Banerjee, P.; Mayes, A.M.; Griffith, L.; Wells, A. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J. Cell Biol. 2001, 154, 459–468.
- Schalkwijk, J.; Steijlen, P.M.; van Vlijmen-Willems, I.M.; Oosterling, B.; Mackie, E.J.; Verstraeten, A.A. Tenascin expression in human dermis is related to epidermal proliferation. Am. J. Pathol. 1991, 139, 1143–1150.
- Hendaoui, I.; Tucker, R.P.; Zingg, D.; Bichet, S.; Schittny, J.; Chiquet-Ehrismann, R. Tenascin-C is required for normal Wnt/beta-catenin signaling in the whisker follicle stem cell niche. Matrix Biol. 2014, 40, 46–53.
- De Laporte, L.; Rice, J.J.; Tortelli, F.; Hubbell, J.A. Tenascin C promiscuously binds growth factors via its fifth fibronectin type III-like domain. PLoS ONE 2013, 8, e62076.
- Martino, M.M.; Briquez, P.S.; Guc, E.; Tortelli, F.; Kilarski, W.W.; Metzger, S.; Rice, J.J.; Kuhn, G.A.; Muller, R.; Swartz, M.A.; et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 2014, 343, 885–888.
- Kuwatsuka, Y.; Murota, H. Involvement of Periostin in Skin Function and the Pathogenesis of Skin Diseases. Adv. Exp. Med. Biol. 2019, 1132, 89–98.
- Egbert, M.; Ruetze, M.; Sattler, M.; Wenck, H.; Gallinat, S.; Lucius, R.; Weise, J.M. The matricellular protein periostin contributes to proper collagen function and is downregulated during skin aging. J. Dermatol. Sci. 2014, 73, 40–48.
- Taniguchi, K.; Arima, K.; Masuoka, M.; Ohta, S.; Shiraishi, H.; Ontsuka, K.; Suzuki, S.; Inamitsu, M.; Yamamoto, K.I.; Simmons, O.; et al. Periostin controls keratinocyte proliferation and differentiation by interacting with the paracrine IL-1alpha/IL-6 loop. J. Investig. Dermatol. 2014, 134, 1295–1304.
- Zhang, X.; Alanazi, Y.F.; Jowitt, T.A.; Roseman, A.M.; Baldock, C. Elastic Fibre Proteins in Elastogenesis and Wound Healing. Int. J. Mol. Sci. 2022, 23, 4087.
- Hucthagowder, V.; Sausgruber, N.; Kim, K.H.; Angle, B.; Marmorstein, L.Y.; Urban, Z. Fibulin-4: A novel gene for an autosomal recessive cutis laxa syndrome. Am. J. Hum. Genet. 2006, 78, 1075–1080.
- Markova, D.; Zou, Y.Q.; Ringpfeil, F.; Sasaki, T.; Kostka, G.; Timpl, R.; Uitto, J.; Chu, M.L. Genetic heterogeneity of cutis laxa: A heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am. J. Hum. Genet. 2003, 72, 998–1004.
- Yanagisawa, H.; Davis, E.C.; Starcher, B.C.; Ouchi, T.; Yanagisawa, M.; Richardson, J.A.; Olson, E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 2002, 415, 168–171.
- Rosini, S.; Pugh, N.; Bonna, A.M.; Hulmes, D.J.S.; Farndale, R.W.; Adams, J.C. Thrombospondin-1 promotes matrix homeostasis by interacting with collagen and lysyl oxidase precursors and collagen cross-linking sites. Sci. Signal. 2018, 11, eaar2566.
- Kyriakides, T.R.; Zhu, Y.H.; Smith, L.T.; Bain, S.D.; Yang, Z.; Lin, M.T.; Danielson, K.G.; Iozzo, R.V.; LaMarca, M.; McKinney, C.E.; et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J. Cell Biol. 1998, 140, 419–430.
- Bradshaw, A.D.; Puolakkainen, P.; Dasgupta, J.; Davidson, J.M.; Wight, T.N.; Helene Sage, E. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J. Investig. Dermatol. 2003, 120, 949–955.
- Bradshaw, A.D.; Graves, D.C.; Motamed, K.; Sage, E.H. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc. Natl. Acad. Sci. USA 2003, 100, 6045–6050.
- Bradshaw, A.D.; Reed, M.J.; Sage, E.H. SPARC-null mice exhibit accelerated cutaneous wound closure. J. Histochem. Cytochem. 2002, 50, 1–10.
- Tremblay, F.; Revett, T.; Huard, C.; Zhang, Y.; Tobin, J.F.; Martinez, R.V.; Gimeno, R.E. Bidirectional modulation of adipogenesis by the secreted protein Ccdc80/DRO1/URB. J. Biol. Chem. 2009, 284, 8136–8147.
- Okada, T.; Nishizawa, H.; Kurata, A.; Tamba, S.; Sonoda, M.; Yasui, A.; Kuroda, Y.; Hibuse, T.; Maeda, N.; Kihara, S.; et al. URB is abundantly expressed in adipose tissue and dysregulated in obesity. Biochem. Biophys. Res. Commun. 2008, 367, 370–376.
- Grill, J.I.; Neumann, J.; Herbst, A.; Ofner, A.; Hiltwein, F.; Marschall, M.K.; Zierahn, H.; Wolf, E.; Schneider, M.R.; Kolligs, F.T. Loss of DRO1/CCDC80 results in obesity and promotes adipocyte differentiation. Mol. Cell. Endocrinol. 2017, 439, 286–296.
- Zhang, H.Y.; Timpl, R.; Sasaki, T.; Chu, M.L.; Ekblom, P. Fibulin-1 and fibulin-2 expression during organogenesis in the developing mouse embryo. Dev. Dyn. 1996, 205, 348–364.
- Fujiwara, H.; Ferreira, M.; Donati, G.; Marciano, D.K.; Linton, J.M.; Sato, Y.; Hartner, A.; Sekiguchi, K.; Reichardt, L.F.; Watt, F.M. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 2011, 144, 577–589.
- Wang, T.; Wagner, A.; Gehwolf, R.; Yan, W.; Passini, F.S.; Thien, C.; Weissenbacher, N.; Lin, Z.; Lehner, C.; Teng, H.; et al. Load-induced regulation of tendon homeostasis by SPARC, a genetic predisposition factor for tendon and ligament injuries. Sci. Transl. Med. 2021, 13, eabe5738.
- Wang, Y.; Jin, S.; Luo, D.; He, D.; Shi, C.; Zhu, L.; Guan, B.; Li, Z.; Zhang, T.; Zhou, Y.; et al. Functional regeneration and repair of tendons using biomimetic scaffolds loaded with recombinant periostin. Nat. Commun. 2021, 12, 1293.
- Jarvinen, T.A.; Jozsa, L.; Kannus, P.; Jarvinen, T.L.; Hurme, T.; Kvist, M.; Pelto-Huikko, M.; Kalimo, H.; Jarvinen, M. Mechanical loading regulates the expression of tenascin-C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J. Cell Sci. 2003, 116, 857–866.
- Sipila, K.; Rognoni, E.; Jokinen, J.; Tewary, M.; Vietri Rudan, M.; Talvi, S.; Jokinen, V.; Dahlstrom, K.M.; Liakath-Ali, K.; Mobasseri, A.; et al. Embigin is a fibronectin receptor that affects sebaceous gland differentiation and metabolism. Dev. Cell 2022, 57, 1453–1465.e7.
- Chen, D.; Chen, X.; Xie, H.T.; Hatton, M.P.; Liu, X.; Liu, Y. Expression of extracellular matrix components in the meibomian gland. Front. Med. 2022, 9, 981610.
- Igoucheva, O.; Alexeev, V.; Halabi, C.M.; Adams, S.M.; Stoilov, I.; Sasaki, T.; Arita, M.; Donahue, A.; Mecham, R.P.; Birk, D.E.; et al. Fibulin-4 E57K Knock-in Mice Recapitulate Cutaneous, Vascular and Skeletal Defects of Recessive Cutis Laxa 1B with both Elastic Fiber and Collagen Fibril Abnormalities. J. Biol. Chem. 2015, 290, 21443–21459.
- Nakamura, T.; Lozano, P.R.; Ikeda, Y.; Iwanaga, Y.; Hinek, A.; Minamisawa, S.; Cheng, C.F.; Kobuke, K.; Dalton, N.; Takada, Y.; et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 2002, 415, 171–175.
- Rognoni, E.; Pisco, A.O.; Hiratsuka, T.; Sipila, K.H.; Belmonte, J.M.; Mobasseri, S.A.; Philippeos, C.; Dilao, R.; Watt, F.M. Fibroblast state switching orchestrates dermal maturation and wound healing. Mol. Syst. Biol. 2018, 14, e8174.
- Lynch, M.D.; Watt, F.M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35.
- Ganier, C.; Rognoni, E.; Goss, G.; Lynch, M.; Watt, F.M. Fibroblast Heterogeneity in Healthy and Wounded Skin. Cold Spring Harb. Perspect. Biol. 2022, 14, a041238.
- Chen, S.X.; Zhang, L.J.; Gallo, R.L. Dermal White Adipose Tissue: A Newly Recognized Layer of Skin Innate Defense. J. Investig. Dermatol. 2019, 139, 1002–1009.
- Zhang, L.J.; Guerrero-Juarez, C.F.; Hata, T.; Bapat, S.P.; Ramos, R.; Plikus, M.V.; Gallo, R.L. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 2015, 347, 67–71.
- Sillat, T.; Saat, R.; Pollanen, R.; Hukkanen, M.; Takagi, M.; Konttinen, Y.T. Basement membrane collagen type IV expression by human mesenchymal stem cells during adipogenic differentiation. J. Cell. Mol. Med. 2012, 16, 1485–1495.
- Mariman, E.C.; Wang, P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 2010, 67, 1277–1292.
- Reggio, S.; Rouault, C.; Poitou, C.; Bichet, J.C.; Prifti, E.; Bouillot, J.L.; Rizkalla, S.; Lacasa, D.; Tordjman, J.; Clement, K. Increased Basement Membrane Components in Adipose Tissue during Obesity: Links with TGFbeta and Metabolic Phenotypes. J. Clin. Endocrinol. Metab. 2016, 101, 2578–2587.
- Mori, S.; Kiuchi, S.; Ouchi, A.; Hase, T.; Murase, T. Characteristic expression of extracellular matrix in subcutaneous adipose tissue development and adipogenesis; comparison with visceral adipose tissue. Int. J. Biol. Sci. 2014, 10, 825–833.
- McCulloch, L.J.; Rawling, T.J.; Sjoholm, K.; Franck, N.; Dankel, S.N.; Price, E.J.; Knight, B.; Liversedge, N.H.; Mellgren, G.; Nystrom, F.; et al. COL6A3 is regulated by leptin in human adipose tissue and reduced in obesity. Endocrinology 2015, 156, 134–146.
- Bolton, K.; Segal, D.; McMillan, J.; Jowett, J.; Heilbronn, L.; Abberton, K.; Zimmet, P.; Chisholm, D.; Collier, G.; Walder, K. Decorin is a secreted protein associated with obesity and type 2 diabetes. Int. J. Obes. 2008, 32, 1113–1121.
- Han, C.Y.; Kang, I.; Harten, I.A.; Gebe, J.A.; Chan, C.K.; Omer, M.; Alonge, K.M.; den Hartigh, L.J.; Gomes Kjerulf, D.; Goodspeed, L.; et al. Adipocyte-Derived Versican and Macrophage-Derived Biglycan Control Adipose Tissue Inflammation in Obesity. Cell Rep. 2020, 31, 107818.
- Daquinag, A.C.; Gao, Z.; Fussell, C.; Sun, K.; Kolonin, M.G. Glycosaminoglycan Modification of Decorin Depends on MMP14 Activity and Regulates Collagen Assembly. Cells 2020, 9, 2646.
- Joost, S.; Annusver, K.; Jacob, T.; Sun, X.; Dalessandri, T.; Sivan, U.; Sequeira, I.; Sandberg, R.; Kasper, M. The Molecular Anatomy of Mouse Skin during Hair Growth and Rest. Cell Stem Cell 2020, 26, 441–457.e7.
- Blanpain, C.; Lowry, W.E.; Geoghegan, A.; Polak, L.; Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004, 118, 635–648.
- Rahmani, W.; Abbasi, S.; Hagner, A.; Raharjo, E.; Kumar, R.; Hotta, A.; Magness, S.; Metzger, D.; Biernaskie, J. Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla, and modulate hair type. Dev. Cell 2014, 31, 543–558.
- Clavel, C.; Grisanti, L.; Zemla, R.; Rezza, A.; Barros, R.; Sennett, R.; Mazloom, A.R.; Chung, C.Y.; Cai, X.; Cai, C.L.; et al. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev. Cell 2012, 23, 981–994.
- Ng, K.J.; Lim, J.; Tan, Y.N.; Quek, D.; Lim, Z.; Pantelireis, N.; Clavel, C. Sox2 in the dermal papilla regulates hair follicle pigmentation. Cell Rep. 2022, 40, 111100.
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; Dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169.
- Harshuk-Shabso, S.; Dressler, H.; Niehrs, C.; Aamar, E.; Enshell-Seijffers, D. Fgf and Wnt signaling interaction in the mesenchymal niche regulates the murine hair cycle clock. Nat. Commun. 2020, 11, 5114.
- Tsutsui, K.; Machida, H.; Nakagawa, A.; Ahn, K.; Morita, R.; Sekiguchi, K.; Miner, J.H.; Fujiwara, H. Mapping the molecular and structural specialization of the skin basement membrane for inter-tissue interactions. Nat. Commun. 2021, 12, 2577.
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.S.; Sawicki, J.A.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004, 22, 411–417.
- Timpl, R.; Brown, J.C. The laminins. Matrix Biol. 1994, 14, 275–281.
- Niemann, C.; Horsley, V. Development and homeostasis of the sebaceous gland. Semin. Cell Dev. Biol. 2012, 23, 928–936.
- Geueke, A.; Niemann, C. Stem and progenitor cells in sebaceous gland development, homeostasis and pathologies. Exp. Dermatol. 2021, 30, 588–597.
- Kobayashi, T.; Voisin, B.; Kim, D.Y.; Kennedy, E.A.; Jo, J.H.; Shih, H.Y.; Truong, A.; Doebel, T.; Sakamoto, K.; Cui, C.Y.; et al. Homeostatic Control of Sebaceous Glands by Innate Lymphoid Cells Regulates Commensal Bacteria Equilibrium. Cell 2019, 176, 982–997.e16.
- Thiele, J.J.; Weber, S.U.; Packer, L. Sebaceous gland secretion is a major physiologic route of vitamin E delivery to skin. J. Investig. Dermatol. 1999, 113, 1006–1010.
- Nishiyama, T.; Kii, I.; Kashima, T.G.; Kikuchi, Y.; Ohazama, A.; Shimazaki, M.; Fukayama, M.; Kudo, A. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS ONE 2011, 6, e18410.
- Liu, S.; Leask, A. CCN2 modulates hair follicle cycling in mice. Mol. Biol. Cell 2013, 24, 3939–3944.
- Smith, A.A.; Li, J.; Liu, B.; Hunter, D.; Pyles, M.; Gillette, M.; Dhamdhere, G.R.; Abo, A.; Oro, A.; Helms, J.A. Activating Hair Follicle Stem Cells via R-spondin2 to Stimulate Hair Growth. J. Investig. Dermatol. 2016, 136, 1549–1558.
- Hagner, A.; Shin, W.; Sinha, S.; Alpaugh, W.; Workentine, M.; Abbasi, S.; Rahmani, W.; Agabalyan, N.; Sharma, N.; Sparks, H.; et al. Transcriptional Profiling of the Adult Hair Follicle Mesenchyme Reveals R-spondin as a Novel Regulator of Dermal Progenitor Function. iScience 2020, 23, 101019.
- Ge, Y.; Miao, Y.; Gur-Cohen, S.; Gomez, N.; Yang, H.; Nikolova, M.; Polak, L.; Hu, Y.; Verma, A.; Elemento, O.; et al. The aging skin microenvironment dictates stem cell behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 5339–5350.
- Yano, K.; Brown, L.F.; Lawler, J.; Miyakawa, T.; Detmar, M. Thrombospondin-1 plays a critical role in the induction of hair follicle involution and vascular regression during the catagen phase. J. Investig. Dermatol. 2003, 120, 14–19.
- Naldaiz-Gastesi, N.; Bahri, O.A.; Lopez de Munain, A.; McCullagh, K.J.A.; Izeta, A. The panniculus carnosus muscle: An evolutionary enigma at the intersection of distinct research fields. J. Anat. 2018, 233, 275–288.
- Naldaiz-Gastesi, N.; Goicoechea, M.; Alonso-Martin, S.; Aiastui, A.; Lopez-Mayorga, M.; Garcia-Belda, P.; Lacalle, J.; San Jose, C.; Arauzo-Bravo, M.J.; Trouilh, L.; et al. Identification and Characterization of the Dermal Panniculus Carnosus Muscle Stem Cells. Stem Cell Rep. 2016, 7, 411–424.
- Machado, M.J.; Watson, M.G.; Devlin, A.H.; Chaplain, M.A.; McDougall, S.R.; Mitchell, C.A. Dynamics of angiogenesis during wound healing: A coupled in vivo and in silico study. Microcirculation 2011, 18, 183–197.
- Pratt, R.L. Hyaluronan and the Fascial Frontier. Int. J. Mol. Sci. 2021, 22, 6845.
- Fede, C.; Pirri, C.; Fan, C.; Petrelli, L.; Guidolin, D.; De Caro, R.; Stecco, C. A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae. Int. J. Mol. Sci. 2021, 22, 1411.
- Stecco, C.; Fede, C.; Macchi, V.; Porzionato, A.; Petrelli, L.; Biz, C.; Stern, R.; De Caro, R. The fasciacytes: A new cell devoted to fascial gliding regulation. Clin. Anat. 2018, 31, 667–676.
- McLaughlin, P.J.; Bakall, B.; Choi, J.; Liu, Z.; Sasaki, T.; Davis, E.C.; Marmorstein, A.D.; Marmorstein, L.Y. Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum. Mol. Genet. 2007, 16, 3059–3070.
- Driver, S.G.W.; Jackson, M.R.; Richter, K.; Tomlinson, P.; Brockway, B.; Halliday, B.J.; Markie, D.M.; Robertson, S.P.; Wade, E.M. Biallelic variants in EFEMP1 in a man with a pronounced connective tissue phenotype. Eur. J. Hum. Genet. 2020, 28, 445–452.
- Correa-Gallegos, D.; Jiang, D.; Christ, S.; Ramesh, P.; Ye, H.; Wannemacher, J.; Kalgudde Gopal, S.; Yu, Q.; Aichler, M.; Walch, A.; et al. Patch repair of deep wounds by mobilized fascia. Nature 2019, 576, 287–292.
- Jiang, D.; Christ, S.; Correa-Gallegos, D.; Ramesh, P.; Kalgudde Gopal, S.; Wannemacher, J.; Mayr, C.H.; Lupperger, V.; Yu, Q.; Ye, H.; et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 2020, 11, 5653.




