
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Petra Petranović Ovčariček | -- | 2562 | 2023-10-05 16:09:32 | | | |
| 2 | Peter Tang | Meta information modification | 2562 | 2023-10-06 03:18:15 | | |
Video Upload Options
Differentiated thyroid cancer (DTC) is the most common subtype of thyroid cancer and has an excellent overall prognosis. However, metastatic DTC in certain cases may have a poor prognosis as it becomes radioiodine-refractory. Molecular imaging is essential for disease evaluation and further management. The most commonly used tracers are [18F]FDG and isotopes of radioiodine. Several other radiopharmaceuticals may be used as well, with different diagnostic performances.
1. Introduction
2. Radioiodine-Refractory Differentiated Thyroid Cancer: Definition and Criteria
|
Main Criteria |
|
No 131I uptake in malignant metastatic tissue outside the thyroid bed on the first post-therapeutic WBS |
|
131I uptake is lost after previous evidence of 131I avid disease |
|
131I is concentrated in some but not in other lesions |
|
Disease progresses despite 131I avidity |
|
Patients have already received 22 GBq or more of 131I |
|
Other Potential Criteria |
|
No 131I uptake in malignant tissue on diagnostic WBS |
|
Significant uptake on [18F]FDG PET/CT |
|
Aggressive tumor histology |
3. Radiopharmaceuticals Used for Radioiodine-Refractory Differentiated Thyroid Cancer Imaging and Therapy
3.1. [18F]FDG
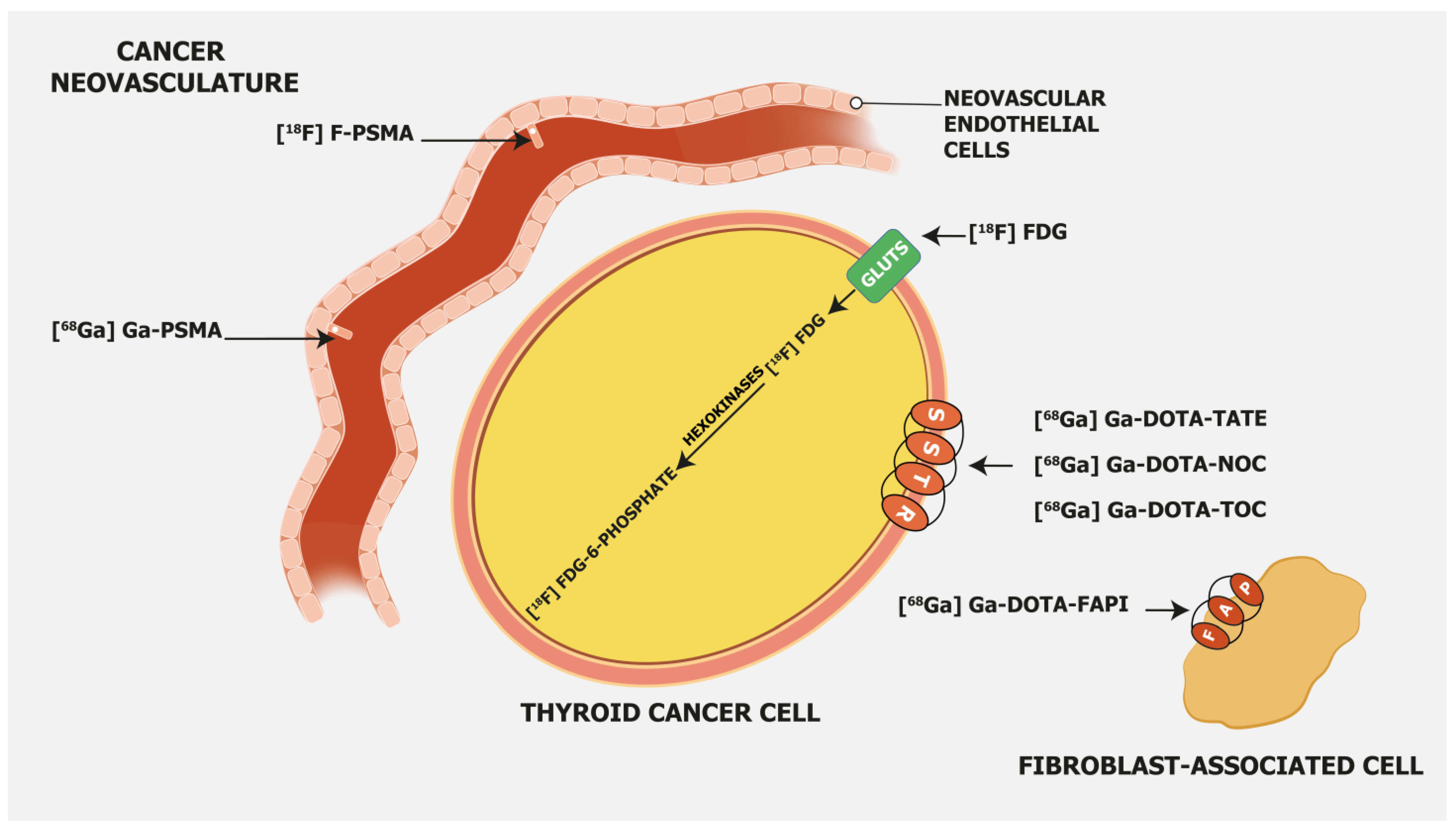
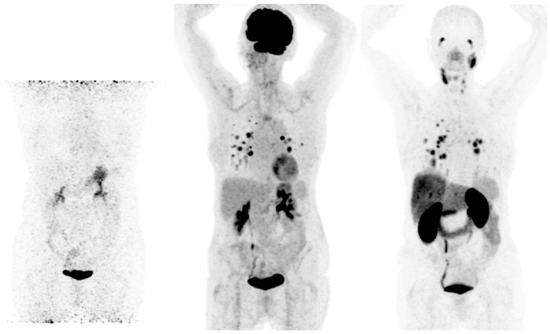
3.2. PSMA-Targeting Radiopharmaceuticals
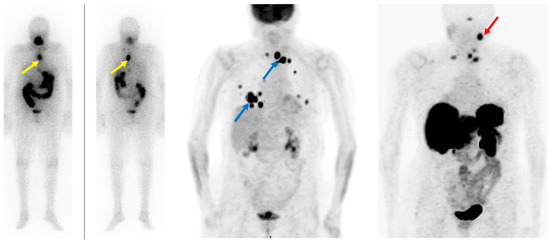
3.3. Somatostatin Receptor-Targeting Radiopharmaceuticals
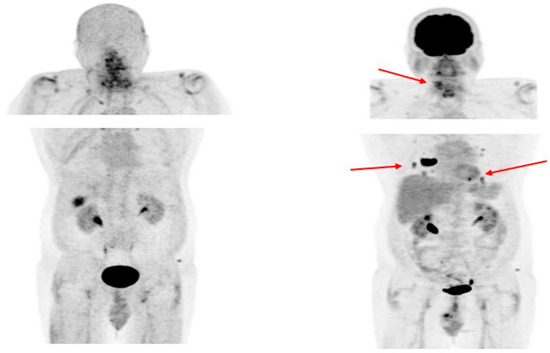
3.4. Fibroblast Activation Protein—Targeting Radiopharmaceuticals
References
- Giovanella, L.; Deandreis, D.; Vrachimis, A.; Campenni, A.; Ovcaricek, P.P. Molecular Imaging and Theragnostics of Thyroid Cancers. Cancers 2022, 14, 1272.
- Schlumberger, M.; Brose, M.; Elisei, R.; Leboulleux, S.; Luster, M.; Pitoia, F.; Pacini, F. Definition and Management of Radioactive Iodine-Refractory Differentiated Thyroid Cancer. Lancet Diabetes Endocrinol. 2014, 2, 356–358.
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133.
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690.
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017, 317, 1338–1348.
- Vaisman, F.; Carvalho, D.P.; Vaisman, M. A New Appraisal of Iodine Refractory Thyroid Cancer. Endocr. Relat. Cancer 2015, 22, R301–R310.
- Van Nostrand, D. Radioiodine Refractory Differentiated Thyroid Cancer: Time to Update the Classifications. Thyroid 2018, 28, 1083–1093.
- Ylli, D.; Van Nostrand, D.; Wartofsky, L. Conventional Radioiodine Therapy for Differentiated Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 181–197.
- Kim, H.; Kim, H.I.; Kim, S.W.; Jung, J.; Jeon, M.J.; Kim, W.G.; Kim, T.Y.; Kim, H.K.; Kang, H.C.; Han, J.M.; et al. Prognosis of Differentiated Thyroid Carcinoma with Initial Distant Metastasis: A Multicenter Study in Korea. Endocrinol. Metab. 2018, 33, 287–295.
- Van Nostrand, D. 131I Treatment of Distant Metastases. In Thyroid Cancer: A Comprehensive Guide to Clinical Management; Springer: New York, NY, USA, 2016; pp. 595–627.
- Giovanella, L.; Van Nostrand, D. Advanced Differentiated Thyroid Cancer: When to Stop Radioiodine? Q. J. Nucl. Med. Mol. Imaging 2019, 63, 267–270.
- Sacks, W.; Braunstein, G.D. Evolving Approaches in Managing Radioactive Iodine-Refractory Differentiated Thyroid Cancer. Endocr. Pract. 2014, 20, 263–275.
- Luo, Y.; Jiang, H.; Xu, W.; Wang, X.; Ma, B.; Liao, T.; Wang, Y. Clinical, Pathological, and Molecular Characteristics Correlating to the Occurrence of Radioiodine Refractory Differentiated Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 549882.
- Hänscheid, H.; Lassmann, M.; Buck, A.K.; Reiners, C.; Verburg, F.A. The Limit of Detection in Scintigraphic Imaging with I-131 in Patients with Differentiated Thyroid Carcinoma. Phys. Med. Biol. 2014, 59, 2353–2368.
- Lee, J.W.; Lee, S.M.; Koh, G.P.; Lee, D.H. The Comparison of (131)I Whole-Body Scans on the Third and Tenth Day after (131)I Therapy in Patients with Well-Differentiated Thyroid Cancer: Preliminary Report. Ann. Nucl. Med. 2011, 25, 439–446.
- Avram, A.M.; Giovanella, L.; Greenspan, B.; Lawson, S.A.; Luster, M.; Van Nostrand, D.; Peacock, J.G.; Ovčariček, P.P.; Silberstein, E.; Tulchinsky, M.; et al. SNMMI Procedure Standard/EANM Practice Guideline for Nuclear Medicine Evaluation and Therapy of Differentiated Thyroid Cancer: Abbreviated Version. J. Nucl. Med. 2022, 63, 15N–35N.
- Sakulpisuti, C.; Charoenphun, P.; Chamroonrat, W. Positron Emission Tomography Radiopharmaceuticals in Differentiated Thyroid Cancer. Molecules 2022, 27, 4936.
- Heydarzadeh, S.; Moshtaghie, A.A.; Daneshpoor, M.; Hedayati, M. Regulators of Glucose Uptake in Thyroid Cancer Cell Lines. Cell Commun. Signal. 2020, 18, 83.
- Leboulleux, S.; Schroeder, P.R.; Busaidy, N.L.; Auperin, A.; Corone, C.; Jacene, H.A.; Ewertz, M.E.; Bournaud, C.; Wahl, R.L.; Sherman, S.I.; et al. Assessment of the Incremental Value of Recombinant Thyrotropin Stimulation before 2--Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography/Computed Tomography Imaging to Localize Residual Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2009, 94, 1310–1316.
- Israeli, R.S.; Powell, C.T.; Fair, W.R.; Heston, W.D.W. Molecular Cloning of a Complementary DNA Encoding a Prostate-Specific Membrane Antigen. Cancer Res. 1993, 53, 227–230.
- Ciappuccini, R.; Saguet-Rysanek, V.; Giffard, F.; Licaj, I.; Dorbeau, M.; Clarisse, B.; Poulain, L.; Bardet, S. PSMA Expression in Differentiated Thyroid Cancer: Association With Radioiodine, 18FDG Uptake, and Patient Outcome. J. Clin. Endocrinol. Metab. 2021, 106, 3536–3545.
- Heitkötter, B.; Steinestel, K.; Trautmann, M.; Grünewald, I.; Barth, P.; Gevensleben, H.; Bögemann, M.; Wardelmann, E.; Hartmann, W.; Rahbar, K.; et al. Neovascular PSMA Expression Is a Common Feature in Malignant Neoplasms of the Thyroid. Oncotarget 2018, 9, 9867–9874.
- Sollini, M.; di Tommaso, L.; Kirienko, M.; Piombo, C.; Erreni, M.; Lania, A.G.; Erba, P.A.; Antunovic, L.; Chiti, A. PSMA Expression Level Predicts Differentiated Thyroid Cancer Aggressiveness and Patient Outcome. EJNMMI Res. 2019, 9, 93.
- Sanchez-Crespo, A. Comparison of Gallium-68 and Fluorine-18 Imaging Characteristics in Positron Emission Tomography. Appl. Radiat. Isot. 2013, 76, 55–62.
- Martiniova, L.; De Palatis, L.; Etchebehere, E.; Ravizzini, G. Gallium-68 in Medical Imaging. Curr. Radiopharm. 2016, 9, 187–207.
- Piron, S.; Verhoeven, J.; Vanhove, C.; De Vos, F. Recent Advancements in 18F-Labeled PSMA Targeting PET Radiopharmaceuticals. Nucl. Med. Biol. 2022, 106–107, 29–51.
- Seifert, R.; Telli, T.; Opitz, M.; Barbato, F.; Berliner, C.; Nader, M.; Umutlu, L.; Stuschke, M.; Hadaschik, B.; Herrmann, K.; et al. Unspecific 18F-PSMA-1007 Bone Uptake Evaluated Through PSMA-11 PET, Bone Scanning, and MRI Triple Validation in Patients with Biochemical Recurrence of Prostate Cancer. J. Nucl. Med. 2023, 64, 738–743.
- Grünig, H.; Maurer, A.; Thali, Y.; Kovacs, Z.; Strobel, K.; Burger, I.A.; Müller, J. Focal Unspecific Bone Uptake on -PSMA-1007 PET: A Multicenter Retrospective Evaluation of the Distribution, Frequency, and Quantitative Parameters of a Potential Pitfall in Prostate Cancer Imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4483–4494.
- Arnfield, E.G.; Thomas, P.A.; Roberts, M.J.; Pelecanos, A.M.; Ramsay, S.C.; Lin, C.Y.; Latter, M.J.; Garcia, P.L.; Pattison, D.A. Clinical Insignificance of PSMA-1007 Avid Non-Specific Bone Lesions: A Retrospective Evaluation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4495–4507.
- Duncan, I.; Ingold, N.; Martinez-Marroquin, E.; Paterson, C. An Australian Experience Using Tc-PSMA SPECT/CT in the Primary Diagnosis of Prostate Cancer and for Staging at Biochemical Recurrence after Local Therapy. Prostate 2023, 83, 970–979.
- Johnbeck, C.B.; Knigge, U.; Kjær, A. PET Tracers for Somatostatin Receptor Imaging of Neuroendocrine Tumors: Current Status and Review of the Literature. Future Oncol. 2014, 10, 2259–2277.
- Pazaitou-Panayiotou, K.; Janson, E.T.; Koletsa, T.; Kotoula, V.; Stridsberg, M.; Karkavelas, G.; Karayannopoulou, G. Somatostatin Receptor Expression in Non-Medullary Thyroid Carcinomas. Hormones 2012, 11, 290–296.
- Ain, K.B.; Taylor, K.D.; Tofiq, S.; Venkataraman, G. Somatostatin Receptor Subtype Expression in Human Thyroid and Thyroid Carcinoma Cell Lines. J. Clin. Endocrinol. Metab. 1997, 82, 1857–1862.
- Ocak, M.; Demirci, E.; Kabasakal, L.; Aygun, A.; Tutar, R.O.; Araman, A.; Kanmaz, B. Evaluation and Comparison of Ga-68 DOTA-TATE and Ga-68 DOTA-NOC PET/CT Imaging in Well-Differentiated Thyroid Cancer. Nucl. Med. Commun. 2013, 34, 1084–1089.
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392.
- Chen, Y.; Zheng, S.; Zhang, J.; Yao, S.; Miao, W. 68Ga-DOTA-FAPI-04 PET/CT Imaging in Radioiodine-Refractory Differentiated Thyroid Cancer (RR-DTC) Patients. Ann. Nucl. Med. 2022, 36, 610–622.
- Fu, H.; Fu, J.; Huang, J.; Pang, Y.; Chen, H. 68Ga-FAPI PET/CT Versus 18F-FDG PET/CT for Detecting Metastatic Lesions in a Case of Radioiodine-Refractory Differentiated Thyroid Cancer. Clin. Nucl. Med. 2021, 46, 940–942.




