
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carmen López Díaz | -- | 2257 | 2023-09-28 14:08:10 | | | |
| 2 | Alfred Zheng | Meta information modification | 2257 | 2023-10-09 08:47:25 | | |
Video Upload Options
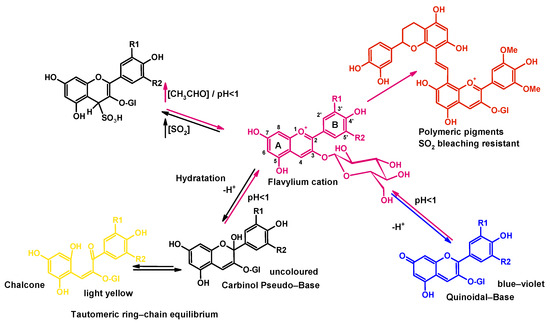
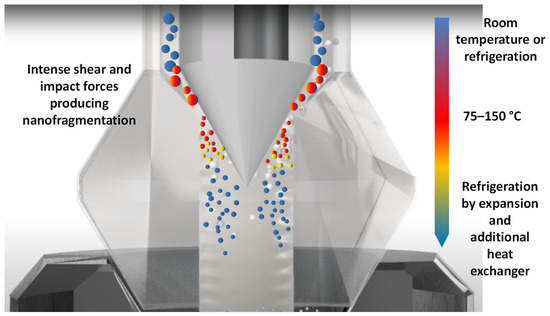
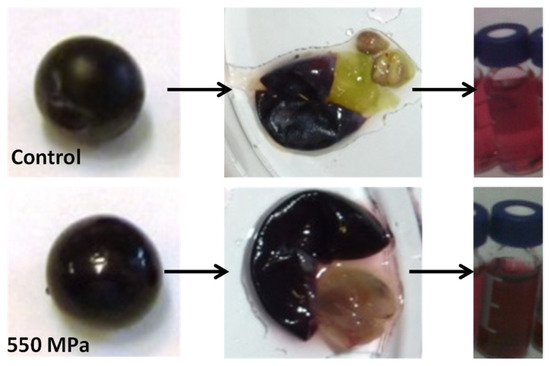
The use of high-pressure technologies is a hot topic in food science because of the potential for a gentle process in which spoilage and pathogenic microorganisms can be eliminated; these technologies also have effects on the extraction, preservation, and modification of some constituents. Anthocyanins can be rapidly extracted from skins using High Hydrostatic Pressure (HHP) and cell fragments using Ultra-High Pressure Homogenization (UHPH), releasing them and facilitating their diffusion into the liquid quickly. High Hydrostatic Pressure (HHP) and Ultra-High Pressure Homogenization (UHPH) techniques are gentle and protective of sensitive molecules such as phenols, terpenes, and vitamins. Both techniques are non-thermal technologies with mild temperatures and residence times.
1. Introduction
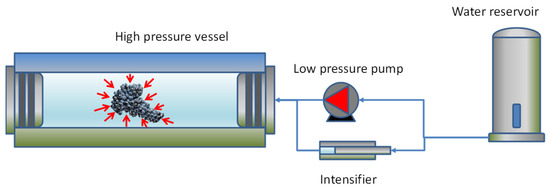
2. Extraction of Anthocyanins by HHP
3. Extraction of Anthocyanins by UHPH
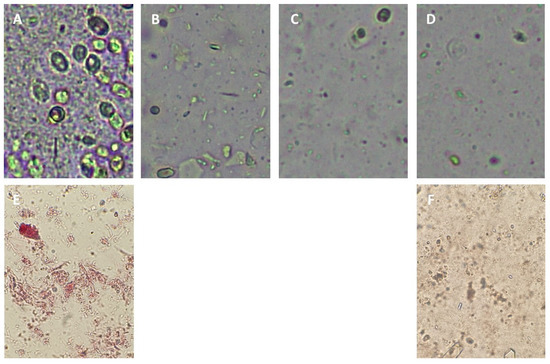
4. HHP vs. UHPH in the Extraction and Stability of Anthocyanins
References
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables, and Grains; CRC Press: Boca Raton, FL, USA, 1993.
- Castañeda-Ovando, A.; Pacheco-Hernández, M.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871.
- Mazza, G.; Francis, D.F.J. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 2009, 35, 341–371.
- Morata, A.; López, C.; Tesfaye, W.; González, C.; Escott, C. Anthocyanins as Natural Pigments in Beverages. In Value-Added Ingredients and Enrichments of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 14, pp. 383–428.
- Santos-Buelga, C.; González-Paramás, A.M. Anthocyanins. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 10–21.
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451.
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933.
- Dangles, O.; Fenger, J.-A. The Chemical Reactivity of Anthocyanins and Its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970.
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11.
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967.
- Morata, A.; Escott, C.; Loira, I.; López, C.; Palomero, F.; González, C. Emerging Non-Thermal Technologies for the Extraction of Grape Anthocyanins. Antioxidants 2021, 10, 1863.
- Tena, N.; Asuero, A.G. Up-To-Date Analysis of the Extraction Methods for Anthocyanins: Principles of the Techniques, Optimization, Technical Progress, and Industrial Application. Antioxidants 2022, 11, 286.
- Buzrul, S.; Alpas, H.; Largeteau, A.; Bozoglu, F.; Demazeau, G. Compression heating of selected pressure transmitting fluids and liquid foods during high hydrostatic pressure treatment. J. Food Eng. 2008, 85, 466–472.
- San Martín, M.F.; Barbosa-Cánovas, G.V.; Swanson, B.G. Food Processing by High Hydrostatic Pressure. Crit. Rev. Food Sci. Nutr. 2002, 42, 627–645.
- Knorr, D.; Froehling, A.; Jaeger, H.; Reineke, K.; Schlueter, O.; Schoessler, K. Emerging Technologies in Food Processing. Annu. Rev. Food Sci. Technol. 2011, 2, 203–235.
- Aganovic, K.; Hertel, C.; Vogel, R.F.; Johne, R.; Schlüter, O.; Schwarzenbolz, U.; Jäger, H.; Holzhauser, T.; Bergmair, J.; Roth, A.; et al. Aspects of high hydrostatic pressure food processing: Perspectives on technology and food safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3225–3266.
- Tiwari, B.K.; O’Donnell, C.P.; Cullen, P.J. Effect of non thermal processing technologies on the anthocyanin content of fruit juices. Trends Food Sci. Technol. 2009, 20, 137–145.
- Varela-Santos, E.; Ochoa-Martinez, A.; Tabilo-Munizaga, G.; Reyes, J.E.; Pérez-Won, M.; Briones-Labarca, V.; Morales-Castro, J. Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innov. Food Sci. Emerg. Technol. 2012, 13, 13–22.
- Zamora, A.; Guamis, B. Opportunities for ultra-high-pressure homogenization (UHPH) for the food industry. Food Eng. Rev. 2015, 7, 130–142.
- Morata, A.; Guamis, B. Use of UHPH to obtain juices with better nutritional quality and healthier wines with low levels of SO2. Front. Nutr. 2020, 7, 598286.
- Bañuelos, M.A.; Loira, I.; Guamis, B.; Escott, C.; Del Fresno, J.M.; Codina-Torrella, I.; Quevedo, J.M.; Gervilla, R.; Rodríguez Chavarría, J.M.; de Lamo, S.; et al. White wine processing by UHPH without SO2. Elimination of microbial populations and effect in oxidative enzymes, colloidal stability and sensory quality. Food Chem. 2020, 332, 127417.
- Vaquero, C.; Escott, C.; Loira, I.; Guamis, B.; del Fresno, J.M.; Quevedo, J.M.; Gervilla, R.; de Lamo, S.; Ferrer-Gallego, R.; González, C.; et al. Cabernet sauvignon red must processing by UHPH to Produce Wine Without SO2: The colloidal structure, microbial and oxidation control, colour protection and sensory quality of the wine. Food Bioprocess Technol. 2022, 15, 620–634.
- Loira, I.; Morata, A.; Bañuelos, M.A.; Puig-Pujol, A.; Guamis, B.; González, C.; Suárez-Lepe, J.A. Use of ultra-high pressure homogenization processing in winemaking: Control of microbial populations in grape musts and effects in sensory quality. Innov. Food Sci. Emerg. Technol. 2018, 50, 50–56.
- Corrales, M.; Fernández García, A.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421.
- Morata, A.; Loira, I.; Vejarano, R.; Bañuelos, M.A.; Sanz, P.D.; Otero, L.; Suárez-Lepe, J.A. Grape processing by High Hydrostatic Pressure: Effect on microbial populations, phenol extraction and wine quality. Food Bioprocess Technol. 2015, 8, 277–286.
- Martín, J.; Asuero, A.G. High hydrostatic pressure for recovery of anthocyanins: Effects, performance, and applications. Sep. Purif. Rev. 2021, 50, 159–176.
- Velázquez-Estrada, R.M.; Hernández-Herrero, M.M.; Guamis-López, B.; Roig-Sagués, A.X. Impact of Ultra High Pressure Homogenization on pectin methylesterase activity and microbial characteristics of orange juice: A comparative study against conventional heat pasteurization. Innov. Food Sci. Emerg. Technol. 2012, 13, 100–106.
- Liu, S.; Xu, Q.; Li, X.; Wang, Y.; Zhu, J.; Ning, C.; Chang, X.; Meng, X. Effects of high hydrostatic pressure on physicochemical properties, enzymes activity, and antioxidant capacities of anthocyanins extracts of wild Lonicera caerulea berry. Innov. Food Sci. Emerg. Technol. 2016, 36, 48–58.
- Yuan, B.; Danao, M.-G.C.; Stratton, J.E.; Weier, S.A.; Weller, C.L.; Lu, M. High pressure processing (HPP) of aronia berry purée: Effects on physicochemical properties, microbial counts, bioactive compounds, and antioxidant capacities. Innov. Food Sci. Emerg. Technol. 2018, 47, 249–255.
- Li, Y.; Padilla-Zakour, O.I. High Pressure Processing vs. Thermal Pasteurization of Whole Concord Grape Puree: Effect on Nutritional Value, Quality Parameters and Refrigerated Shelf Life. Foods 2021, 10, 2608.
- Zhang, L.; Xiao, G.; Yu, Y.; Xu, Y.; Wu, J.; Zou, B.; Li, L. Low-oxygen pulping combined with high hydrostatic pressure improve the stability of blueberry pulp anthocyanins and color during storage. Food Control 2022, 138, 108991.
- Zhang, W.; Shen, Y.; Li, Z.; Xie, X.; Gong, E.S.; Tian, J.; Si, X.; Wang, Y.; Gao, N.; Shu, C.; et al. Effects of high hydrostatic pressure and thermal processing on anthocyanin content, polyphenol oxidase and β-glucosidase activities, color, and antioxidant activities of blueberry (Vaccinium Spp.) puree. Food Chem. 2021, 342, 128564.
- Zhang, L.; Zhu, C.; Chen, X.; Xu, X.; Wang, H. Resistance of detached-cells of biofilm formed by Staphylococcus aureus to ultra high pressure homogenization. Food Res. Int. 2021, 139, 109954.
- Ferragut, V.; Hernández-Herrero, M.; Veciana-Nogués, M.T.; Borras-Suarez, M.; González-Linares, J.; Vidal-Carou, M.C.; Guamis, B. Ultra-high-pressure homogenization (UHPH) system for producing high-quality vegetable-based beverages: Physicochemical, microbiological, nutritional and toxicological characteristics. J. Sci. Food Agric. 2015, 95, 953–961.
- Ferragut, V.; Valencia-Flores, D.C.; Pérez-González, M.; Gallardo, J.; Hernández-Herrero, M. Quality Characteristics and Shelf-Life of Ultra-High Pressure Homogenized (UHPH) Almond Beverage. Foods 2015, 4, 159–172.
- Patrignani, F.; Mannozzi, C.; Tappi, S.; Tylewicz, U.; Pasini, F.; Castellone, V.; Riciputi, Y.; Rocculi, P.; Romani, S.; Caboni, M.F.; et al. (Ultra) High Pressure Homogenization Potential on the shelf-life and functionality of kiwi fruit juice. Front. Microbiol. 2019, 10, 246.
- Salehi, F. Physico-chemical and rheological properties of fruit and vegetable juices as affected by high pressure homogenization: A review. Int. J. Food Prop. 2020, 23, 1136–1149.
- Comuzzo, P.; Calligaris, S. Potential applications of High Pressure Homogenization in winemaking: A Review. Beverages 2019, 5, 56.
- Levy, R.; Okun, Z.; Shpigelman, A. High-Pressure Homogenization: Principles and applications beyond microbial inactivation. Food Eng. Rev. 2021, 13, 490–508.
- Singh, S.V.; Singh, R.; Singh, A.; Chinchkar, A.V.; Kamble, M.G.; Dutta, S.J.; Singh, S.B. A review on green pressure processing of fruit juices using microfluidization: Quality, safety and preservation. Appl. Food Res. 2022, 2, 100235.
- Suárez-Jacobo, Á.; Saldo, J.; Rüfer, C.E.; Guamis, B.; Roig-Sagués, A.X.; Gervilla, R. Aseptically packaged UHPH-treated apple juice: Safety and quality parameters during storage. J. Food Eng. 2012, 109, 291–300.
- Valencia-Flores, D.C.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparing the effects of Ultra-High-Pressure Homogenization and conventional thermal treatments on the microbiological, physical, and chemical quality of almond beverages. J. Food Sci. 2013, 78, E199–E205.
- Codina-Torrella, I.; Gallardo-Chacón, J.J.; Juan, B.; Guamis, B.; Trujillo, A.J. Effect of Ultra-High Pressure Homogenization (UHPH) and Conventional Thermal Pasteurization on the Volatile Composition of Tiger Nut Beverage. Foods 2023, 12, 683.
- Benjamin, O.; Gamrasni, D. Microbial, nutritional, and organoleptic quality of pomegranate juice following high-pressure homogenization and low-temperature pasteurization. J. Food Sci. 2020, 85, 592–599.
- Kruszewski, B.; Zawada, K.; Karpiński, P. Impact of High-Pressure Homogenization parameters on physicochemical characteristics, bioactive compounds content, and antioxidant capacity of blackcurrant juice. Molecules 2021, 26, 1802.
- Yu, Y.; Xu, Y.; Wu, J.; Xiao, G.; Fu, M.; Zhang, Y. Effect of ultra-high pressure homogenisation processing on phenolic compounds, antioxidant capacity and anti-glucosidase of mulberry juice. Food Chem. 2014, 153, 114–120.
- Joubran, A.M.; Katz, I.H.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. The effect of pressure level and cycling in high-pressure homogenization on physicochemical, structural and functional properties of filtered and non-filtered strawberry nectar. Innov. Food Sci. Emerg. Technol. 2019, 57, 102203.
- Karacam, C.H.; Sahin, S.; Oztop, M.H. Effect of high pressure homogenization (microfluidization) on the quality of Ottoman Strawberry (F. Ananassa) juice. LWT 2015, 64, 932–937.
- Patrignani, F.; Lanciotti, R. Applications of High and Ultra High Pressure Homogenization for Food Safety. Front. Microbiol. 2016, 7, 1132.
- Tinello, F.; Lante, A. Recent advances in controlling polyphenol oxidase activity of fruit and vegetable products. Innov. Food Sci. Emerg. Technol. 2018, 50, 73–83.
- Hendrickx, M.; Ludikhuyze, L.; Van den Broeck, I.; Weemaes, C. Effects of High Pressure on enzymes related to food quality. Trends Food Sci. Technol. 1998, 9, 197–203.
- Cascaes Teles, A.S.; Hidalgo Chávez, D.W.; Zarur Coelho, M.A.; Rosenthal, A.; Fortes Gottschalk, L.M.; Tonon, R.V. Combination of enzyme-assisted extraction and high hydrostatic pressure for phenolic compounds recovery from grape pomace. J. Food Eng. 2021, 288, 110128.
- Ding, Y.; Ban, Q.; Wu, Y.; Sun, Y.; Zhou, Z.; Wang, Q.; Cheng, J.; Xiao, H. Effect of high hydrostatic pressure on the edible quality, health and safety attributes of plant-based foods represented by cereals and legumes: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 4636–4654.
- Iqbal, A.; Murtaza, A.; Hu, W.; Ahmad, I.; Ahmed, A.; Xu, X. Activation and inactivation mechanisms of polyphenol oxidase during thermal and non-thermal methods of food processing. Food Bioprod. Process. 2019, 117, 170–182.
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of Innovative Food Processing Technologies on the Physicochemical and Nutritional Properties and Quality of Non-Dairy Plant-Based Beverages. Foods 2020, 9, 288.
- Ahmad, T.; Butt, M.Z.; Aadil, R.M.; Inam-ur-Raheem, M.; Abdullah; Bekhit, A.E.-D.; Guimarães, J.T.; Balthazar, C.F.; Rocha, R.S.; Esmerino, E.A.; et al. Impact of nonthermal processing on different milk enzymes. Int. J. Dairy Technol. 2019, 72, 481–495.
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High pressure processing of carrot juice: Effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2020, 307, 125549.
- Navarro-Baez, J.E.; Martínez, L.M.; Welti-Chanes, J.; Buitimea-Cantúa, G.V.; Escobedo-Avellaneda, Z. High Hydrostatic Pressure to Increase the Biosynthesis and Extraction of Phenolic Compounds in Food: A Review. Molecules 2022, 27, 1502.




