Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexandra Eleftheria Menni | -- | 4612 | 2023-09-28 10:10:14 | | | |
| 2 | Jason Zhu | Meta information modification | 4612 | 2023-10-07 04:39:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Menni, A.; Stavrou, G.; Tzikos, G.; Shrewsbury, A.D.; Kotzampassi, K. Endoscopic Salvage of Gastrointestinal Anastomosis Leaks. Encyclopedia. Available online: https://encyclopedia.pub/entry/49759 (accessed on 07 February 2026).
Menni A, Stavrou G, Tzikos G, Shrewsbury AD, Kotzampassi K. Endoscopic Salvage of Gastrointestinal Anastomosis Leaks. Encyclopedia. Available at: https://encyclopedia.pub/entry/49759. Accessed February 07, 2026.
Menni, Alexandra, George Stavrou, Georgios Tzikos, Anne D. Shrewsbury, Katerina Kotzampassi. "Endoscopic Salvage of Gastrointestinal Anastomosis Leaks" Encyclopedia, https://encyclopedia.pub/entry/49759 (accessed February 07, 2026).
Menni, A., Stavrou, G., Tzikos, G., Shrewsbury, A.D., & Kotzampassi, K. (2023, September 28). Endoscopic Salvage of Gastrointestinal Anastomosis Leaks. In Encyclopedia. https://encyclopedia.pub/entry/49759
Menni, Alexandra, et al. "Endoscopic Salvage of Gastrointestinal Anastomosis Leaks." Encyclopedia. Web. 28 September, 2023.
Copy Citation
Anastomotic leakage, which is defined as a defect in the integrity of a surgical join between two hollow viscera leading to communication between the intraluminal and extraluminal compartments, continues to be of high incidence and one of the most feared complications following gastrointestinal surgery, with a significant potential for a fatal outcome. Surgical options for management are limited and carry a high risk of morbidity and mortality; thus, surgeons are urged to look for alternative options which are minimally invasive, repeatable, non-operative, and do not require general anesthesia.
anastomotic leakage
tissue sealants
clips
stents
endoscopic vacuum therapy
1. Introduction
Anastomotic leakage is a dreaded complication after major surgery in hollow viscera of the gastrointestinal tract (GI), which is associated with prolonged stay in the intensive care unit and increased mortality [1]. It is defined as a pathological communication between intra- and extra-luminal compartments as a result of the dehiscence of the anastomotic suture line, which can occur in near proximity to surgery, usually on post-operative days 3 to 5 or in the later part of the first 3 weeks [1][2]. This anastomotic “full-thickness” defect often leads to leakage of the luminal contents into the peritoneal cavity or the mediastinum, possibly leading to sepsis and death, occurring in up to 60% of cases if treatment is delayed [1][3], and is thus responsible for the preponderance of surgical mortality [4][5][6].
Despite the advances in surgical techniques and the significant decrease in surgery-related mortality and morbidity of whatever etiology, anastomotic leakage still occurs in a significant number of patients [7]; the percentage varies depending on the type of oncological surgery. It occurs in 8% to 26% of patients after esophagectomy [5][8][9][10][11] and in 3% to 12% after gastrectomy [5][8][12][13], while in colon surgery the case occurrence is 5% to 15% following colorectal anastomosis [14][15], rising to 15% to 28% after low anterior resection in large cohorts [2][16][17][18][19].
In bariatric surgery, on the other hand, related leaks have been reported in only 0.6% to 5.25% of cases after a Roux-en-Y gastric bypass [20][21] and in 1% to 3.9% following sleeve gastrectomy [22][23][24][25][26].
Intra-abdominal or intrathoracic leaks are generally the most complex in relation to the extraperitoneal ones, with treatment options varying from the conservative to open surgery re-operation, depending on the patient’s clinical condition and their hemodynamic stability, the leak size, the anatomical site of leakage, the presence of a pus-filled cavity near the dehiscence, and the time elapsed since surgery [27][28].
Over the last 30 years, interventional endoscopy has evolved as an effective and less invasive alternative to surgery, progressively changing the management model for anastomotic leaks. Presently, a variety of techniques are available to reestablish the continuity of the hollow viscera in a less invasive manner, thus minimizing patient morbidity related to re-operation.
2. History of Endoscopic Sealing
The Cuffed Stent
Historically, the first successful endoscopic attempt to seal an esophago-tracheal defect was performed by Lux et al. [29] in the University of Erlangen in 1987 using a modification of the typical Wilson–Cook Medical esophageal silicone tube, a cuffed stent. This commercially available esophageal cuffed tube comprises a silicone shaft with a metal spiral wire embedded in its wall to strengthen it. The proximal and the distal ends, constructed from softer silicone rubber, are funnel shaped to prevent tube displacement. This tube is a standard silicone rubber Wilson–Cook medical tube with an internal diameter of 12 mm and length varying between 4.4 cm and 16.4 cm.
The modification consists of a balloon-type addition around the tube shaft. Indeed, the shaft is surrounded by a polyurethane foam, tightly air-sealed with a silicone rubber sheath, forming a cuff for the prosthesis. The foam shrinks when a vacuum is created in the cuffed portion by means of a syringe connected to a plastic fine-suction catheter, the end of which is impacted into the foam. When the cuff has totally shrunk, the tube’s outer diameter is only 2.6 cm. After insertion of the tube, by means of a suitable introducer, into the pre-measured position in the esophagus, which is the center of the cuff strictly against the center of the wall defect, the suction-induced vacuum is released; the self-inflating cuff fills with air through the fine catheter, allowing the foam rubber to expand to a final diameter of 4 cm, this being adequate to seal the anastomosis opening. The natural elasticity of the polyurethane foam allows its shape to conform to that of the esophagus without excessive pressure risking tissue necrosis. To further ensure that the cuff is fully expanded, it is possible to inject additional air with the syringe until pressure resistance is felt.
This modified tube cuffed stent has only been reported four times in the literature, in a total of 28 cases with malignant esophagogastric communication, since its production was officially discontinued in 1990 [29][30][31][32]. One such tube, from laboratory museum, is illustrated in Figure 1.
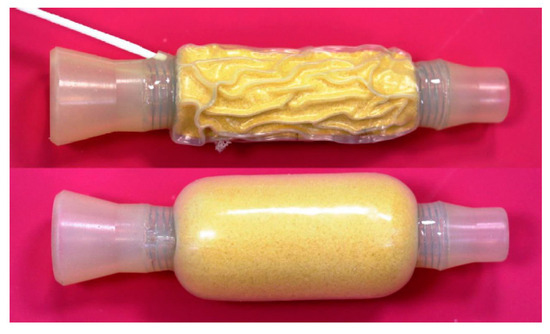
Figure 1. The Wilson–Cook cuffed stent.
3. Current Technology
3.1. Tissue Adhesives
One of the initial techniques used to cope with anastomotic leaks, usually less than 5 mm in length, was the use of adhesives, namely fibrin glue and cyanoacrylates.
The commercially available fibrin glue (Tisseel VH Fibrin sealant, Baxter AG, Vienna, Austria) consists of two frozen components: fibronectin, reconstituted with aprotinin, and thrombin, reconstituted with calcium chloride. They are delivered via a dual-barrel syringe and combined at the site of the anastomotic defect through a specially designed long double-lumen catheter inserted through the biopsy channel of the endoscope [33]; upon contact of the two components, thrombin converts fibronectin into fibrin, forming a stable clot within 10–60 s in a manner similar to that of the coagulation cascade. This fibrin clot initially acts like an acellular clot, the aprotinin component increasing its resistance to degradation in a fibrinolytic environment, while, within the next two weeks of application, it is fully reabsorbed progressively by macrophages and fibroblasts [33][34]. It is advised that fibrin glue be applied after a thorough debridement of the area and coating with normal saline [33][35], while others consider the fibrin clot to be most effective when applied to dry areas [3].
N-butyl-2-cyanoacrylate (Histoacryl; B. Braun, Melsungen, Germany) is a synthetic adhesive which is polymerized upon contact with damp surfaces [3]. This is why it is advised that, just before injection, both the catheter and the delivery syringe be flushed with 5% dextrose solution and that the catheter be as short as possible in order to avoid the premature polymerization of the glue within the catheter. For the same reason, both the biopsy channel and the distal part of the endoscope must be lubricated with silicon oil to prevent the permanent attachment of glue [33]. When cyanoacrylate comes in contact with the tissue, it initially generates a localized foreign body reaction, leading to an inflammatory response which promotes angiogenesis and, finally, tissue healing while the glue itself sloughs off spontaneously within the next five to ten days [33][34].
In cases of larger anastomotic defects, there are few reports of Vicryl meshes being used to create a backbone to keep the fibrin glue in place at the leak edges [36]. Fiber adhesives are also generally applied as a combination of adjunctive treatment after endo-sponge placement, before temporary stent placement, or as a complementary treatment after leak repair with clip placement [37].
3.2. Endoclips and Over-the-Scope Clips
Endoscopic clips, originally used in the context of an emergency hemostasis or for mucosal marking, were first used in 1990 in an effort to close gastric and colonic perforations, mainly iatrogenic ones [38]. However, their small size opening makes them incapable of successfully treating large mucosal defects since they grasp only the mucosal layer margins and exhibit weak closing force and limited mucosal tissue apposition [34][39]. Today, the newly available endoclips, also called through-the-scope clips (TTS), are fully rotatable and have a wingspan of 11 mm; thus, a success rate of 60 to 80% is reported [3][40], mainly in cases of upper GI tract perforations and esophageal defects, while in cases of inflammation and fibrosis of tissue around the perforation, their placement is a struggle due to their limited closure potential [28].
On the other hand, large clips that are loaded over the endoscope, over-the-scope clips (OTSC—Ovesco Endoscopy, AG, Tubingen, Germany), have become particularly popular for the closure of larger, full-thickness GI tract defects since their first appearance in 2007 [15][41] (Figure 2). OTSCs are full-thickness suturing devices, designed precisely for flexible endoscopes and made of biocompatible elastic shape-memory nitinol alloy; the clip, with its four prongs, allows continuous pressure to be applied to the grasped area so that even in cases of swelling and sinking of the grasped tissue, there is sufficient pressure to maintain tissue apposition. On the other hand, blood flow is maintained through the inter-prong space of the clip, thus enabling the OTSC to prevent tissue necrosis and allowing unimpeded wound healing.
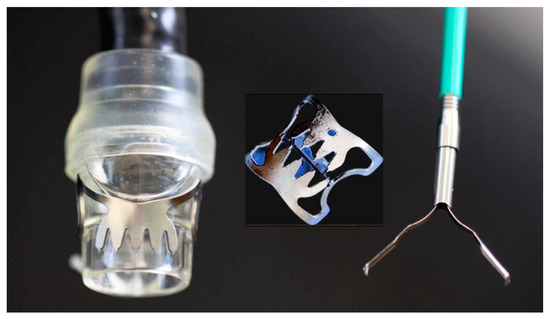
Figure 2. The TTS and OTSC clips.
The OTSC system consists of an applicator cap with a mounted nitinol clip, a hand wheel, and a thread retriever. The cap, which is attached to the tip of the endoscope in a manner similar to that of a band-ligation cap, comes in three sizes with respect to diameter, 11 mm, 12 mm, and 14 mm, to ensure proper attachment to the tip of endoscopes of different diameters. Caps are also available in two depths, 3 mm or 6 mm, related to the tissue grasping capacity appropriate to the thickness of the tissue to which they will be applied. In addition, there are three different clip shapes: pointed, which is used for perforation and fistula closure, as is ideal for inflammatory and fibrotic tissues; round, which facilitates tissue compression and is used for hemostasis, especially in the esophagus and colon; and a longer pointed clip, mainly used for the thicker stomach wall [42]. Of similar philosophy is the more recent hexagonal-shaped padlock clip (Steris, Mentor, OH, USA) with a full circumferential closing system [3][43].
There are also three additional devices facilitating clip application: (i) the three-pronged tissue anchor, which has three stretchable needles opening simultaneously to grasp a lesion, as in the cases of difficult chronic ulcers and fistulas [44]; (ii) the twin grasper, which grasps both sides of a lesion through the separate and alternative opening of its blades, thus facilitating tissue apposition; and (iii) the reloader, in case more than one clip is needed to close the gap [42]. An endoscope with a 3.2 mm working channel is recommended, while a double channel one may better facilitate the handling of additional devices [45].
For OTSC positioning, when the endoscope, having loaded the cup with the clip, is against and in touch with the defect, the surrounding tissue can be sufficiently aspirated within the cap and, by turning the hand wheel, the clip is deployed. If the entire defect can’t be suctioned within the cup, the tissue anchor or the twin grasper is also used [34][41].
3.3. Stents
The rationale for placing a temporary stent over a GI anastomotic leakage is to “seal” the defect and divert the contents of the lumen, thus enabling the defect to heal. Although there are many reported “side-effects” after a stent placement in cases of anastomotic leaks—the main being stent migration—these devices still remain an effective, safe, and easily applied therapeutic option, even when applied by an inexperienced endoscopist [46][47][48][49].
Although plastic stents were the first introduced in the 1990s for the repair of esophageal perforations [50], today they have been successfully replaced with the more flexible and more easily handled metal self-expandable stents. These are placed endoscopically over a guidewire, preferably under fluoroscopic control to ensure proper positioning, while frequent imaging monitoring is advised due to the high migration rate [49].
Metal stents are made of either Elgiloy, an alloy of cobalt, nickel, and chromium, or of Nitinol, an alloy of nickel and titanium [3]. Those stents, used for sealing defects, are either fully or partially covered by a polyurethane, polyethylene, or silicone rubber membrane, either along their full length or leaving uncovered the distal and proximal ends [3][34][49]. It is well understood that the use of a totally non-covered stent, although having the advantage of not migrating, is not finally a wise decision, since it is unable to seal the leakage and, additionally, the tissue overgrowth through the stent metal grid results in it rapidly becoming impacted in the mucosa and thus totally impossible to remove.
When totally covered stents are used, they must be secured in place, either by clips in at least two, diametrically opposite sites or by sutures [49][51] due to the high probability of migration [49]. On the other hand, partially covered stents embed in the mucosa at their proximal and distal ends, making migration difficult/less likely (Figure 3) but stent removal a little more difficult. Thus, in no case should such a stent be placed over an Endo-Vac sponge, as a prerequisite of this treatment is frequent (twice a week) stent removal to change the sponge.

Figure 3. A self-expandable metal stent.
Physicians should also always keep in mind that the digestive tract is not a geometric cylinder, so no matter how well the stent is placed, there will almost always be microleakages from the peripheral part, at least in the first few days [52]. Regarding colon anastomoses dehiscence, stents may be used only in end-to-end anastomoses; while in the case of an anastomosis after low anterior resection of the rectum, it is generally contra-indicated, since its peripheral end must be terminated at least 1 cm above the dentate line [53]. Additionally, prior drainage of any nearby collection is mandatory and, should sepsis occur, stent placement is strictly prohibited [54].
The use of stents has also proved to be particularly effective in bariatric surgery-related leakages. Specially designed stents are now available, such as the Mega Stent (Taewoong Medical, Seoul, Republic of Korea)—a fully covered stent of large diameter and length (18 cm to 24 cm) with a special design to avoid migration and increased elasticity. The Niti-S-Beta stent (Taewoong Medical, Seoul, Republic of Korea) is also a fully covered stent with a proximal flange and a double-bump in the proximal third to reduce the likelihood of migration [3].
3.4. Endoscopic Vacuum Therapy (EVT)
The technological knowledge and experience gained from the successful application of negative pressure therapy to treat open abdominal wounds and/or entero-atmospheric fistulas over the last 20 years, known as the vacuum-assisted closure technique [49][55], is easily transferred for the restoration of anastomotic dehiscence after GI surgery. This technique was first described by Nagell and Holte [56] in 2006 as vacuum-assisted closure for the treatment of anastomotic leakage after rectal resection by means of digitally inserting the sponge into the rectum and, through the defect of the anastomosis, into the presacral space (Figure 4). Two years later, Weidenhagen et al. presented their experience with 29 patients with anastomotic leakage after anterior resection of the rectum, and, in 2010, the first small series of six patients after esophageal resection [57][58].

Figure 4. A custom-made endo-vac.
In order to insert the Endo-Vac sponge—an open-pored polyurethane foam—into the peri-anastomotic cavity, an introducer “sleeve” is first advanced under endoscopic control until the entrance of the cavity. The introducer sleeve is fixed in this position, the endoscope is withdrawn, and the sponge attached to the evacuation tube—having already been cut to exactly fit the geometry of the cavity—is compressed and inserted into the introducer sleeve. A pusher is then used to advance and totally insert the sponge into the peri-anastomotic cavity; then, the introducer sleeve is finally withdrawn, leaving the sponge fully deployed in the cavity, with the peripheral end of the 14Fr evacuation tube exiting from the mouth and connected to the vacuum wound drainage system under a negative pressure of approximately 125–150 mmHg [34][57][58][59].
It is strongly advised, prior to starting treatment, that the cavity and the anastomosis defect orifice be thoroughly irrigated and debrided using every available instrument, from standard biopsy forceps to an over-the-scope grasper and a cytology brush [59]. Continuous negative pressure reduces tissue swelling, promoting microcirculation and leading to the formation of granulation tissue and bacterial clearance [60], but frequent sponge changes, every two to three days, are strictly required [61].
Finally, the use of a self-expandable metal stent placed over an Endo-Vac sponge—named “the stent-over-sponge [SOS] approach”—seems to be another suitable therapeutic option for treating leaks [62]. The stent optimizes the vacuum force by sealing the sponge toward the GI lumen, thus maximizing the suction efficacy while avoiding dislodgement from the correct position [63][64]. However, the sponge should be replaced with a new one every three to five days in order to prevent the ingrowth of granulation tissue, and thus the stent also needs to be removed and replaced, significantly affecting the cost of treatment [64].
3.5. Endoscopic Internal Drainage (EID)
Fluid or pus collection outside the anastomosis defect remains a serious problem for uneventful healing, leading sometimes to peritonitis or mediastinitis or even sepsis, especially when there is insufficient drainage of this “cavity” through an external drainage tube placed at the time of operation. Although this cavity is a closed space among the surrounding viscera, formed mainly by granulomatous inflammatory tissues and pseudo-membranes and communicating only with the lumen of the GI tract through the existing leakage orifice [34], it must be emptied, since its occlusion may result in pus formation and possible sepsis. Pequignot et al. [65], in 2012, were the first to attempt the insertion of a double pigtail stent, or a naso-biliary drain, through the leak orifice into the “cavity” in order to drain fluid/purulent contents into the gut lumen. (Figure 5).

Figure 5. A pig-tail stent.
Whatever the technique used for dehiscence, this extra-luminal cavity must be inspected carefully, washed-out as far as possible, and every effort must be made to drain internally, towards the lumen, or externally, through the existing drainage tube fistulus tract. According to the endoscopic internal drainage technique, the cavity is catheterized with a straight catheter and a guidewire over which one or more final single- or double-pigtail catheters—depending on the cavity size—are placed [28], changes of which need to be carried out every three weeks [66]. By such means, tissue granulation and re-epithelialization occurs [28][67], progressively closing this dead space. Additionally, a naso-duodenal feeding tube may be inserted into the third part of the duodenum to ensure adequate intestinal feeding for at least the first four weeks, while, in the case of a heavily purulent collection, a nasobiliary tube should be inserted into the cavity to ensure the interchange of irrigation and drainage procedures.
In cases of large defects, the pigtail catheter should be combined with a simultaneous stent placement. In this way, the anastomosis opening is sealed with the stent while at the same time the collection is drained towards the lumen by the use of the pigtail.
3.6. Vac-Stent Technique
The Vac-Stent technique is a relatively new one (2019), combining the advantages of the self-expandable covered metal stent, for the coverage of the anastomotic defect, with those of improving wound healing through the endoscopic vacuum technique (EVT) in one medical device, thus optimizing the suction efficacy for sealing the leak and keeping the stent in position while maintaining intestinal passage.
It would be considered a transformation or an evolution of the previous described technique of “SOS” (stent-over-sponge) [63][64] but for the significant difference that the vacuum sponge—the Endo-Vac device—applied in the former is placed within the peri-anastomotic cavity while the present device—the Vac-stent—remains within the GI lumen, making application much easier.
The VACStent® (VAC Stent Medtec AG, Steinhausen, Switzerland) is 7 mm long with a diameter of 14 mm in the center and 30 mm at the flare ends. The device consists of a self-expandable stent (Micro-Tech Co. Ltd. Nanjing, Republic of China) covered with a 50 mm long open-pore cylindrical polyurethane foam (10 mm thick) fixed to the outer layer of the stent and connected via a catheter to a vacuum source (Möller Medical GmbH Fulda, Germany). The VACStent® is made of nitinol wire and is fully covered with a silicone–parylene layer to prevent tissue ingrowth and seal the sponge toward the esophageal lumen [68].
At present, the Vac-Stent is indicated only for the treatment of esophageal leaks which can be reached endoscopically. Using the vacuum stent enables drainage of inflammatory wound secretions by means of negative-pressure wound therapy and sealing of the leak through the liquid-tight coated stent, taking preservation of the passage into account.
A continuous suction of between 40 and a maximum of 125 mmHg is applied to the sponge, keeping it in place due to suction, even in difficult sites of the GI tract, while it allows for the direct passage of endoscopic instruments, enteral nutrition, and intestinal contents [28][68][69].
The Vac-Stent device is placed over a guidewire inserted through the working channel of the endoscope under fluoroscopic or endoscopic guidance in order to center the body of the stent and, subsequently, the sponge on the defect, which is then deployed via a distal release system. Finally, the suction catheter attached to the sponge is guided through the nose and connected to the vacuum negative-pressure system, which should be flushed with water three times per day in order to keep the suction catheter open. Depending on the size of the contact with the wound, the manufacturers recommend removing the vacuum stent after two to seven days and replacing it with a new system. If the wound contact covers a large area, the system should be changed after a maximum of 72 h [70]. Removal is facilitated by a tapered hood distal attachment cap, or simply by means of endoscopic foreign body grasping forceps. To prevent the edge of the wound from tearing open again, it is recommended that the stent be rinsed prior to extraction in order to more effectively remove any adhering dressing (see instructions for use, 2023, on www.vac-stent.com, accessed on 1 May 2023).
The Vac-Stent and similar devices are indicated for defects of approximately 30 mm, with definite contraindications being defects with diameters of more than 50 mm, leaks within a distance of less than 20 mm from the upper esophageal sphincter, and a contaminated extraluminal cavity. However, further studies are required to establish it in the armamentarium of devices for the salvage of anastomotic leaks.
3.7. The Suturing System
The idea of designing a suture machine small enough to be loaded onto the tip of an endoscope is at least 20 years old. Perhaps the first successful attempt was that of the BARD EndoCinch-I endoscopic suturing system, used for endoluminal gastroplication as an alternative to surgical fundoplication in patients with gastroesophageal reflux disease [71], which was quickly replaced with the newly designed ESD [72], both devices being able to perform plications involving only gastric mucosa. Following these, several other attempts were made, focusing on full-thickness suturing; the majority of these, however, have revealed major limitations mitigating against their widespread clinical use.
The OverStitch system (Apollo Endosurgery, Austin, TX, USA) was first developed in 2009 and is currently the most common endoscopic suturing device [73][74]. The first version of this device could only be loaded onto an Olympus dual-channel therapeutic endoscope; however, the next generation, the newly introduced Over-Stitch SX, can be mounted on any channel endoscope, enabling single-operator surgical suturing.
The main components of this suturing platform are: the needle driver handle, attached to the endoscope controls, the cap with the metallic needle, mounted on tip of the endoscope, and an anchor exchange catheter. Grasping forceps and a tissue retracting helix device may be used to help tissue apposition, while a specially designed non-absorbable suture accompanying the device is used for full-thickness uninterrupted or continuous suturing [3][28][75][76].
However, its relatively large size and its reduced maneuverability have made its use challenging in the narrow or angulated GI areas such as the gastric fundus, the duodenum, and the sigmoid colon. To date, it has been effectively used mainly for closure of mucosal defects after endoscopic resections, transoral outlet reduction after bariatric surgery, and in stent fixation in order to prevent migration. However, there are few studies explicitly evaluating the role/use of OverStitch in primary closure of GI leaks and fistula, mainly in stapler line leaks after bariatric surgery. Therefore, no conclusions or recommendations can be drawn for this indication [77][78][79].
It is of great importance to emphasize that robust and healthy mucosa is essential to hold the sutures when tissues are approximated and that before attempting an endoscopic closure it is paramount that the involving tissues be de-epithelialized in order to guarantee a reliable closure. Thus, various techniques such as coagulation of the defect perimeter, mechanical abrasion of the fistula tract, modified endoscopic submucosal dissection to completely ablate the mucosa, or multiple endoscopic mucosal resections around the fistula opening have been reported as a “must” [80][81].
Although the results from the OverStitch device seem encouraging, it remains a complex procedure and a high level of expertise and proper training is required for its operation, limiting its use to a few tertiary centers.
4. The Possible Future
4.1. Stem Cells
Just two years ago, the Costamagna group in collaboration with thoracic surgeons [82] presented a case series of successfully treated difficult esophageal fistulas after initial failures to respond to other endoscopic or surgical treatments. A stromal vascular fraction obtained after mechanical emulsification of autologous adipose tissue, known as tSVFem, was endoscopically injected with the objective of exploiting its regenerative capacity for fistula closure—a technique being widely used in other medical fields, mainly plastic surgery.
The tSVFem comprises mesenchymal stromal cells and fragments of the extracellular matrix obtained after proper harvesting of fat obtained from the superficial layer of subcutaneous tissue as well as from oil released after the mechanical disruption of mature adipocytes. By means of endoscopy, 10 mL of fat was initially injected to completely fill the fistula and, thereafter, 1–2 mL of tSVFem was injected into the submucosa of the 4 quadrants of the fistula borders to obliterate it completely. Seven days later, endoscopy revealed complete healing of the fistula [82][83].
The same technique has also been applied for successful closure of a gastro-bronchial fistula after a 25 mm leak at a laparoscopic sleeve gastrectomy suture line, followed by a huge fluid collection in the left subphrenic area, communicated with another intrapulmonary collection [84].
These initial references to using autologous stem cell transplantation seem promising, since there is already knowledge and experience of the technique, even in other fields of reconstructive and regenerative medicine.
4.2. Modification of Luminal Microbiome
Over the last decade, Alverdy’s laboratory group have undertaken in-depth research on the mechanisms of low anterior anastomosis dehiscence beyond the broadly believed etiologies of tissue ischemia, anastomotic tension, malnutrition, hypo-albuminaemia, etc. There have been multiple confirmations, experimentally and clinically, that a low microbial diversity within the gut lumen allows the overgrowth of mucin-degrading members of the Bacteroidaceae and Lachnospiraceae families. Pathogens such as Pseudomonas aeruginosa, Enterococcus faecalis, and Serratia marcescens, with their capacity to proliferate when the microbiota become depleted, can produce collagenase and elicit intestinal inflammation, leading to anastomotic leaks [85][86]. It has also been found experimentally that anastomotic dehiscence develops when Pseudomonas aeruginosa, normally colonizing anastomotic sites, becomes transformed in vivo after a single nucleotide polymorphism (SNP) mutation to express a tissue-destroying, more virulent phenotype [86]. Similarly, they demonstrate that anastomotic dehiscence sites in rats were colonized by Enterococcus faecalis strains, which exhibited an increased collagen-degrading activity and an increased ability to activate host MMP9 through the expression of the gelE and sprE genes, both of which contributed to anastomotic leakage; elimination of E. faecalis strains through topical application of proper antibiotics or pharmacological suppression of intestinal MMP9 activation prevents anastomotic leaks in rats [85].
Although recent work has revealed that, in humans, the presence of collagenolytic bacteria is the most deterministic cause, alone it is not sufficient to cause anastomotic leakage [87].
However, taken together, various other interventions performed in colon surgery-subjected humans, such as mechanical bowel preparation, oral and intravenous antibiotics, inotropes, opioid analgesics, and even the presence of diabetes mellitus, could have a negative influence on the microbiome [88].
On the other hand, looking at the beneficial effects of probiotics in enhancing epithelial barrier function, preventing systematic infections, reducing surgical site infections and all surgery-related complications, and in improving wound healing [88][89][90][91][92][93], a promising approach might be the “dietary” manipulation of patients prior to surgery by means of boosting their gut with beneficial probiotic species whose immune-modulatory, anti-inflammatory, and healing properties are well-documented [94][95].
References
- Kähler, G. Anastomotic Leakage after Upper Gastrointestinal Surgery: Endoscopic Treatment. Visc. Med. 2017, 33, 202–206.
- Munshi, E.; Dahlbäck, C.; Johansson, S.; Lydrup, M.L.; Jutesten, H.; Buchwald, P. Long-term Outcomes of Endoscopic Vacuum Therapy and Transanal Drainage for Anastomotic Leakage After Anterior Resection. Vivo 2022, 36, 2275–2278.
- Cereatti, F.; Grassia, R.; Drago, A.; Conti, C.B.; Donatelli, G. Endoscopic management of gastrointestinal leaks and fistulae: What option do we have? World J. Gastroenterol. 2020, 26, 4198–4217.
- Rutegård, M.; Lagergren, P.; Rouvelas, I.; Lagergren, J. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: A population-based study. Ann. Surg. Oncol. 2012, 19, 99–103.
- Lang, H.; Piso, P.; Stukenborg, C.; Raab, R.; Jähne, J. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur. J. Surg. Oncol. 2000, 26, 168–171.
- Messager, M.; Warlaumont, M.; Renaud, F.; Marin, H.; Branche, J.; Piessen, G.; Mariette, C. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur. J. Surg. Oncol. 2017, 43, 258–269.
- Scognamiglio, P.; Reeh, M.; Melling, N.; Kantowski, M.; Eichelmann, A.-K.; Chon, S.-H.; El-Sourani, N.; Schön, G.; Höller, A.; Izbicki, J.R.; et al. Management of intra-thoracic anastomotic leakages after esophagectomy: Updated systematic review and meta-analysis of endoscopic vacuum therapy versus stenting. BMC Surg. 2022, 22, 309.
- Blencowe, N.S.; Strong, S.; Mcnair, A.G.; Brookes, S.T.; Crosby, T.; Griffin, S.M.; Blazeby, J.M. Reporting of short-term clinical outcomes after esophagectomy: A systematic review. Ann. Surg. 2012, 255, 658–666.
- Kuppusamy, M.K.; Low, D.E. Evaluation of International Contemporary Operative Outcomes and Management Trends Associated With Esophagectomy: A 4-Year Study of >6000 Patients Using ECCG Definitions and the Online Esodata Database. Ann. Surg. 2022, 275, 515–525.
- Aiolfi, A.; Asti, E.; Rausa, E.; Bonavina, G.; Bonitta, G.; Bonavina, L. Use of C-reactive protein for the early prediction of anastomotic leak after esophagectomy: Systematic review and Bayesian meta-analysis. PLoS ONE 2018, 13, e0209272.
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking Complications Associated with Esophagectomy. Ann. Surg. 2019, 269, 291–298.
- Watanabe, M.; Miyata, H.; Gotoh, M.; Baba, H.; Kimura, W.; Tomita, N.; Nakagoe, T.; Shimada, M.; Kitagawa, Y.; Sugihara, K.; et al. Total gastrectomy risk model: Data from 20,011 Japanese patients in a nationwide internet-based database. Ann. Surg. 2014, 260, 1034–1039.
- Van Der Werf, L.R.; Busweiler, L.a.D.; Van Sandick, J.W.; Van Berge Henegouwen, M.I.; Wijnhoven, B.P.L. Reporting National Outcomes After Esophagectomy and Gastrectomy According to the Esophageal Complications Consensus Group (ECCG). Ann. Surg. 2020, 271, 1095–1101.
- Vermeer, T.A.; Orsini, R.G.; Daams, F.; Nieuwenhuijzen, G.A.; Rutten, H.J. Anastomotic leakage and presacral abscess formation after locally advanced rectal cancer surgery: Incidence, risk factors and treatment. Eur. J. Surg. Oncol. 2014, 40, 1502–1509.
- Clifford, R.E.; Fowler, H.; Govindarajah, N.; Vimalachandran, D.; Sutton, P.A. Early anastomotic complications in colorectal surgery: A systematic review of techniques for endoscopic salvage. Surg. Endosc. 2019, 33, 1049–1065.
- Yu, J.H.; Huang, X.W.; Song, Y.C.; Lin, H.Z.; Zheng, F.W. Analysis of Prevention and Treatment of Anastomotic Leakage after Sphincter-Preserving Surgery for Middle- and Low-Grade Rectal Cancer under Laparoscopy. Int. J. Clin. Pract. 2022, 2022, 6231880.
- Penna, M.; Hompes, R.; Arnold, S.; Wynn, G.; Austin, R.; Warusavitarne, J.; Moran, B.; Hanna, G.B.; Mortensen, N.J.; Tekkis, P.P. Incidence and Risk Factors for Anastomotic Failure in 1594 Patients Treated by Transanal Total Mesorectal Excision: Results From the International TaTME Registry. Ann. Surg. 2019, 269, 700–711.
- Okoshi, K.; Masano, Y.; Hasegawa, S.; Hida, K.; Kawada, K.; Nomura, A.; Kawamura, J.; Nagayama, S.; Yoshimura, T.; Sakai, Y. Efficacy of transanal drainage for anastomotic leakage after laparoscopic low anterior resection of the rectum. Asian J. Endosc. Surg. 2013, 6, 90–95.
- Yang, L.; Huang, X.E.; Zhou, J.N. Risk assessment on anastomotic leakage after rectal cancer surgery: An analysis of 753 patients. Asian Pac. J. Cancer Prev. 2013, 14, 4447–4453.
- Marshall, J.S.; Srivastava, A.; Gupta, S.K.; Rossi, T.R.; Debord, J.R. Roux-en-Y gastric bypass leak complications. Arch. Surg. 2003, 138, 520–523; discussion 523–524.
- Csendes, A.; Burgos, A.M.; Braghetto, I. Classification and management of leaks after gastric bypass for patients with morbid obesity: A prospective study of 60 patients. Obes. Surg. 2012, 22, 855–862.
- Morales, M.P.; Miedema, B.W.; Scott, J.S.; De La Torre, R.A. Management of postsurgical leaks in the bariatric patient. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 295–304.
- Gonzalez, R.; Sarr, M.G.; Smith, C.D.; Baghai, M.; Kendrick, M.; Szomstein, S.; Rosenthal, R.; Murr, M.M. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J. Am. Coll. Surg. 2007, 204, 47–55.
- Abou Rached, A.; Basile, M.; El Masri, H. Gastric leaks post sleeve gastrectomy: Review of its prevention and management. World J. Gastroenterol. 2014, 20, 13904–13910.
- Rosenthal, R.J.; Diaz, A.A.; Arvidsson, D.; Baker, R.S.; Basso, N.; Bellanger, D.; Boza, C.; El Mourad, H.; France, M.; Gagner, M.; et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: Best practice guidelines based on experience of >12,000 cases. Surg. Obes. Relat. Dis. 2012, 8, 8–19.
- Bashah, M.; Khidir, N.; El-Matbouly, M. Management of leak after sleeve gastrectomy: Outcomes of 73 cases, treatment algorithm and predictors of resolution. Obes. Surg. 2020, 30, 515–520.
- Mandarino, F.V.; Barchi, A.; Fanti, L.; D’amico, F.; Azzolini, F.; Esposito, D.; Biamonte, P.; Lauri, G.; Danese, S. Endoscopic vacuum therapy for post-esophagectomy anastomotic dehiscence as rescue treatment: A single center case series. Esophagus 2022, 19, 417–425.
- Binda, C.; Jung, C.F.M.; Fabbri, S.; Giuffrida, P.; Sbrancia, M.; Coluccio, C.; Gibiino, G.; Fabbri, C. Endoscopic Management of Postoperative Esophageal and Upper GI Defects-A Narrative Review. Medicina 2023, 59, 136.
- Lux, G.; Wilson, D.; Wilson, J.; Demling, L. A cuffed tube for the treatment of oesophago-bronchial fistulae. Endoscopy 1987, 19, 28–30.
- Irving, J.D.; Simson, J.N. A new cuffed oesophageal prosthesis for the management of malignant oesophago-respiratory fistula. Ann. R. Coll. Surg. Engl. 1988, 70, 13–15.
- Hordijk, M.L.; Dees, J.; Van Blankenstein, M. The management of malignant esophago-respiratory fistulas with a cuffed prosthesis. Endoscopy 1990, 22, 241–244.
- Sargeant, I.R.; Thorpe, S.; Bown, S.G. Cuffed esophageal prosthesis: A useful device in desperate situations in esophageal malignancy. Gastrointest. Endosc. 1992, 38, 669–675.
- Kotzampassi, K.; Eleftheriadis, E. Tissue sealants in endoscopic applications for anastomotic leakage during a 25-year period. Surgery 2015, 157, 79–86.
- Shehab, H. Endoscopic management of postsurgical leaks. Int. J. Gastrointest. Interv. 2016, 5, 6–14.
- Hua, F.; Sun, D.; Zhao, X.; Song, X.; Yang, W. Update on therapeutic strategy for esophageal anastomotic leak: A systematic literature review. Thorac. Cancer 2023, 14, 339–347.
- Truong, S.; Böhm, G.; Klinge, U.; Stumpf, M.; Schumpelick, V. Results after endoscopic treatment of postoperative upper gastrointestinal fistulas and leaks using combined Vicryl plug and fibrin glue. Surg. Endosc. 2004, 18, 1105–1108.
- Blumetti, J.; Abcarian, H. Management of low colorectal anastomotic leak: Preserving the anastomosis. World J. Gastrointest. Surg. 2015, 7, 378–383.
- Binmoeller, K.F.; Grimm, H.; Soehendra, N. Endoscopic closure of a perforation using metallic clips after snare excision of a gastric leiomyoma. Gastrointest. Endosc. 1993, 39, 172–174.
- Li, C.; Zhao, Y.; Han, Z.; Zhou, Y. Anastomotic leaks following gastrointestinal surgery: Updates on diagnosis and interventions. Int. J. Clin. Exp. Med. 2016, 9, 7031–7040.
- Cho, S.B.; Lee, W.S.; Joo, Y.E.; Kim, H.R.; Park, S.W.; Park, C.H.; Kim, H.S.; Choi, S.K.; Rew, J.S. Therapeutic options for iatrogenic colon perforation: Feasibility of endoscopic clip closure and predictors of the need for early surgery. Surg. Endosc. 2012, 26, 473–479.
- Al Ghossaini, N.; Lucidarme, D.; Bulois, P. Endoscopic treatment of iatrogenic gastrointestinal perforations: An overview. Dig. Liver Dis. 2014, 46, 195–203.
- Banerjee, S.; Barth, B.A.; Bhat, Y.M.; Desilets, D.J.; Gottlieb, K.T.; Maple, J.T.; Pfau, P.R.; Pleskow, D.K.; Siddiqui, U.D.; Tokar, J.L.; et al. Endoscopic closure devices. Gastrointest. Endosc. 2012, 76, 244–251.
- Goenka, M.K.; Rodge, G.A.; Tiwary, I.K. Endoscopic Management with a Novel Over-The-Scope Padlock Clip System. Clin. Endosc. 2019, 52, 574–580.
- Von Renteln, D.; Denzer, U.W.; Schachschal, G.; Anders, M.; Groth, S.; Rösch, T. Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos). Gastrointest. Endosc. 2010, 72, 1289–1296.
- Kobara, H.; Mori, H.; Nishiyama, N.; Fujihara, S.; Okano, K.; Suzuki, Y.; Masaki, T. Over-the-scope clip system: A review of 1517 cases over 9 years. J. Gastroenterol. Hepatol. 2019, 34, 22–30.
- D’alessandro, A.; Galasso, G.; Zito, F.P.; Giardiello, C.; Cereatti, F.; Arienzo, R.; Pacini, F.; Chevallier, J.-M.; Donatelli, G. Role of Endoscopic Internal Drainage in Treating Gastro-Bronchial and Gastro-Colic Fistula After Sleeve Gastrectomy. Obes. Surg. 2022, 32, 342–348.
- Keller, D.S.; Talboom, K.; Van Helsdingen, C.P.M.; Hompes, R. Treatment Modalities for Anastomotic Leakage in Rectal Cancer Surgery. Clin. Colon Rectal Surg. 2021, 34, 431–438.
- Evrard, S.; Le Moine, O.; Lazaraki, G.; Dormann, A.; El Nakadi, I.; Devière, J. Self-expanding plastic stents for benign esophageal lesions. Gastrointest. Endosc. 2004, 60, 894–900.
- Gelbmann, C.M.; Ratiu, N.L.; Rath, H.C.; Rogler, G.; Lock, G.; Schölmerich, J.; Kullmann, F. Use of self-expandable plastic stents for the treatment of esophageal perforations and symptomatic anastomotic leaks. Endoscopy 2004, 36, 695–699.
- Symonds, C.J. The Treatment of Malignant Stricture of the Œsophagus by Tubage or Permanent Catheterism. Br. Med. J. 1887, 1, 870–873.
- Vanbiervliet, G.; Filippi, J.; Karimdjee, B.S.; Venissac, N.; Iannelli, A.; Rahili, A.; Benizri, E.; Pop, D.; Staccini, P.; Tran, A.; et al. The role of clips in preventing migration of fully covered metallic esophageal stents: A pilot comparative study. Surg. Endosc. 2012, 26, 53–59.
- Winder, J.S.; Pauli, E.M. Comprehensive management of full-thickness luminal defects: The next frontier of gastrointestinal endoscopy. World J. Gastrointest. Endosc. 2015, 7, 758–768.
- Tsai, Y.Y.; Chen, W.T. Management of anastomotic leakage after rectal surgery: A review article. J. Gastrointest. Oncol. 2019, 10, 1229–1237.
- Venezia, L.; Michielan, A.; Condino, G.; Sinagra, E.; Stasi, E.; Galeazzi, M.; Fabbri, C.; Anderloni, A. Feasibility and safety of self-expandable metal stent in nonmalignant disease of the lower gastrointestinal tract. World J. Gastrointest. Endosc. 2020, 12, 60–71.
- Erdmann, D.; Drye, C.; Heller, L.; Wong, M.S.; Levin, S.L. Abdominal wall defect and enterocutaneous fistula treatment with the Vacuum-Assisted Closure (V.A.C.) system. Plast. Reconstr. Surg. 2001, 108, 2066–2068.
- Nagell, C.F.; Holte, K. Treatment of anastomotic leakage after rectal resection with transrectal vacuum-assisted drainage (VAC). A method for rapid control of pelvic sepsis and healing. Int. J. Color. Dis. 2006, 21, 657–660.
- Weidenhagen, R.; Gruetzner, K.U.; Wiecken, T.; Spelsberg, F.; Jauch, K.W. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: A new method. Surg. Endosc. 2008, 22, 1818–1825.
- Weidenhagen, R.; Hartl, W.H.; Gruetzner, K.U.; Eichhorn, M.E.; Spelsberg, F.; Jauch, K.W. Anastomotic leakage after esophageal resection: New treatment options by endoluminal vacuum therapy. Ann. Thorac. Surg. 2010, 90, 1674–1681.
- Reimer, S.; Seyfried, F.; Flemming, S.; Brand, M.; Weich, A.; Widder, A.; Plaßmeier, L.; Kraus, P.; Döring, A.; Hering, I.; et al. Evolution of endoscopic vacuum therapy for upper gastrointestinal leakage over a 10-year period: A quality improvement study. Surg. Endosc. 2022, 36, 9169–9178.
- Krokowicz, L.; Borejsza-Wysocki, M.; Mackiewicz, J.; Iqbal, A.; Drews, M. 10 years of Negative Pressure Wound Therapy (NPWT): Evolution of Indications for its Use. Negat. Press. Wound Ther. 2014, 1, 27–32.
- Weidenhagen, R.; Gruetzner, K.U.; Wiecken, T.; Spelsberg, F.; Jauch, K.W. Endoluminal vacuum therapy for the treatment of anastomotic leakage after anterior rectal resection. Rozhl. Chir. 2008, 87, 397–402.
- Gubler, C.; Schneider, P.M.; Bauerfeind, P. Complex anastomotic leaks following esophageal resections: The new stent over sponge (SOS) approach. Dis. Esophagus 2013, 26, 598–602.
- Bartella, I.; Mallmann, C.; Bürger, M.; Toex, U.; Goeser, T.; Bruns, C.; Chon, S.H. Stent-over-sponge (SOS): A rescue option in patients with complex postoperative anastomotic leaks after esophagectomy. Endoscopy 2019, 51, E227–E228.
- Morell, B.; Murray, F.; Vetter, D.; Bueter, M.; Gubler, C. Endoscopic vacuum therapy (EVT) for early infradiaphragmal leakage after bariatric surgery-outcomes of six consecutive cases in a single institution. Langenbecks Arch. Surg. 2019, 404, 115–121.
- Pequignot, A.; Fuks, D.; Verhaeghe, P.; Dhahri, A.; Brehant, O.; Bartoli, E.; Delcenserie, R.; Yzet, T.; Regimbeau, J.M. Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleeve gastrectomy? Obes. Surg. 2012, 22, 712–720.
- Donatelli, G.; Dumont, J.L.; Cereatti, F.; Ferretti, S.; Vergeau, B.M.; Tuszynski, T.; Pourcher, G.; Tranchart, H.; Mariani, P.; Meduri, A.; et al. Treatment of Leaks Following Sleeve Gastrectomy by Endoscopic Internal Drainage (EID). Obes. Surg. 2015, 25, 1293–1301.
- Gonzalez, J.M.; Lorenzo, D.; Guilbaud, T.; Bège, T.; Barthet, M. Internal endoscopic drainage as first line or second line treatment in case of postsleeve gastrectomy fistulas. Endosc. Int. Open 2018, 6, E745–E750.
- Chon, S.H.; Scherdel, J.; Rieck, I.; Lorenz, F.; Dratsch, T.; Kleinert, R.; Gebauer, F.; Fuchs, H.F.; Goeser, T.; Bruns, C.J. A new hybrid stent using endoscopic vacuum therapy in treating esophageal leaks: A prospective single-center experience of its safety and feasibility with mid-term follow-up. Dis. Esophagus 2022, 35, doab067.
- Chon, S.H.; Töx, U.; Lorenz, F.; Rieck, I.; Wagner, B.J.; Kleinert, R.; Fuchs, H.F.; Goeser, T.; Quaas, A.; Bruns, C.J. A Novel Hybrid Stent with Endoscopic Vacuum Therapy for Treating Leaks of the Upper Gastrointestinal Tract. Visc. Med. 2021, 37, 403–409.
- Pattynama, L.M.D.; Eshuis, W.J.; Van Berge Henegouwen, M.I.; Bergman, J.; Pouw, R.E. Vacuum-stent: A combination of endoscopic vacuum therapy and an intraluminal stent for treatment of esophageal transmural defects. Front. Surg. 2023, 10, 1145984.
- Mahmood, Z.; Mcmahon, B.P.; Arfin, Q.; Byrne, P.J.; Reynolds, J.V.; Murphy, E.M.; Weir, D.G. Endocinch therapy for gastro-oesophageal reflux disease: A one year prospective follow up. Gut 2003, 52, 34–39.
- Schiefke, I.; Neumann, S.; Zabel-Langhennig, A.; Moessner, J.; Caca, K. Use of an Endoscopic Suturing Device (the ”ESD”) to Treat Patients with Gastroesophageal Reflux Disease, After Unsuccessful EndoCinch Endoluminal Gastroplication: Another Failure. Endoscopy 2005, 37, 700–705.
- Moran, E.A.; Gostout, C.J.; Bingener, J. Preliminary performance of a flexible cap and catheter-based endoscopic suturing system. Gastrointest. Endosc. 2009, 69, 1375–1383.
- Muniraj, T.; Harry, R. The use of OverStitchTM for the treatment of intestinal perforation, fistulas and leaks. Int. J. Gastrointest. Interv. 2017, 6, 151–156.
- Zhang, L.Y.; Bejjani, M.; Ghandour, B.; Khashab, M.A. Endoscopic through-the-scope suturing. VideoGIE 2022, 7, 46–51.
- Bi, D.; Zhang, L.Y.; Alqaisieh, M.; Shrigiriwar, A.; Farha, J.; Mahmoud, T.; Akiki, K.; Almario Jose, A.; Shah-Khan, S.M.; Gordon, S.R.; et al. Novel Through-the-Scope Suture Closure of Colonic Endoscopic Mucosal Resection Defects. Gastrointest. Endosc. 2023, 98, 122–129.
- Chon, S.H.; Toex, U.; Plum, P.S.; Kleinert, R.; Bruns, C.J.; Goeser, T.; Berlth, F. Efficacy and feasibility of OverStitch suturing of leaks in the upper gastrointestinal tract. Surg. Endosc. 2020, 34, 3861–3869.
- Mohan, B.; Khan, S.R.; Madhu, D.; Kassab, L.; Jonnadula, S.; Chandan, S.; Facciorusso, A.; Adler, D. Clinical Outcomes of Endoscopic Suturing in Gastrointestinal Fistulas, Leaks, Perforations and Mucosal Defects: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2021, 116, S470–S471.
- Granata, A.; Amata, M.; Ligresti, D.; Martino, A.; Tarantino, I.; Barresi, L.; Traina, M. Endoscopic management of post-surgical GI wall defects with the overstitch endosuturing system: A single-center experience. Surg. Endosc. 2020, 34, 3805–3817.
- Ge, P.S.; Thompson, C.C. The Use of the Overstitch to Close Perforations and Fistulas. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 147–161.
- Pang, M.; Mousa, O.; Werlang, M.; Brahmbhatt, B.; Woodward, T. A hybrid endoscopic technique to close tracheoesophageal fistula. VideoGIE 2018, 3, 15–16.
- Nachira, D.; Trivisonno, A.; Costamagna, G.; Toietta, G.; Margaritora, S.; Pontecorvi, V.; Punzo, G.; Porziella, V.; Boskoski, I. Successful Therapy of Esophageal Fistulas by Endoscopic Injection of Emulsified Adipose Tissue Stromal Vascular Fraction. Gastroenterology 2021, 160, 1026–1028.
- Porziella, V.; Nachira, D.; Boškoski, I.; Trivisonno, A.; Costamagna, G.; Margaritora, S. Emulsified stromal vascular fraction tissue grafting: A new frontier in the treatment of esophageal fistulas. Gastrointest. Endosc. 2020, 92, 1262–1263.
- Mongardini, F.M.; Cacciatore, C.; Catauro, A.; Maglione, F.; Picardi, F.; Lauro, A.; Gambardella, C.; Allaria, A.; Docimo, L. Stemming the Leak: A Novel Treatment for Gastro-Bronchial Fistula. Dig. Dis. Sci. 2022, 67, 5425–5432.
- Shogan, B.D.; Belogortseva, N.; Luong, P.M.; Zaborin, A.; Lax, S.; Bethel, C.; Ward, M.; Muldoon, J.P.; Singer, M.; An, G.; et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci. Transl. Med. 2015, 7, 286ra68.
- Olivas, A.D.; Shogan, B.D.; Valuckaite, V.; Zaborin, A.; Belogortseva, N.; Musch, M.; Meyer, F.; Trimble, W.L.; An, G.; Gilbert, J.; et al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: Possible role in anastomotic leak. PLoS ONE 2012, 7, e44326.
- Guyton, K.; Belogortseva, N.; Levine, Z.; Kaiser, B.D.; Sangwan, N.; Hyman, N.; Shogan, B.D.; Zaborina, O.; Alverdy, J.C. Patient Acceptance of Routine Serial Postoperative Endoscopy Following Low Anterior Resection (LAR) and Its Ability to Detect Biomarkers in Anastomotic Lavage Fluid. World J. Surg. 2021, 45, 2227–2234.
- Kotzampassi, K. Why Give My Surgical Patients Probiotics. Nutrients 2022, 14, 4389.
- Kotzampassi, K. What Surgeon Should Know about Probiotics. Nutrients 2022, 14, 4374.
- Tarapatzi, G.; Filidou, E.; Kandilogiannakis, L.; Spathakis, M.; Gaitanidou, M.; Arvanitidis, K.; Drygiannakis, I.; Valatas, V.; Kotzampassi, K.; Manolopoulos, V.G.; et al. The Probiotic Strains Bifidοbacterium lactis, Lactobacillus acidophilus, Lactiplantibacillus plantarum and Saccharomyces boulardii Regulate Wound Healing and Chemokine Responses in Human Intestinal Subepithelial Myofibroblasts. Pharmaceuticals 2022, 15, 1293.
- Filidou, E.; Kolios, G. Probiotics in Intestinal Mucosal Healing: A New Therapy or an Old Friend? Pharmaceuticals 2021, 14, 1181.
- Williamson, A.J.; Alverdy, J.C. Influence of the Microbiome on Anastomotic Leak. Clin. Colon Rectal Surg. 2021, 34, 439–446.
- Moysidis, M.; Stavrou, G.; Cheva, A.; Abba Deka, I.; Tsetis, J.K.; Birba, V.; Kapoukranidou, D.; Ioannidis, A.; Tsaousi, G.; Kotzampassi, K. The 3-D configuration of excisional skin wound healing after topical probiotic application. Injury 2022, 53, 1385–1393.
- Kelm, M.; Anger, F. Mucosa and microbiota—The role of intrinsic parameters on intestinal wound healing. Front. Surg. 2022, 9, 905049.
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 2015, 39, 2776–2783.
More
Information
Subjects:
Surgery; Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
502
Revisions:
2 times
(View History)
Update Date:
07 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




