
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liuxiong Wang | -- | 2456 | 2023-09-28 12:06:53 | | | |
| 2 | Alfred Zheng | Meta information modification | 2456 | 2023-10-09 08:45:59 | | |
Video Upload Options
Pigeon is an important economic poultry species in many countries. Pigeon milk is generally characterized by having high concentrations of proteins and lipids, and a complicated regulatory network is involved in the milk formation. Hormones, especially prolactin, could promote the proliferation of crop epidermal cells and nutrient accumulation. The expression of target genes associated with these important biological processes in the crop epidermis is affected by non-coding RNAs. Meanwhile, signaling pathways, such as target of rapamycin (TOR), Janus kinase/signal transducer and activator of transcription proteins (JAK/STAT), protein kinase B (Akt), etc., influence the production of crop milk by either enhancing protein synthesis in crop cells or inducing apoptosis of crop epidermal cells.
1. Introduction
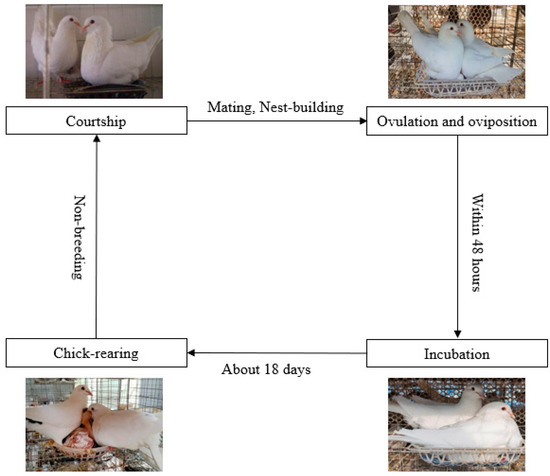
2. The Formation Mechanism of Pigeon Milk
2.1. The Proliferation of Pigeon Crop Epidermal Cells
2.1.1. Morphological Changes in the Crop
2.1.2. The Regulation of Hormones on Crop Proliferation
2.1.3. The Regulation of Crop Proliferation by Non-Coding RNA
2.2. Accumulation of Nutrients in Pigeon Crop Epithelial Cells
2.2.1. Synthesis of Protein in Crop Epidermal Cells
2.2.2. Synthesis of Lipid in Crop Epidermal Cells
2.2.3. Synthesis of Carbohydrates in Crop Epidermal Cells
2.3. Shedding of Crop Epidermal Cells
References
- Ding, J.; Liao, N.; Zheng, Y.; Yang, L.; Zhou, H.; Xu, K.; Han, C.; Luo, H.; Qin, C.; Tang, C.; et al. The composition and function of pigeon milk microbiota transmitted from parent pigeons to squabs. Front. Microbiol. 2020, 11, 1789.
- Fattah, A.F.A. Parental Care during Incubation, Brooding and Growth Rates of Egyptian Baladi Pigeon Nestlings. Brit. J. Poult. Sci. 2015, 4, 29–33.
- Silver, R.; Andrews, H.; Ball, G.F. Parental care in an ecological perspective: A quantitative analysis of avian subfamilies. Integ. Comp. Biol. 1985, 25, 823–840.
- Gayathri, K.L.; Hegde, S.N. Influence of breeding activity on the haematology of domestic pigeons, Columba livia, Pavo. Indian J. Ornithol. 1994, 32, 39–45.
- Nepote, K.H. Pigeons as Laboratory Animals. Poult. Avian. Biol. Rev. 1999, 10, 109–115.
- Shetty, S.; Jacob, R.T.; Shenoy, K.B.; Hegde, S.N. Patterns of Breeding Behaviour in the Domestic Pigeon. Bird Behav. 1990, 9, 14–19.
- Shapiro, M.D.; Domyan, E.T. Domestic pigeons. Curr. Biol. 2013, 23, 302–303.
- Gillespie, M.J.; Haring, V.R.; McColl, K.A.; Monaghan, P.; Donald, J.A.; Nicholas, K.R.; Moore, R.J.; Crowley, T.M. Histological and global gene expression analysis of the ‘lactating’ pigeon crop. BMC Genom. 2011, 12, 452.
- Gillespie, M.J.; Crowley, T.M.; Haring, V.R.; Wilson, S.L.; Harper, J.A.; Payne, J.S.; Green, D.; Monaghan, P.; Donald, J.A.; Nicholas, K.R.; et al. Transcriptome analysis of pigeon milk production-role of cornification and triglyceride synthesis genes. BMC Genom. 2013, 14, 169.
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29.
- Rendón, M.A.; Garrido, A.; Rendón-Martos, M.; Ramirez, J.M.; Amat, J.A. Assessing sex-related chick provisioning in greater flamingo Phoenicopterus roseus parents using capture-recapture models. J. Anim. Ecol. 2014, 83, 479–490.
- Fretwell, P.T.; Trathan, P.N.; Wienecke, B.; Kooyman, G.L. Emperor penguins breeding on iceshelves. PLoS ONE 2014, 9, 85285.
- Beams, H.W.; Meyer, R.K. The formation of pigeon “milk”. Physiol. Zool. 1931, 4, 486–500.
- Riddle, O.; Bates, R.W.; Dykshorn, S. The preparation, identification and assay of prolactin-a hormone of the anterior pituitary. Am. J. Physiol. 1933, 105, 191–216.
- Dumont, J.N. Prolactin-induced cytologic changes in the mucosa of the pigeon crop during crop-milk formation. Z. Zellforsch. Mikrosk. Anat. 1965, 68, 755–782.
- Riddle, O.; Bates, W.R.; Dykshorn, W.S. A new hormone of the anterior pituitary. Exp. Biol. Med. 1932, 29, 1211–1212.
- Horseman, N.D.; Buntin, J.D. Regulation of pigeon cropmilk secretion and parental behaviors by prolactin. Annu. Rev. Nutr. 1995, 15, 213–238.
- Bates, R.W.; Riddle, O. Effect of Route of Administration on the Bioassay of Prolactin. Proc. Soc. Exp. Biol. Med. 1936, 34, 847–849.
- Horseman, N.D.; Nollin, L.J. The mitogenic, but not differentiative, response of crop tissue to prolactin is circadian phase dependent. Endocrinology 1985, 116, 2085–2089.
- Nicoll, C.S.; Bern, H.A. Further analysis of the occurrence of pigeon crop sac-stimulating activity (Prolactin) in the vertebrate adenohypophysis. Gen. Comp. Endocrinol. 1968, 11, 5–20.
- Pukac, L.A.; Horseman, N.D. Regulation of pigeon crop gene expression by prolactin. Endocrinology 1984, 114, 1718–1724.
- Anderson, T.R.; Pitts, D.S.; Nicoll, C.S. Prolactin’s mitogenic action on the pigeon crop-sac mucosal epithelium involves direct and indirect mechanisms. Gen. Comp. Endocrinol. 1984, 54, 236–246.
- Hirvonen, A. Ornithine decarboxylase activity and the accumulation of its mRNA during early stages of liver regeneration. Biochim. Biophys. Acta. 1989, 1007, 120–123.
- Beyer, H.S.; Zieve, L. Effects of partial and sham hepatectomy on ornithine decarboxylase and thymidine kinase activities and mRNA contents. Biochem. Int. 1990, 20, 761–765.
- Nishiguchi, Y.; Hibasami, H.; Komada, Y.; Sakurai, M.; Nakashima, K. Human promyelocytic cell line hl60 has the specific binding sites for prolactin and its ornithine decarboxylase, dna synthesis and cellular proliferation are induced by prolactin. Leuk. Res. 1993, 17, 633–637.
- Bani, D. Relaxin: A pleiotropic hormone. Gen. Pharmacol. 1997, 28, 13–22.
- Bani, G.; Bigazzi, M. Morphological changes induced in mouse mammary gland by porcine and human relaxin. Actu. Anat. 1984, 119, 149–154.
- Bani, G.; Bigazzi, M.; Bani, D. Effects of relaxin on the mouse mammary gland. I. The myoepithelial cells. J. Endocrinol. Investig. 1985, 8, 207–215.
- Bani, G.; Bigazzi, M.; Bani, D. Effects of relaxin on the mouse mammary gland. II. The epithelial cells. J. Endocrinol. Investig. 1986, 9, 145–152.
- Bigazzi, M.; Bani, G.; Sacchi, T.B.; Petrucci, F.; Bianchi, S. Relaxin: A mammotropic hormone promoting growth and differentiation of the pigeon crop sac mucosa. Acta Endocrinol. 1988, 117, 181–188.
- Bani, G.; Sacchi, T.B.; Cecchi, R.; Bigazzi, M. The effects of relaxin on the pigeon crop sac mucosa: Light and electron microscopic study. Z. Mikrosk. Annt. Forsch. 1987, 101, 577–596.
- Bani, G.; Sacchi, T.B.; Bigazzi, M. Response of the pigeon crop sac to mammotrophic hormones: Comparison between relaxin and prolactin. Gen. Comp. Endocr. 1990, 80, 16–23.
- Xie, P.; Wan, X.P.; Bu, Z.; Diao, E.J.; Gong, D.Q.; Zou, X.T. Changes in hormone profiles, growth factors, and mRNA expression of the related receptors in crop tissue, relative organ weight, and serum biochemical parameters in the domestic pigeon (Columba livia) during incubation and chick-rearing periods under artificial farming conditions. Poult. Sci. 2018, 97, 2189–2202.
- Anderson, T.R.; Mayer, G.L.; Hebert, N.; Nicoll, C.S. Interactions among prolactin, epidermal growth factor, and proinsulin on the growth and morphology of the pigeon crop-sac mucosal epithelium in vivo. Endocrinology 1987, 120, 1258–1264.
- Tanaka, T.; Haneda, S.; Imakawa, K.; Sakai, S.; Nagaoka, K. A microRNA, miR-101a, controls mammary gland development by regulating cyclooxygenase-2 expression. Differentiation 2009, 77, 181–187.
- Dysin, A.P.; Barkova, O.Y.; Pozovnikova, M.V. The role of microRNAs in the mammary gland development, health, and function of cattle, goats, and sheep. Non-Coding RNA 2021, 7, 78.
- Wang, J.Q.; Zhou, H.T.; Hickford, J.G.H.; Hao, Z.Y.; Gong, H.; Hu, J.; Liu, X.; Li, S.B.; Shen, J.Y.; Ke, N.; et al. Identification and characterization of circular RNAs in mammary gland tissue from sheep at peak lactation and during the nonlactating period. J. Dairy Sci. 2021, 104, 2396–2409.
- Ge, P.; Ma, H.; Li, Y.; Ni, A.; Isa, A.M.; Wang, P.; Bian, S.; Shi, L.; Zong, Y.; Wang, Y.; et al. Identification of microRNA-Associated-ceRNA Networks Regulating Crop Milk Production in Pigeon (Columba livia). Genes 2020, 12, 39.
- Ma, H.; Ge, P.; Bian, S.; Li, Y.; Ni, A.; Zhang, R.; Wang, Y.; Zhao, J.; Zong, Y.; Yuan, J.; et al. miR-193-5p negatively regulates PIK3CD to promote crop fibrocyte proliferation in pigeon (Columba livia). Poult. Sci. 2023, 102, 102378.
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17.
- Rezaei, R.; Wu, Z.; Hou, Y.; Bazer, F.W.; Wu, G. Amino acids and mammary gland development: Nutritional implications for milk production and neonatal growth. J. Anim. Sci. Biotechnol. 2016, 7, 20.
- Sciascia, Q.L.; Pacheco, D.; Mccoard, S.A. 2015. Administration of exogenous growth hormone is associated with changes in plasma and intracellular mammary amino acid profiles and abundance of the mammary gland amino acid transporter SLC3A2 in mid-lactation dairy cows. PLoS ONE 2015, 10, e0134323.
- Xie, P.; Han, M.X.; Chen, W.X.; Wan, X.P.; Xu, Y.G.; Gong, D.Q. The profiling of amino acids in crop milk and plasma and mRNA abundance of amino acid transporters and enzymes related to amino acid synthesis in the crop tissue of male and female pigeons during incubation and chick-rearing periods. Poult. Sci. 2020, 99, 1628–1642.
- Apelo, S.I.; Singer, L.M.; Ray, W.K.; Helm, R.F.; Lin, X.Y.; Mcgilliard, M.L.; St-pierre, N.R.; Hanigan, M.D. Casein synthesis is independently and additively related to individual essential amino acid supply. J. Dairy Sci. 2014, 97, 2998–3005.
- Appuhamy, J.A.; Nayananjalie, W.A.; England, E.M.; Gerrard, D.E.; Akers, R.M.; Hanigan, M.D. Effects of AMP-activated protein kinase (AMPK) signaling and essential amino acids on mammalian target of rapamycin (mTOR) signaling and protein synthesis rates in mammary cells. J. Dairy Sci. 2014, 97, 419–429.
- Appuhamy, J.A.D.R.N.; Bell, A.L.; Nayananjalie, W.A.D.; Escobar, J.; Hanigan, M.D. Essential amino acids regulate both initiation and elongation of mRNA translation independent of insulin in MAC-T cells and bovine mammary tissue slices. J. Nutr. 2011, 141, 1209–1215.
- Chiu, M.; Tardito, S.; Barilli, A.; Bianchi, M.G.; Dall, A.V.; Bussolati, O. Glutamine stimulates mTORC1 independent of the cell content of essential amino acids. Amino Acids 2012, 43, 2561–2567.
- Dunlop, E.A.; Tee, A.R. Mammalian target of rapamycin complex 1: Signalling inputs, substrates and feedback mechanisms. Cell. Sig. 2009, 21, 827–835.
- Chen, M.J.; Fu, Z.; Jiang, S.G.; Wang, X.Q.; Yan, H.C.; Gao, C.Q. Targeted disruption of TORC1 retards young squab growth by inhibiting the synthesis of crop milk protein in breeding pigeon (Columba livia). Poult. Sci. 2020, 99, 416–422.
- Xie, W.Y.; Fu, Z.; Pan, N.X.; Yan, H.C.; Wang, X.Q.; Gao, C.Q. Leucine promotes the growth of squabs by increasing crop milk protein synthesis through the TOR signaling pathway in the domestic pigeon (Columba livia). Poult. Sci. 2019, 98, 5514–5524.
- Arriola Apelo, S.I.; Singer, L.M.; Lin, X.Y.; McGilliard, M.L.; St-Pierre, N.R.; Haniga, M.D. Isoleucine, leucine, methionine, and threonine effects on mammalian target of rapamycin signaling in mammary tissue. J. Dairy Sci. 2014, 97, 1047–1056.
- Chen, M.J.; Pan, N.X.; Wang, X.Q.; Yan, H.C.; Gao, C.Q. Methionine promotes crop milk protein synthesis through the JAK2-STAT5 signaling during lactation of domestic pigeons (Columba livia). Food Funct. 2020, 11, 10786–10798.
- Hu, X.C.; Gao, C.Q.; Wang, X.H.; Yan, H.C.; Chen, Z.S.; Wang, X.Q. Crop milk protein is synthesised following activation of the IRS1/Akt/TOR signalling pathway in the domestic pigeon (Columba livia). Br. Poult. Sci. 2016, 57, 855–862.
- Futter, C.E.; Felder, S.; Schlessinger, J.; Ullrich, A.; Hopkins, C.R. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J. Cell. Biol. 1993, 120, 77–83.
- Emans, N.; Gorvel, J.P.; Walter, C.; Gerke, V.; Kellner, R.; Griffiths, G.; Gruenberg, J. Annexin II is a major component of fusogenic endosomal vesicles. J. Cell. Biol. 1993, 120, 1357–1369.
- Horseman, N.D. A prolactin-inducible gene product which is a member of the Calpactin/Lipocortin family. Mol. Endocrinol. 1989, 3, 773–779.
- Cai, J.; Wang, D.M.; Zhao, F.Q.; Liang, S.L.; Liu, J.X. AMPK-mTOR pathway is involved in glucose-modulated amino acid sensing and utilization in the mammary glands of lactating goats. J. Anim. Sci. Biotechnol. 2020, 11, 32.
- Ma, H.; Bian, S.; Li, Y.; Ni, A.; Zhang, R.; Ge, P.; Han, P.; Wang, Y.; Zhao, J.; Zong, Y.; et al. Analyses of circRNAs profiles of the lactating and nonlactating crops in pigeon (Columba livia). Poult. Sci. 2023, 102, 102464.
- Ma, H.; Ni, A.; Ge, P.; Li, Y.; Shi, L.; Wang, P.; Fan, J.; Isa, A.M.; Sun, Y.; Chen, J. Analysis of Long Non-Coding RNAs and mRNAs Associated with Lactation in the Crop of Pigeons (Columba livia). Genes 2020, 11, 201.
- Zhao, W.S.; Hu, S.L.; Yu, K.; Wang, H.; Wang, W.; Loor, J.; Luo, J. Lipoprotein lipase, tissue expression and effects on genes related to fatty acid synthesis in goat mammary epithelial cells. Int. J. Mol. Sci. 2014, 15, 22757–22771.
- Rudolph, M.C.; McManaman, J.L.; Phang, T.; Russell, T.; Kominsky, D.J.; Serkova, N.J.; Stein, T.; Anderson, S.M.; Neville, M.C. Metabolic regulation in the lactating mammary gland: A lipid synthesizing machine. Physiol. Genom. 2007, 28, 323–336.
- Maningat, P.D.; Sen, P.; Rijnkels, M.; Sunehag, A.L.; Hadsell, D.L.; Bray, M.; Haymond, M.W. Gene expression in the human mammary epithelium during lactation: The milk fat globule transcriptome. Physiol. Genom. 2008, 37, 12–22.
- Garrison, M.M.; Scow, R.O. Effect of prolactin on lipoprotein lipase in crop sac and adipose tissue of pigeons. Am. J. Physiol. 1975, 228, 1542–1544.
- Clegg, R.A.; Barber, M.C.; Pooley, L.; Ernens, I.; Larondelle, Y.; Travers, M.T. Milk fat synthesis and secretion: Molecular and cellular aspects. Livest. Prod. Sci. 2001, 70, 3–14.
- Xie, P.; Wang, X.P.; Bu, Z.; Zou, X.T. Differential expression of fatty acid transporters and fatty acid synthesis-related genes in crop tissues of male and female pigeons (Columba livia domestica) during incubation and chick rearing. Br. Poult. Sci. 2017, 58, 594–602.
- Ohno, Y.; Suto, S.; Yamanaka, M.; Mizutani, Y.; Mitsutake, S.; Igarashi, Y.; Sassa, T.; Kihara, A. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18439–18444.
- Fielding, B.A.; Frayn, K.N. Lipoprotein lipase and the disposition of dietary fatty acids. Br. J. Nutr. 1998, 80, 495–502.
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366.
- Xie, P.; Zhu, J.G.; Wang, L.X.; Liu, Y.; Diao, E.J.; Gong, D.Q.; Liu, T.W. Lipid accumulation and oxidative stress in the crop tissues of male and female pigeons during incubation and chick-rearing periods. Poult. Sci. 2023, 102, 102289.
- Feng, X.; Cai, Z.; Mu, T.; Yu, B.; Wang, Y.; Ma, R.; Liu, J.; Wang, C.; Zhang, J.; Gu, Y. CircRNA screening and ceRNA network construction for milk fat metabolism in dairy cows. Front. Vet. Sci. 2022, 9, 995629.
- Bian, Y.; Lei, Y.; Wang, C.; Wang, J.; Wang, L.; Liu, L.; Liu, L.; Gao, X.; Li, Q. Epigenetic regulation of miR-29s affects the lactation activity of dairy cow mammary epithelial cells. J. Cell. Physiol. 2015, 230, 2152–2163.
- Braun, E.J.; Sweazea, K.L. Glucose regulation in birds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 1–9.
- Witteveen, M.; Brown, M.; Downs, C.T. Does sugar content matter? Blood plasma glucose levels in an occasional and a specialist avian nectarivore. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 167, 40–44.
- Zhu, J.G.; Xie, P.; Song, C.; Liu, T.W.; Gong, D.Q. Differential expression of glucose metabolism-related genes and AMP-activated protein kinases in crop tissue of male and female pigeons (Columba livia domestica) during the incubation and chick-rearing periods. J. Anim. Physiol. Anim. Nutr. 2023, 107, 680–690.
- Lin, S.C.; Hardie, D.G. AMPK: Sensing glucose as well as cellular energy status. Cell. Metab. 2018, 27, 299–313.
- Halse, R.; Fryer, L.G.; McCormack, J.G.; Carling, D.; Yeaman, S.J. Regulation of glycogen synthase by glucose and glycogen: A possible role for AMP-activated protein kinase. Diabetes 2003, 52, 9–15.
- Xie, P.; Zhu, J.G.; Liu, Y.; Liu, T.W.; Xu, Y.G.; Gong, D.Q. Effect of Akt activation on apoptosis-related gene expression in the crop tissues of male and female pigeons (Columba livia). Poult. Sci. 2021, 100, 101392.
- Luedde, T.; Schwabe, R.F. Nf-κB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118.
- Shoshan, B.Y.; De, S. Mitochondrial VDAC, the Na+/Ca2+ exchanger, and the Ca2+ uniporter in Ca2+ dynamics and signaling. Adv. Exp. Med. Biol. 2017, 9, 323–347.
- Rutkowski, D.T.; Kaufman, R.J. A trip to the ER: Coping with stress. Trends Cell Biol. 2004, 14, 20–28.
- Steffen, J.; Koehler, C.M. ER-mitochondria contacts: Actin dynamics at the ER control mitochondrial fission via calcium release. J. Cell. Biol. 2018, 217, 15–17.




