1. Epidermis
The construction of the epidermis begins with the basal epidermal stem cells that undergo a continuous and balanced process of symmetric self-renewal and asymmetric division to produce progenitors and differentiated cells. These provide a constant supply of keratinocytes committed to terminal differentiation which form the suprabasal layers and the cornified skin barrier [
61]. Through hemidesmosomes and integrin receptors, the basal epidermal stem cells are anchored to the basement membrane (BM), which connects the epidermis to the underlying dermis. The BM’s primary constituents are structural scaffolding matrix proteins such as collagens IV, VII, XVII, laminin 332, and laminin 511 [
62]. Fibronectin is also present in the lamina lucida of the BM (the laminin-rich area) [
63]. Matricellular proteins, such as fibulin 2 [
17,
64], fibulin 7 [
18], SPARC [
19,
20], hemicentin 1 (fibulin 6) [
22,
23], THBS1 [
24], and thrombospondin 5 (THBS5 or COMP) [
65], are contained in the epidermal BM via interactions with structural proteins or the integrin receptors.
Functionally, fibulin 2 binds to laminin 332 to stabilize the epidermal BM in neonatal skin. The absence of fibulin 2 results in separation of the dermal–epidermal junction (DEJ) and skin blisters [
17]. Fibulin 7 is also expressed in the epidermal BM and has been shown to bind to collagen IV in vitro [
18], although its role in the BM arrangement remains unknown. SPARC was similarly shown to bind to collagen IV in vitro and induce collagen IV and VII expression in the epidermal BM in reconstructed human three-dimensional skin culture models [
19,
20,
21]. Hemicentin 1 co-localizes with laminin α2 at the epidermal BM [
22].
Hmcn1 null mice exhibit unevenly widened lamina lucida and lamina densa and compromised hemidesmosomes [
22], which suggests its function in BM organization. Hemicentin 1 was further shown to compete with laminins for its binding site to the nidogen 2 proteoglycan during BM formation and maintenance [
23]. In contrast to other matricellular proteins in the BM, THBS1 is believed to act as an endogenous angiogenesis inhibitor, forming a barrier between the nonvascular epidermis and the vascularized dermis [
25,
26]. Matricellular proteins at the BM/DEJ, such as fibulin 2 and SPARC, are produced by both keratinocytes and fibroblasts [
17,
66,
67,
68,
69], with hemicentin 1 and COMP mainly being secreted by fibroblasts [
22,
70]. In contrast, a subset of basal keratinocytes expresses
Thbs1, albeit at low abundance during homeostasis [
71]
In addition to keratinocytes, the epidermis is home to resident immune cells and melanocytes, which provide pigmentation to the skin and hair [
16]. Melanocytes are regenerated by melanocyte stem cells, which are neural-crest-derived cells. Melanocytes produce melanin and have long dendrites that can make physical connections with up to 40 keratinocytes. Melanin is transferred to keratinocytes via caveolae-dependent internalization (lipid-raft-mediated endocytosis) to protect their nuclei from ultraviolet (UV) radiation [
72]. Melanocyte survival also depends on its attachment to the epidermal BM via binding between its discoidin domain receptor 1 (DDR1) and collagen IV [
27]. During stress or UV radiation, keratinocytes secrete paracrine factors such as IL-1β that, in turn, induce melanocytes to secrete the matricellular protein CCN3. CCN3 promotes DDR1 binding to collagen IV and inhibits melanocyte proliferation [
27,
28].
2. Dermis
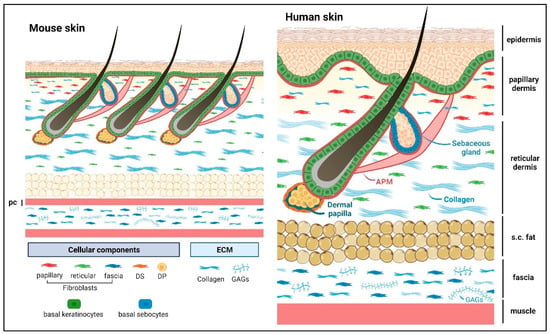
Underneath the epidermal BM are the papillary and reticular dermal compartments, which are distinct in their cellularization and ECM components (
Figure 1) [
73]. The upper dermis/papillary dermis is densely populated with papillary fibroblasts that reside within thin and loose networks of fibrillar collagens (I and III) and elastic fibers. They produce ECMs such as collagen VI [
74], fibronectin, and the matricellular proteins tenascin C and fibulin 2 [
66]. These ECM proteins interact with each other to maintain structural integrity and provide biological cues for the surrounding cells. Fibronectin is a glycoprotein required for the formation of microfibrils. It acts as a scaffold for the elastic fibers, modulates the balance of skin rigidity and elasticity, and supports cell attachment and migration along the ECM matrix, such as collagen fibers [
75,
76]. Fibronectin also possesses growth-factor-binding ability [
35,
36]. Collagen VI fibrils interact with both fibronectin and collagen I [
77] and may promote the tensile strength of the skin [
78]. While fibulin 2 binds to fibrillin 1 and regulates the homeostasis of elastic fibers [
37,
38], further maintenance of the dermal ECM may come from the binding of tenascin C to fibronectin, collagen, and periostin [
29]. Tenascin C has been reported to control cell proliferation by acting as a constitutive ligand to activate the cell surface epidermal growth factor receptor [
30,
31] and by sequestration of soluble growth factors such as Wnt3a, TGFβ, and VEGF [
29,
32,
33,
34]. Similarly, periostin is expressed in the papillary dermis, interacts with fibronectin, collagen I, and tenascin C [
79] and is reported to modulate collagen structure and stability [
43]. Periostin also impacts keratinocyte proliferation via fibroblast paracrine secretion of IL-6 [
44].
Figure 1. Schematic presentation of mouse and human dorsal skin. Abbreviations: DP, dermal papilla; DS, dermal sheath; pc, panniculus carnosus muscle; s.c. fat, subcutaneous fat; APM, arrector pili muscle; GAGs, glycosaminoglycans. The sweat gland is not shown in the image. Matricellular protein constituents and their roles are summarized in
Table 1. Created with
BioRender.com (accessed on 13 February 2023).
Table 1. Matricellular proteins in different compartments of the skin.
Skin
Compartment |
Matricellular
Protein |
Function |
References & Study Model |
| Epidermis |
Fibulin 2 |
binds to laminin 332 for BM Stability |
[17] (mouse) |
| Fibulin 7 |
BM localization, binds to collagen IV in vitro |
[18] (mouse) |
| SPARC |
binds to collagen IV in vitro and induces expression of collagen IV and VII |
[19] (human skin & 3D culture), [20] (molecular structure), [21] (summary of works in mouse) |
Hemicentin 1
(Fibulin 6) |
BM stability |
[22] (mouse), [23] (mouse & zebrafish) |
| Thrombospondin 1 |
inhibits angiogenesis |
[24] (human skin), [25] (summary of works in mouse), [26] (mouse xenograft) |
| CCN3 |
promotes DDR1 binding to collagen IV and inhibits melanocytes proliferation in UV-mediated stress |
[27] (human skin reconstructs), [28] (summary of works in human skin & cell culture) |
| Dermis |
Tenascin C |
growth factors sequestration,
regulates cell proliferation |
[29] (summary), [30] (mouse NR6 cells), [31] (human skin), [32] (mouse), [33,34] (molecular interactions) |
| Fibronectin * |
growth factor sequestration,
supports cell attachment and migration |
[35] (summary), [36] (mouse) |
| Fibrillin 1 * |
supports elastic fiber formation and homeostasis |
[37] (human & bovine tissue) |
| Fibulin 2 |
[38] (mouse), [37] (human & bovine tissue) |
| [39] (summary in human & mouse), [40,41] (human genetic mutations), [42] (mouse) |
| Fibulin 4 |
| Fibulin 5 |
| Periostin |
modulates collagen structure and stability, regulates keratinocyte proliferation |
[43] (human skin), [44] (mouse skin 3D culture) |
| Thrombospondin 1 |
dermal vascularization balance,
interacts with collagen I |
[25] (summary), [26] (mouse xenograft), [45] (in vivo mouse & human dermal fibroblasts) |
| Thrombospondin 2 |
collagen structural arrangement and abundance |
[46] (mouse) |
| SPARC |
[47] (mouse) |
| Dermal adipose tissue |
SPARC |
wound healing, modulates adipogenesis |
[48,49] (mouse) |
| CCDC80/URB/DRO1 |
modulates adipogenesis |
[50] (mouse tissue and 3T3-L1 cells), [51] (human & mouse tissue), [52] (mouse) |
| Hair follicle |
Fibulin 1 |
BM homeostasis |
[53] (mouse), [54] [summary] |
| Periostin |
tendon-related genes, may provide stability for arrector pili muscle and hair follicle connection |
[54,55] (mouse), [56,57] (rat) |
| Tenascin C |
| SPARC |
| Sebaceous glands |
Fibronectin* |
regulates cell differentiation |
[58] (mouse) |
| Tenascin C |
growth factor sequestration |
[59] (human & mouse eyelids) |
Panniculus
carnosus muscle |
Fibulin 4 |
Panniculus carnosus muscle homeostasis |
[60] (mouse) |
The softer papillary dermis serves as a cushion connecting the stiffer epidermal BM to the lower reticular dermis, a major part of the dermal compartment. Reticular dermis contains more sparsely distributed reticular fibroblasts, thick and highly organized collagen bundles that contribute to skin tensile strength [
62,
73]. Reticular fibroblasts produce ECMs such as collagen I, fibrillin 1, fibronectin, emilin 1, and the matricellular proteins THBS1, tenascin C, and fibulin 2 [
66]. Fibrillin 1, elastin, emilin 1, fibronectin, and matricellular proteins such as fibulin 2, 4, and 5 are expressed in the dermis and crucial for the formation of elastic fibers, which provide the skin with elastic properties [
39,
40,
41,
42,
80]. Aside from regulating the balance of dermal vascularization, THBS1 is physically associated with the collagen I KGHR motif, which is important for cross-linking. The loss of this interaction results in abnormal collagen I and human dermal fibroblast differentiation into myofibroblasts [
45]. Similarly,
Thbs2 knockout mice have disarranged collagen fibril sizes and patterns in the dermis, which correlate with reduced skin tensile strength [
46]. Abnormal collagen fibrils have also been observed in SPARC null mice, which exhibit decreases in the amount of dermal collagen I, the fibril diameter, and the tensile strength [
47].
Fibroblasts in the embryonic/neonatal mouse skin are proliferative. However, in the mature skin, fibroblasts cease to divide and produce more ECM in the dermis. This ECM (collagen) enrichment in the adult skin acts as negative feedback to further inhibit fibroblast proliferation, although this quiescent state is reversible and fibroblasts are activated again upon skin injury [
81]. Resident dermal immune cells are also activated to produce various cytokines that modulate fibroblast differentiation and proliferation [
81,
82]. Dermal fibroblasts originate from a common multipotent mesenchymal cell progenitor (expressing Pdgfrα, Dlk1, and Lrig1) that give rise to papillary and reticular fibroblast progenitors [
82].
3. Dermal Adipose Tissue
Reticular fibroblast progenitors can give rise to adipocyte precursors and mature, lipid-filled adipocytes [
82,
83], which constitute the dermal fat layer. Dermal adipose functions as energy storage, thermal insulation, and mechanical support. Furthermore, it harbors innate immune antimicrobial functions [
84,
85]. Mature adipocytes express the collagen IV BM protein, which is deposited pericellularly and is believed to act as a strong scaffold to protect cells from mechanical stress in this loose connective tissue [
86,
87]. Collagen IV expression around these adipocytes is increased in obese compared with lean human subcutaneous adipose tissue [
88], possibly to counterbalance the physical changes associated with an increase in adipocyte cell size. Other ECMs associated with skin adipose are collagen I, III, V [
89], and VI [
90]. Proteoglycans such as versican, biglycan, and decorin are also expressed in adipose tissue in order to bind to collagens, support their scaffolding function, and counteract compressive forces. However, in cases of obesity, these proteoglycans are present in excessive amounts, resulting in ECM defects and the promotion of tissue inflammation [
91,
92,
93]. Adipogenesis is influenced by the matricellular protein SPARC, as its loss of expression leads to increased dermal adipose tissue [
48] and accelerated wound healing [
49]. Similarly, the secreted protein coiled-coil domain containing 80 (CCDC80/URB/DRO1) is highly expressed by adipocytes to modulate adipogenesis, and its genetic deletion in mice promotes body fat deposition, including subcutaneous fat [
50,
51,
52].
4. The Hair Follicle
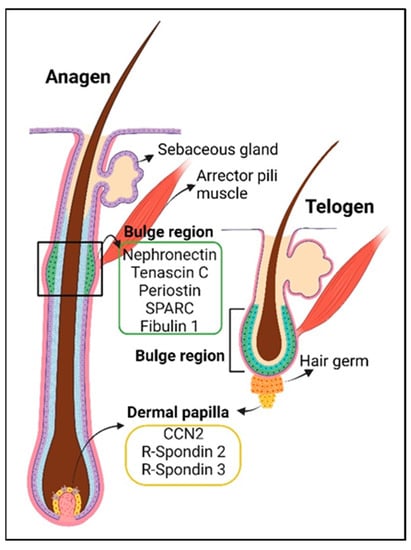
The hair follicle structure spans from the hypodermis (dermal adipose) up to the epidermis. Its size changes according to the hair cycle, which consists of the telogen (resting), anagen (growing), and catagen (regression) phases [
94]. Hair follicles are connected to the sebaceous glands, arrector pili muscles, and dermal papilla at the base of the follicle (
Figure 1 and
Figure 2). During homeostasis, the hair follicle is mainly regenerated from hair follicle stem cells in the bulge region, with coordination from signals originating from mesenchymal cells in the dermal papilla, which control the exit from telogen and the duration of anagen [
95,
96,
97,
98,
99,
100] (
Figure 2). The dermal papilla cells are, in turn, replenished by a subset of dermal sheath cells, which have self-renewal ability, also referred to as hair follicle dermal stem cells [
96]. Hair follicle BM proteins are expressed by both epithelial cells and fibroblasts, with distinct contributions [
101]. They consist of core structural BM proteins such as laminins, collagens IV, VI, VII, XVII, and XVIII, netrins 1 and 4, fibronectin, nephronectin, and matricellular proteins such as periostin, tenascin C, fibulin 1, SPARC, SMOC1, spondin-1, and R-spondin 3 [
75,
101,
102]. The function of fibulin 1 is likely related to the regulation of BM homeostasis through its binding to laminin and collagen IV [
53,
103]. Nephronectin is produced by hair follicle stem cells to form an adhesion point for the arrector pili muscle cells expressing α8β1 integrins. Arrector pili muscles and the sympathetic nervous system cooperate with hair follicles to achieve piloerections to keep warm air closer to the skin in response to cold temperatures or emotions [
54]. It has been proposed that hair follicle stem cells expressing periostin, tenascin C, and SPARC resemble tendon-related gene functions, i.e., they are expressed in the tendonous part of muscle tissue, which strengthens the bone–muscle connection and allows for skeletal movement [
55,
56,
57]. Similarly, their deposition in the hair bulge matrix may stabilize the connection between arrector pili muscles and hair follicles during piloerections [
54].
Figure 2. The hair follicle in anagen (growth phase, left) and telogen (resting phase, right) and the associated matricellular proteins in the hair follicle stem cell niche (bulge region, green box) and dermal papilla niche (yellow box). Adapted from
BioRender.com (accessed on 2 August 2023).
5. The Sebaceous Gland
Sebaceous glands are located in the upper and permanent area of the hair follicle (junctional zone) in hair-associated skin. However, they may also be present in the skin independently of hair follicles such as in the meibomian glands of the eyelid [
104,
105]. The peripheral (basal) epidermal cells of these glands are proliferative and contain sebaceous gland stem cells. The differentiated sebocytes reside in the inner layer of the gland. Through cell death and lysis, lipids/sebum are released onto the skin surface to promote barrier functions, water repulsion, and antimicrobial- and antioxidant activities (vitamin E) [
105,
106,
107]. To our knowledge, documentation regarding the ECM of the sebaceous gland is limited. A recent report indicated expression and interaction of fibronectin with the sebaceous gland basal cells to regulate its differentiation [
58]. Another report discussed the ECM in the meibomian glands such as collagen IV, laminin α2, and β1, which may serve as BM scaffolding proteins, and the matricellular protein tenascin C, which may modulate growth factor bioavailability around sebaceous gland stem cells [
59].
Table 2. Matricellular proteins for maintenance of hair follicles and sebaceous gland stem cells.
6. The Panniculus Carnosus Muscle
The PC muscle is a layer of skeletal muscle located directly under the dermal adipose tissue in some mammals to facilitate skin movement independently of deeper muscle mass. The PC muscle has been suggested to mediate skin twitching during irritation, facial skin movement for social expressions, shivering thermogenesis, and skin contraction to promote wound closure [
114]. In humans, PC muscle remnants exist only in certain anatomical regions, such as the craniofacial muscle, the platysma muscle (ventral region of the neck), and the palmaris brevis in the hand [
114]. The PC muscle is reported to be highly regenerative [
115] and vascularized [
116]. The fibulin 4 matricellular protein may regulate PC muscle homeostasis, as the
Fbln4 E57K homozygous mutation (the equivalent of the human mutation causing cutis laxa) in mice exhibits a thinner dermis and PC muscle layer, possibly due to elastic fiber defects and abnormal collagen fibrils [
60]. However, it is unknown whether this mutation compromises PC muscle function.
7. Subcutaneous Fascia
The subcutaneous fascia ECM is enriched in an aqueous matrix containing glycosaminoglycans such as hyaluronan to facilitate smooth gliding between the skin and muscle. Fibroblasts and fasciacytes contribute to ECM production in the fascia [
117]. Fibroblasts secrete fibrous matrix components such as fibrillin, elastin, fibronectin, and collagens I and III, whereas hyaluronan is produced by fasciacytes [
118]. Fasciacytes are round, fibroblast-like cells expressing vimentin [
119]. Little is known about the role of matricellular proteins in subcutaneous fascia maintenance, although the loss of fibulin 3 compromises elastic fibers in the visceral fascia [
120,
121]. Interestingly, lineage tracing experiments have demonstrated that, during wound healing, fascia fibroblasts are mainly responsible for generating scar tissue and not dermal fibroblasts [
122,
123].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241814274