Differentiated thyroid cancer (DTC) is the most common subtype of thyroid cancer and has an excellent overall prognosis. However, metastatic DTC in certain cases may have a poor prognosis as it becomes radioiodine-refractory. Molecular imaging is essential for disease evaluation and further management. The most commonly used tracers are [18F]FDG and isotopes of radioiodine. Several other radiopharmaceuticals may be used as well, with different diagnostic performances.
- radioiodine-refractory DTC
- molecular imaging
- FDG
- PSMA
- FAPI
- somatostatin analogues
- PRRT
- radioligand therapy
- theranostics
1. Introduction
2. Radioiodine-Refractory Differentiated Thyroid Cancer: Definition and Criteria
Among DTC patients, about 30% have or will develop metastatic disease at loco-regional lymph nodes (5-year survival rate > 90%) or, more rarely, in distant organs (mainly lungs and bones) with a significantly worse prognosis (5-year survival approx. 55%). Additionally, an increase in the overall mortality rate of DTC patients has been observed lately, probably due to an increased incidence of metastatic DTC patients [5]. Luckily, many patients with advanced DTC still exhibit 131I avidity, and therefore approximately 40% will achieve remission after 131I treatment [6][7][8][9]. Consequently, repeated courses of 131I therapy, in addition to TSH suppression, are the standard of care to manage metastasized DTC when the disease remains iodine-avid [10][11]. Unfortunately, 131I therapy becomes ineffective in a fraction of patients and should be stopped when a patient no longer responds to treatment. However, considering that RAI-R DTC patients still have limited therapeutic options, premature stopping of 131I therapy (i.e., when still able to obtain disease stabilization and symptom relief) should be avoided. The correct identification of RAI-R disease remains controversial (Table 1). Currently, different criteria to define an RAI-R disease are reported in the literature. In 2014, an international expert panel proposed stopping 131I therapy when “at least one lesion becomes 131I negative and continues to grow” [2]. Sacks and colleagues supported stopping 131I therapy when diagnostic scintigraphy (i.e., low 131I or 123I activity administered) is negative in the presence of structural disease, in the case of a positive [18F]FDG PET scan or cumulative administered activities of >18.5–22 GBq 131I [12].|
Main Criteria |
|
No 131I uptake in malignant metastatic tissue outside the thyroid bed on the first post-therapeutic WBS |
|
131I uptake is lost after previous evidence of 131I avid disease |
|
131I is concentrated in some but not in other lesions |
|
Disease progresses despite 131I avidity |
|
Patients have already received 22 GBq or more of 131I |
|
Other Potential Criteria |
|
No 131I uptake in malignant tissue on diagnostic WBS |
|
Significant uptake on [18F]FDG PET/CT |
|
Aggressive tumor histology |
3. Radiopharmaceuticals Used for Radioiodine-Refractory Differentiated Thyroid Cancer Imaging and Therapy
3.1. [
18
F]FDG
[18F]FDG is, besides radioiodine, the most commonly employed molecular imaging tracer in RAI-R DTC. It is considered especially appropriate in DTC patients with elevated serum Tg and negative radioiodine whole-body imaging [16], and its sensitivity depends on tumor differentiation, which has a superior detection rate in DTC patients with aggressive histopathology/biological behavior. [18F]FDG, as a glucose analogue, is transported into the cells across the glucose transporter (GLUT) protein and phosphorylated by hexokinase into [18F]FDG-6-phosphate, which is not metabolized but trapped within the cell (Figure 1).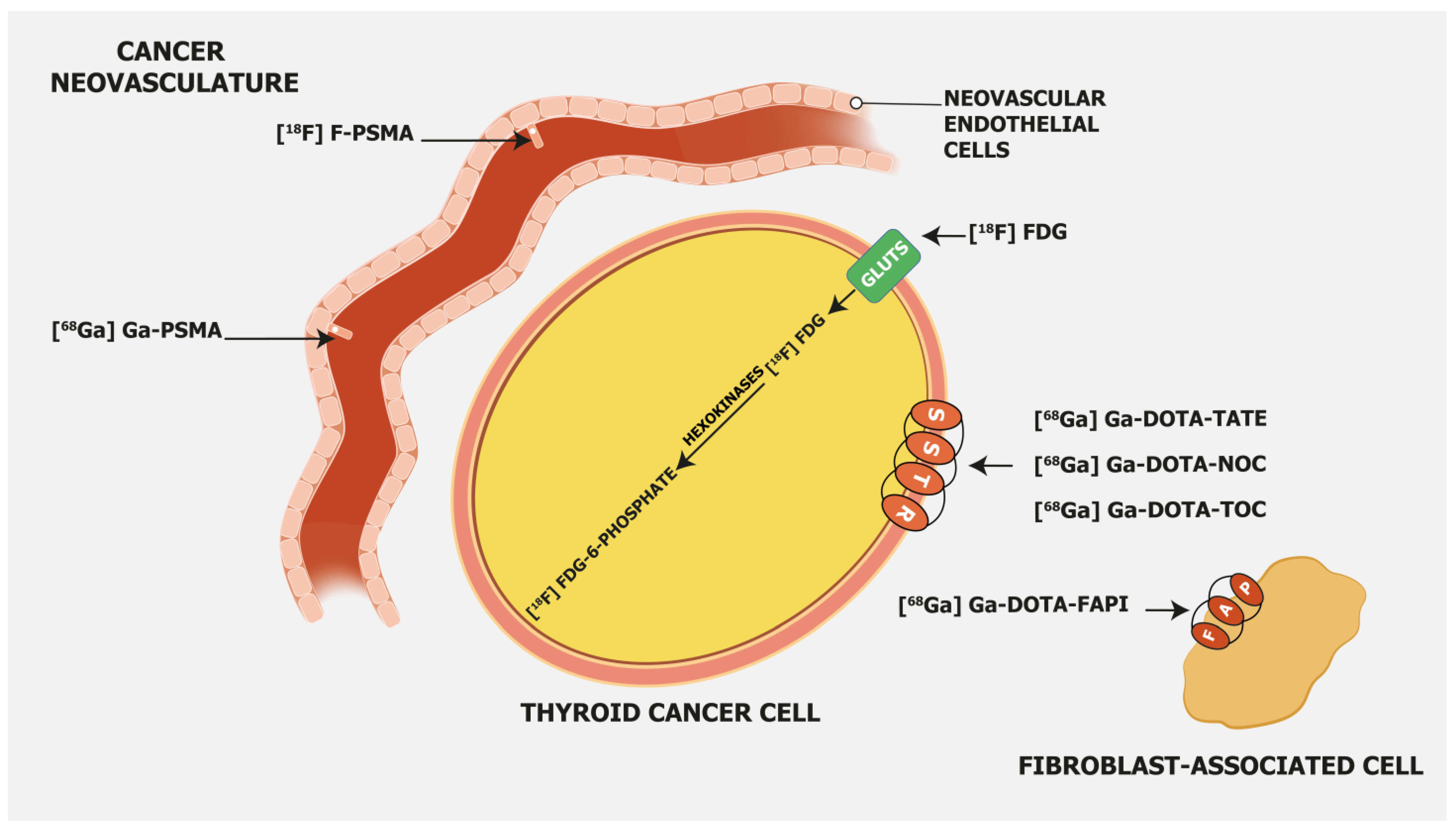
3.2. PSMA-Targeting Radiopharmaceuticals
Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein type II encoded by the Folate Hydrolase 1 gene [20] expressed in prostate cancer but also on the membrane of neovascular endothelial cells of various solid tumors, such as thyroid, head, bladder, lung, breast, gynecologic, gastric, and colorectal cancers [21] (Figure 1). Immunohistochemical studies have demonstrated that high PSMA expression in the neovasculature of thyroid cancer positively correlates with a more clinically aggressive course of the disease. Moreover, it was shown that DTC patients with lesions of moderate and strong PSMA expression have a higher risk of developing radioiodine-refractory disease or a higher risk of disease-specific mortality [22][23] (Figure 2).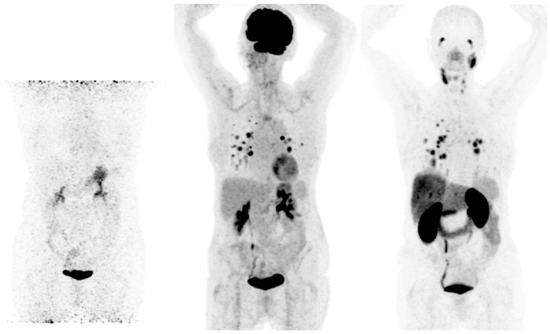
3.3. Somatostatin Receptor-Targeting Radiopharmaceuticals
Somatostatin receptor-targeting radiopharmaceuticals are used for imaging tumors with high expression of somatostatin receptors (SSTR) (Figure 1). Most commonly, somatostatin analogs are labeled with 68Ga, such as [68Ga]Ga-DOTA-TATE, [68Ga]Ga-DOTA-NOC, and [68Ga]Ga-DOTA-TOC. They have different binding affinities for different SSTR subtypes. [68Ga]Ga-DOTA-TATE has a quite selective and very high affinity for SSTR 2, [68Ga]Ga-DOTA-NOC binds SSTR 2 and 3, while [68Ga]Ga-DOTA-TOC has an affinity for SSTR 2 and SSTR 5 [31]. DTC cells may exhibit high expression of SSTR 2, 3, and 5 [32][33] (Figure 3).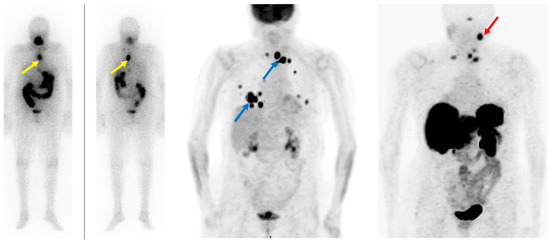
3.4. Fibroblast Activation Protein—Targeting Radiopharmaceuticals
Fibroblast activation protein (FAP) expression is very low in normal human fibroblasts. However, cancer-related fibroblasts are characterized by high expression of FAP (Figure 1) since they carry both exopeptidase and endopeptidase activity [17]. A large fraction of the total mass in various tumors is made of tumor fibroblasts and extracellular fibrosis, while on many occasions less than 10% of tumor cells are involved [35]. Thus, radiolabeled fibroblast activation protein inhibitors (FAPIs) are suitable for tumor imaging. Chen et al. recently conducted a study to evaluate the performance of [68Ga]Ga-DOTA-FAPI-04 PET/CT in the detection of RAI-R DTC lesions [36]. They enrolled 24 RAI-R DTC patients and demonstrated that 21 (87.5%) patients have FAPI-positive lesions, with a mean SUVmax of 4.25 and a growth rate of 6.51%. SUVmax was positively correlated with the lesions’ growth rates. In certain cases, [68Ga]Ga-DOTA-labeled FAPI radiopharmaceuticals showed better detection of metastatic RAI-R DTC lesions compared to [18F]FDG, also due to a better target-to-background ratio [37]. Detectable strong expression of FAP opens an opportunity for new therapeutic options in RAI-R DTC patients. Like PSMA labeling, several attempts have been made for 18F labeling of FAPI ligands to overcome 68Ga limitations as stated above (e.g., longer positron range, higher maximum +energy, lower positron yield, higher costs, etc.), with [18F]FAPI-74 being the most promising candidate. Studies are currently ongoing, e.g., for the diagnostic comparison of [18F]FDG with [18F]FAPI-74 in patients with TENIS syndrome (EudraCT Number: 2022-001997-70; Figure 4).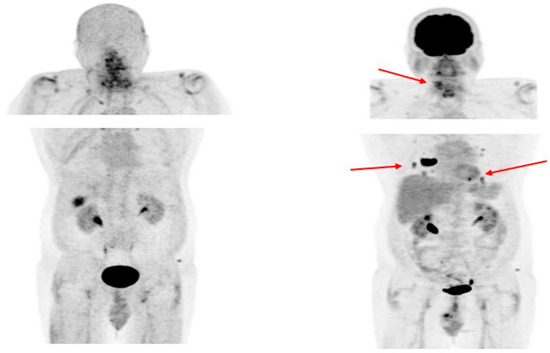
References
- Giovanella, L.; Deandreis, D.; Vrachimis, A.; Campenni, A.; Ovcaricek, P.P. Molecular Imaging and Theragnostics of Thyroid Cancers. Cancers 2022, 14, 1272.
- Schlumberger, M.; Brose, M.; Elisei, R.; Leboulleux, S.; Luster, M.; Pitoia, F.; Pacini, F. Definition and Management of Radioactive Iodine-Refractory Differentiated Thyroid Cancer. Lancet Diabetes Endocrinol. 2014, 2, 356–358.
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133.
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690.
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017, 317, 1338–1348.
- Vaisman, F.; Carvalho, D.P.; Vaisman, M. A New Appraisal of Iodine Refractory Thyroid Cancer. Endocr. Relat. Cancer 2015, 22, R301–R310.
- Van Nostrand, D. Radioiodine Refractory Differentiated Thyroid Cancer: Time to Update the Classifications. Thyroid 2018, 28, 1083–1093.
- Ylli, D.; Van Nostrand, D.; Wartofsky, L. Conventional Radioiodine Therapy for Differentiated Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 181–197.
- Kim, H.; Kim, H.I.; Kim, S.W.; Jung, J.; Jeon, M.J.; Kim, W.G.; Kim, T.Y.; Kim, H.K.; Kang, H.C.; Han, J.M.; et al. Prognosis of Differentiated Thyroid Carcinoma with Initial Distant Metastasis: A Multicenter Study in Korea. Endocrinol. Metab. 2018, 33, 287–295.
- Van Nostrand, D. 131I Treatment of Distant Metastases. In Thyroid Cancer: A Comprehensive Guide to Clinical Management; Springer: New York, NY, USA, 2016; pp. 595–627.
- Giovanella, L.; Van Nostrand, D. Advanced Differentiated Thyroid Cancer: When to Stop Radioiodine? Q. J. Nucl. Med. Mol. Imaging 2019, 63, 267–270.
- Sacks, W.; Braunstein, G.D. Evolving Approaches in Managing Radioactive Iodine-Refractory Differentiated Thyroid Cancer. Endocr. Pract. 2014, 20, 263–275.
- Luo, Y.; Jiang, H.; Xu, W.; Wang, X.; Ma, B.; Liao, T.; Wang, Y. Clinical, Pathological, and Molecular Characteristics Correlating to the Occurrence of Radioiodine Refractory Differentiated Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 549882.
- Hänscheid, H.; Lassmann, M.; Buck, A.K.; Reiners, C.; Verburg, F.A. The Limit of Detection in Scintigraphic Imaging with I-131 in Patients with Differentiated Thyroid Carcinoma. Phys. Med. Biol. 2014, 59, 2353–2368.
- Lee, J.W.; Lee, S.M.; Koh, G.P.; Lee, D.H. The Comparison of (131)I Whole-Body Scans on the Third and Tenth Day after (131)I Therapy in Patients with Well-Differentiated Thyroid Cancer: Preliminary Report. Ann. Nucl. Med. 2011, 25, 439–446.
- Avram, A.M.; Giovanella, L.; Greenspan, B.; Lawson, S.A.; Luster, M.; Van Nostrand, D.; Peacock, J.G.; Ovčariček, P.P.; Silberstein, E.; Tulchinsky, M.; et al. SNMMI Procedure Standard/EANM Practice Guideline for Nuclear Medicine Evaluation and Therapy of Differentiated Thyroid Cancer: Abbreviated Version. J. Nucl. Med. 2022, 63, 15N–35N.
- Sakulpisuti, C.; Charoenphun, P.; Chamroonrat, W. Positron Emission Tomography Radiopharmaceuticals in Differentiated Thyroid Cancer. Molecules 2022, 27, 4936.
- Heydarzadeh, S.; Moshtaghie, A.A.; Daneshpoor, M.; Hedayati, M. Regulators of Glucose Uptake in Thyroid Cancer Cell Lines. Cell Commun. Signal. 2020, 18, 83.
- Leboulleux, S.; Schroeder, P.R.; Busaidy, N.L.; Auperin, A.; Corone, C.; Jacene, H.A.; Ewertz, M.E.; Bournaud, C.; Wahl, R.L.; Sherman, S.I.; et al. Assessment of the Incremental Value of Recombinant Thyrotropin Stimulation before 2--Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography/Computed Tomography Imaging to Localize Residual Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2009, 94, 1310–1316.
- Israeli, R.S.; Powell, C.T.; Fair, W.R.; Heston, W.D.W. Molecular Cloning of a Complementary DNA Encoding a Prostate-Specific Membrane Antigen. Cancer Res. 1993, 53, 227–230.
- Ciappuccini, R.; Saguet-Rysanek, V.; Giffard, F.; Licaj, I.; Dorbeau, M.; Clarisse, B.; Poulain, L.; Bardet, S. PSMA Expression in Differentiated Thyroid Cancer: Association With Radioiodine, 18FDG Uptake, and Patient Outcome. J. Clin. Endocrinol. Metab. 2021, 106, 3536–3545.
- Heitkötter, B.; Steinestel, K.; Trautmann, M.; Grünewald, I.; Barth, P.; Gevensleben, H.; Bögemann, M.; Wardelmann, E.; Hartmann, W.; Rahbar, K.; et al. Neovascular PSMA Expression Is a Common Feature in Malignant Neoplasms of the Thyroid. Oncotarget 2018, 9, 9867–9874.
- Sollini, M.; di Tommaso, L.; Kirienko, M.; Piombo, C.; Erreni, M.; Lania, A.G.; Erba, P.A.; Antunovic, L.; Chiti, A. PSMA Expression Level Predicts Differentiated Thyroid Cancer Aggressiveness and Patient Outcome. EJNMMI Res. 2019, 9, 93.
- Sanchez-Crespo, A. Comparison of Gallium-68 and Fluorine-18 Imaging Characteristics in Positron Emission Tomography. Appl. Radiat. Isot. 2013, 76, 55–62.
- Martiniova, L.; De Palatis, L.; Etchebehere, E.; Ravizzini, G. Gallium-68 in Medical Imaging. Curr. Radiopharm. 2016, 9, 187–207.
- Piron, S.; Verhoeven, J.; Vanhove, C.; De Vos, F. Recent Advancements in 18F-Labeled PSMA Targeting PET Radiopharmaceuticals. Nucl. Med. Biol. 2022, 106–107, 29–51.
- Seifert, R.; Telli, T.; Opitz, M.; Barbato, F.; Berliner, C.; Nader, M.; Umutlu, L.; Stuschke, M.; Hadaschik, B.; Herrmann, K.; et al. Unspecific 18F-PSMA-1007 Bone Uptake Evaluated Through PSMA-11 PET, Bone Scanning, and MRI Triple Validation in Patients with Biochemical Recurrence of Prostate Cancer. J. Nucl. Med. 2023, 64, 738–743.
- Grünig, H.; Maurer, A.; Thali, Y.; Kovacs, Z.; Strobel, K.; Burger, I.A.; Müller, J. Focal Unspecific Bone Uptake on -PSMA-1007 PET: A Multicenter Retrospective Evaluation of the Distribution, Frequency, and Quantitative Parameters of a Potential Pitfall in Prostate Cancer Imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4483–4494.
- Arnfield, E.G.; Thomas, P.A.; Roberts, M.J.; Pelecanos, A.M.; Ramsay, S.C.; Lin, C.Y.; Latter, M.J.; Garcia, P.L.; Pattison, D.A. Clinical Insignificance of PSMA-1007 Avid Non-Specific Bone Lesions: A Retrospective Evaluation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4495–4507.
- Duncan, I.; Ingold, N.; Martinez-Marroquin, E.; Paterson, C. An Australian Experience Using Tc-PSMA SPECT/CT in the Primary Diagnosis of Prostate Cancer and for Staging at Biochemical Recurrence after Local Therapy. Prostate 2023, 83, 970–979.
- Johnbeck, C.B.; Knigge, U.; Kjær, A. PET Tracers for Somatostatin Receptor Imaging of Neuroendocrine Tumors: Current Status and Review of the Literature. Future Oncol. 2014, 10, 2259–2277.
- Pazaitou-Panayiotou, K.; Janson, E.T.; Koletsa, T.; Kotoula, V.; Stridsberg, M.; Karkavelas, G.; Karayannopoulou, G. Somatostatin Receptor Expression in Non-Medullary Thyroid Carcinomas. Hormones 2012, 11, 290–296.
- Ain, K.B.; Taylor, K.D.; Tofiq, S.; Venkataraman, G. Somatostatin Receptor Subtype Expression in Human Thyroid and Thyroid Carcinoma Cell Lines. J. Clin. Endocrinol. Metab. 1997, 82, 1857–1862.
- Ocak, M.; Demirci, E.; Kabasakal, L.; Aygun, A.; Tutar, R.O.; Araman, A.; Kanmaz, B. Evaluation and Comparison of Ga-68 DOTA-TATE and Ga-68 DOTA-NOC PET/CT Imaging in Well-Differentiated Thyroid Cancer. Nucl. Med. Commun. 2013, 34, 1084–1089.
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392.
- Chen, Y.; Zheng, S.; Zhang, J.; Yao, S.; Miao, W. 68Ga-DOTA-FAPI-04 PET/CT Imaging in Radioiodine-Refractory Differentiated Thyroid Cancer (RR-DTC) Patients. Ann. Nucl. Med. 2022, 36, 610–622.
- Fu, H.; Fu, J.; Huang, J.; Pang, Y.; Chen, H. 68Ga-FAPI PET/CT Versus 18F-FDG PET/CT for Detecting Metastatic Lesions in a Case of Radioiodine-Refractory Differentiated Thyroid Cancer. Clin. Nucl. Med. 2021, 46, 940–942.
