Anastomotic leakage, which is defined as a defect in the integrity of a surgical join between two hollow viscera leading to communication between the intraluminal and extraluminal compartments, continues to be of high incidence and one of the most feared complications following gastrointestinal surgery, with a significant potential for a fatal outcome. Surgical options for management are limited and carry a high risk of morbidity and mortality; thus, surgeons are urged to look for alternative options which are minimally invasive, repeatable, non-operative, and do not require general anesthesia.
- anastomotic leakage
- tissue sealants
- clips
- stents
- endoscopic vacuum therapy
1. Introduction
2. History of Endoscopic Sealing
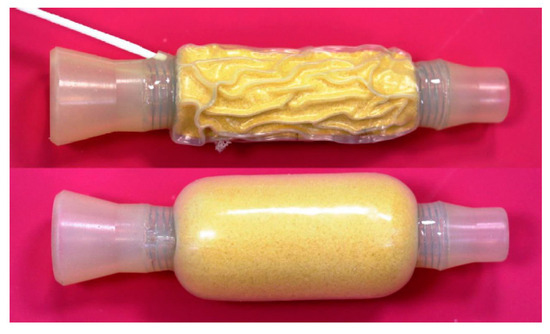
The Cuffed Stent
3. Current Technology
3.1. Tissue Adhesives
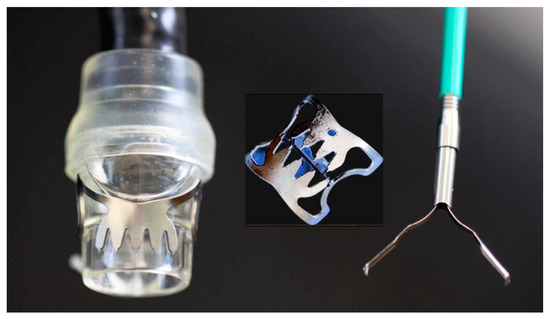
3.2. Endoclips and Over-the-Scope Clips
3.3. Stents

3.4. Endoscopic Vacuum Therapy (EVT)

3.5. Endoscopic Internal Drainage (EID)

3.6. Vac-Stent Technique
3.7. The Suturing System
4. The Possible Future
4.1. Stem Cells
4.2. Modification of Luminal Microbiome
This entry is adapted from the peer-reviewed paper 10.3390/gidisord5030032
References
- Kähler, G. Anastomotic Leakage after Upper Gastrointestinal Surgery: Endoscopic Treatment. Visc. Med. 2017, 33, 202–206.
- Munshi, E.; Dahlbäck, C.; Johansson, S.; Lydrup, M.L.; Jutesten, H.; Buchwald, P. Long-term Outcomes of Endoscopic Vacuum Therapy and Transanal Drainage for Anastomotic Leakage After Anterior Resection. Vivo 2022, 36, 2275–2278.
- Cereatti, F.; Grassia, R.; Drago, A.; Conti, C.B.; Donatelli, G. Endoscopic management of gastrointestinal leaks and fistulae: What option do we have? World J. Gastroenterol. 2020, 26, 4198–4217.
- Rutegård, M.; Lagergren, P.; Rouvelas, I.; Lagergren, J. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: A population-based study. Ann. Surg. Oncol. 2012, 19, 99–103.
- Lang, H.; Piso, P.; Stukenborg, C.; Raab, R.; Jähne, J. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur. J. Surg. Oncol. 2000, 26, 168–171.
- Messager, M.; Warlaumont, M.; Renaud, F.; Marin, H.; Branche, J.; Piessen, G.; Mariette, C. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur. J. Surg. Oncol. 2017, 43, 258–269.
- Scognamiglio, P.; Reeh, M.; Melling, N.; Kantowski, M.; Eichelmann, A.-K.; Chon, S.-H.; El-Sourani, N.; Schön, G.; Höller, A.; Izbicki, J.R.; et al. Management of intra-thoracic anastomotic leakages after esophagectomy: Updated systematic review and meta-analysis of endoscopic vacuum therapy versus stenting. BMC Surg. 2022, 22, 309.
- Blencowe, N.S.; Strong, S.; Mcnair, A.G.; Brookes, S.T.; Crosby, T.; Griffin, S.M.; Blazeby, J.M. Reporting of short-term clinical outcomes after esophagectomy: A systematic review. Ann. Surg. 2012, 255, 658–666.
- Kuppusamy, M.K.; Low, D.E. Evaluation of International Contemporary Operative Outcomes and Management Trends Associated With Esophagectomy: A 4-Year Study of >6000 Patients Using ECCG Definitions and the Online Esodata Database. Ann. Surg. 2022, 275, 515–525.
- Aiolfi, A.; Asti, E.; Rausa, E.; Bonavina, G.; Bonitta, G.; Bonavina, L. Use of C-reactive protein for the early prediction of anastomotic leak after esophagectomy: Systematic review and Bayesian meta-analysis. PLoS ONE 2018, 13, e0209272.
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking Complications Associated with Esophagectomy. Ann. Surg. 2019, 269, 291–298.
- Watanabe, M.; Miyata, H.; Gotoh, M.; Baba, H.; Kimura, W.; Tomita, N.; Nakagoe, T.; Shimada, M.; Kitagawa, Y.; Sugihara, K.; et al. Total gastrectomy risk model: Data from 20,011 Japanese patients in a nationwide internet-based database. Ann. Surg. 2014, 260, 1034–1039.
- Van Der Werf, L.R.; Busweiler, L.a.D.; Van Sandick, J.W.; Van Berge Henegouwen, M.I.; Wijnhoven, B.P.L. Reporting National Outcomes After Esophagectomy and Gastrectomy According to the Esophageal Complications Consensus Group (ECCG). Ann. Surg. 2020, 271, 1095–1101.
- Vermeer, T.A.; Orsini, R.G.; Daams, F.; Nieuwenhuijzen, G.A.; Rutten, H.J. Anastomotic leakage and presacral abscess formation after locally advanced rectal cancer surgery: Incidence, risk factors and treatment. Eur. J. Surg. Oncol. 2014, 40, 1502–1509.
- Clifford, R.E.; Fowler, H.; Govindarajah, N.; Vimalachandran, D.; Sutton, P.A. Early anastomotic complications in colorectal surgery: A systematic review of techniques for endoscopic salvage. Surg. Endosc. 2019, 33, 1049–1065.
- Yu, J.H.; Huang, X.W.; Song, Y.C.; Lin, H.Z.; Zheng, F.W. Analysis of Prevention and Treatment of Anastomotic Leakage after Sphincter-Preserving Surgery for Middle- and Low-Grade Rectal Cancer under Laparoscopy. Int. J. Clin. Pract. 2022, 2022, 6231880.
- Penna, M.; Hompes, R.; Arnold, S.; Wynn, G.; Austin, R.; Warusavitarne, J.; Moran, B.; Hanna, G.B.; Mortensen, N.J.; Tekkis, P.P. Incidence and Risk Factors for Anastomotic Failure in 1594 Patients Treated by Transanal Total Mesorectal Excision: Results From the International TaTME Registry. Ann. Surg. 2019, 269, 700–711.
- Okoshi, K.; Masano, Y.; Hasegawa, S.; Hida, K.; Kawada, K.; Nomura, A.; Kawamura, J.; Nagayama, S.; Yoshimura, T.; Sakai, Y. Efficacy of transanal drainage for anastomotic leakage after laparoscopic low anterior resection of the rectum. Asian J. Endosc. Surg. 2013, 6, 90–95.
- Yang, L.; Huang, X.E.; Zhou, J.N. Risk assessment on anastomotic leakage after rectal cancer surgery: An analysis of 753 patients. Asian Pac. J. Cancer Prev. 2013, 14, 4447–4453.
- Marshall, J.S.; Srivastava, A.; Gupta, S.K.; Rossi, T.R.; Debord, J.R. Roux-en-Y gastric bypass leak complications. Arch. Surg. 2003, 138, 520–523; discussion 523–524.
- Csendes, A.; Burgos, A.M.; Braghetto, I. Classification and management of leaks after gastric bypass for patients with morbid obesity: A prospective study of 60 patients. Obes. Surg. 2012, 22, 855–862.
- Morales, M.P.; Miedema, B.W.; Scott, J.S.; De La Torre, R.A. Management of postsurgical leaks in the bariatric patient. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 295–304.
- Gonzalez, R.; Sarr, M.G.; Smith, C.D.; Baghai, M.; Kendrick, M.; Szomstein, S.; Rosenthal, R.; Murr, M.M. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J. Am. Coll. Surg. 2007, 204, 47–55.
- Abou Rached, A.; Basile, M.; El Masri, H. Gastric leaks post sleeve gastrectomy: Review of its prevention and management. World J. Gastroenterol. 2014, 20, 13904–13910.
- Rosenthal, R.J.; Diaz, A.A.; Arvidsson, D.; Baker, R.S.; Basso, N.; Bellanger, D.; Boza, C.; El Mourad, H.; France, M.; Gagner, M.; et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: Best practice guidelines based on experience of >12,000 cases. Surg. Obes. Relat. Dis. 2012, 8, 8–19.
- Bashah, M.; Khidir, N.; El-Matbouly, M. Management of leak after sleeve gastrectomy: Outcomes of 73 cases, treatment algorithm and predictors of resolution. Obes. Surg. 2020, 30, 515–520.
- Mandarino, F.V.; Barchi, A.; Fanti, L.; D’amico, F.; Azzolini, F.; Esposito, D.; Biamonte, P.; Lauri, G.; Danese, S. Endoscopic vacuum therapy for post-esophagectomy anastomotic dehiscence as rescue treatment: A single center case series. Esophagus 2022, 19, 417–425.
- Binda, C.; Jung, C.F.M.; Fabbri, S.; Giuffrida, P.; Sbrancia, M.; Coluccio, C.; Gibiino, G.; Fabbri, C. Endoscopic Management of Postoperative Esophageal and Upper GI Defects-A Narrative Review. Medicina 2023, 59, 136.
- Lux, G.; Wilson, D.; Wilson, J.; Demling, L. A cuffed tube for the treatment of oesophago-bronchial fistulae. Endoscopy 1987, 19, 28–30.
- Irving, J.D.; Simson, J.N. A new cuffed oesophageal prosthesis for the management of malignant oesophago-respiratory fistula. Ann. R. Coll. Surg. Engl. 1988, 70, 13–15.
- Hordijk, M.L.; Dees, J.; Van Blankenstein, M. The management of malignant esophago-respiratory fistulas with a cuffed prosthesis. Endoscopy 1990, 22, 241–244.
- Sargeant, I.R.; Thorpe, S.; Bown, S.G. Cuffed esophageal prosthesis: A useful device in desperate situations in esophageal malignancy. Gastrointest. Endosc. 1992, 38, 669–675.
- Kotzampassi, K.; Eleftheriadis, E. Tissue sealants in endoscopic applications for anastomotic leakage during a 25-year period. Surgery 2015, 157, 79–86.
- Shehab, H. Endoscopic management of postsurgical leaks. Int. J. Gastrointest. Interv. 2016, 5, 6–14.
- Hua, F.; Sun, D.; Zhao, X.; Song, X.; Yang, W. Update on therapeutic strategy for esophageal anastomotic leak: A systematic literature review. Thorac. Cancer 2023, 14, 339–347.
- Truong, S.; Böhm, G.; Klinge, U.; Stumpf, M.; Schumpelick, V. Results after endoscopic treatment of postoperative upper gastrointestinal fistulas and leaks using combined Vicryl plug and fibrin glue. Surg. Endosc. 2004, 18, 1105–1108.
- Blumetti, J.; Abcarian, H. Management of low colorectal anastomotic leak: Preserving the anastomosis. World J. Gastrointest. Surg. 2015, 7, 378–383.
- Binmoeller, K.F.; Grimm, H.; Soehendra, N. Endoscopic closure of a perforation using metallic clips after snare excision of a gastric leiomyoma. Gastrointest. Endosc. 1993, 39, 172–174.
- Li, C.; Zhao, Y.; Han, Z.; Zhou, Y. Anastomotic leaks following gastrointestinal surgery: Updates on diagnosis and interventions. Int. J. Clin. Exp. Med. 2016, 9, 7031–7040.
- Cho, S.B.; Lee, W.S.; Joo, Y.E.; Kim, H.R.; Park, S.W.; Park, C.H.; Kim, H.S.; Choi, S.K.; Rew, J.S. Therapeutic options for iatrogenic colon perforation: Feasibility of endoscopic clip closure and predictors of the need for early surgery. Surg. Endosc. 2012, 26, 473–479.
- Al Ghossaini, N.; Lucidarme, D.; Bulois, P. Endoscopic treatment of iatrogenic gastrointestinal perforations: An overview. Dig. Liver Dis. 2014, 46, 195–203.
- Banerjee, S.; Barth, B.A.; Bhat, Y.M.; Desilets, D.J.; Gottlieb, K.T.; Maple, J.T.; Pfau, P.R.; Pleskow, D.K.; Siddiqui, U.D.; Tokar, J.L.; et al. Endoscopic closure devices. Gastrointest. Endosc. 2012, 76, 244–251.
- Goenka, M.K.; Rodge, G.A.; Tiwary, I.K. Endoscopic Management with a Novel Over-The-Scope Padlock Clip System. Clin. Endosc. 2019, 52, 574–580.
- Von Renteln, D.; Denzer, U.W.; Schachschal, G.; Anders, M.; Groth, S.; Rösch, T. Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos). Gastrointest. Endosc. 2010, 72, 1289–1296.
- Kobara, H.; Mori, H.; Nishiyama, N.; Fujihara, S.; Okano, K.; Suzuki, Y.; Masaki, T. Over-the-scope clip system: A review of 1517 cases over 9 years. J. Gastroenterol. Hepatol. 2019, 34, 22–30.
- D’alessandro, A.; Galasso, G.; Zito, F.P.; Giardiello, C.; Cereatti, F.; Arienzo, R.; Pacini, F.; Chevallier, J.-M.; Donatelli, G. Role of Endoscopic Internal Drainage in Treating Gastro-Bronchial and Gastro-Colic Fistula After Sleeve Gastrectomy. Obes. Surg. 2022, 32, 342–348.
- Keller, D.S.; Talboom, K.; Van Helsdingen, C.P.M.; Hompes, R. Treatment Modalities for Anastomotic Leakage in Rectal Cancer Surgery. Clin. Colon Rectal Surg. 2021, 34, 431–438.
- Evrard, S.; Le Moine, O.; Lazaraki, G.; Dormann, A.; El Nakadi, I.; Devière, J. Self-expanding plastic stents for benign esophageal lesions. Gastrointest. Endosc. 2004, 60, 894–900.
- Gelbmann, C.M.; Ratiu, N.L.; Rath, H.C.; Rogler, G.; Lock, G.; Schölmerich, J.; Kullmann, F. Use of self-expandable plastic stents for the treatment of esophageal perforations and symptomatic anastomotic leaks. Endoscopy 2004, 36, 695–699.
- Symonds, C.J. The Treatment of Malignant Stricture of the Œsophagus by Tubage or Permanent Catheterism. Br. Med. J. 1887, 1, 870–873.
- Vanbiervliet, G.; Filippi, J.; Karimdjee, B.S.; Venissac, N.; Iannelli, A.; Rahili, A.; Benizri, E.; Pop, D.; Staccini, P.; Tran, A.; et al. The role of clips in preventing migration of fully covered metallic esophageal stents: A pilot comparative study. Surg. Endosc. 2012, 26, 53–59.
- Winder, J.S.; Pauli, E.M. Comprehensive management of full-thickness luminal defects: The next frontier of gastrointestinal endoscopy. World J. Gastrointest. Endosc. 2015, 7, 758–768.
- Tsai, Y.Y.; Chen, W.T. Management of anastomotic leakage after rectal surgery: A review article. J. Gastrointest. Oncol. 2019, 10, 1229–1237.
- Venezia, L.; Michielan, A.; Condino, G.; Sinagra, E.; Stasi, E.; Galeazzi, M.; Fabbri, C.; Anderloni, A. Feasibility and safety of self-expandable metal stent in nonmalignant disease of the lower gastrointestinal tract. World J. Gastrointest. Endosc. 2020, 12, 60–71.
- Erdmann, D.; Drye, C.; Heller, L.; Wong, M.S.; Levin, S.L. Abdominal wall defect and enterocutaneous fistula treatment with the Vacuum-Assisted Closure (V.A.C.) system. Plast. Reconstr. Surg. 2001, 108, 2066–2068.
- Nagell, C.F.; Holte, K. Treatment of anastomotic leakage after rectal resection with transrectal vacuum-assisted drainage (VAC). A method for rapid control of pelvic sepsis and healing. Int. J. Color. Dis. 2006, 21, 657–660.
- Weidenhagen, R.; Gruetzner, K.U.; Wiecken, T.; Spelsberg, F.; Jauch, K.W. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: A new method. Surg. Endosc. 2008, 22, 1818–1825.
- Weidenhagen, R.; Hartl, W.H.; Gruetzner, K.U.; Eichhorn, M.E.; Spelsberg, F.; Jauch, K.W. Anastomotic leakage after esophageal resection: New treatment options by endoluminal vacuum therapy. Ann. Thorac. Surg. 2010, 90, 1674–1681.
- Reimer, S.; Seyfried, F.; Flemming, S.; Brand, M.; Weich, A.; Widder, A.; Plaßmeier, L.; Kraus, P.; Döring, A.; Hering, I.; et al. Evolution of endoscopic vacuum therapy for upper gastrointestinal leakage over a 10-year period: A quality improvement study. Surg. Endosc. 2022, 36, 9169–9178.
- Krokowicz, L.; Borejsza-Wysocki, M.; Mackiewicz, J.; Iqbal, A.; Drews, M. 10 years of Negative Pressure Wound Therapy (NPWT): Evolution of Indications for its Use. Negat. Press. Wound Ther. 2014, 1, 27–32.
- Weidenhagen, R.; Gruetzner, K.U.; Wiecken, T.; Spelsberg, F.; Jauch, K.W. Endoluminal vacuum therapy for the treatment of anastomotic leakage after anterior rectal resection. Rozhl. Chir. 2008, 87, 397–402.
- Gubler, C.; Schneider, P.M.; Bauerfeind, P. Complex anastomotic leaks following esophageal resections: The new stent over sponge (SOS) approach. Dis. Esophagus 2013, 26, 598–602.
- Bartella, I.; Mallmann, C.; Bürger, M.; Toex, U.; Goeser, T.; Bruns, C.; Chon, S.H. Stent-over-sponge (SOS): A rescue option in patients with complex postoperative anastomotic leaks after esophagectomy. Endoscopy 2019, 51, E227–E228.
- Morell, B.; Murray, F.; Vetter, D.; Bueter, M.; Gubler, C. Endoscopic vacuum therapy (EVT) for early infradiaphragmal leakage after bariatric surgery-outcomes of six consecutive cases in a single institution. Langenbecks Arch. Surg. 2019, 404, 115–121.
- Pequignot, A.; Fuks, D.; Verhaeghe, P.; Dhahri, A.; Brehant, O.; Bartoli, E.; Delcenserie, R.; Yzet, T.; Regimbeau, J.M. Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleeve gastrectomy? Obes. Surg. 2012, 22, 712–720.
- Donatelli, G.; Dumont, J.L.; Cereatti, F.; Ferretti, S.; Vergeau, B.M.; Tuszynski, T.; Pourcher, G.; Tranchart, H.; Mariani, P.; Meduri, A.; et al. Treatment of Leaks Following Sleeve Gastrectomy by Endoscopic Internal Drainage (EID). Obes. Surg. 2015, 25, 1293–1301.
- Gonzalez, J.M.; Lorenzo, D.; Guilbaud, T.; Bège, T.; Barthet, M. Internal endoscopic drainage as first line or second line treatment in case of postsleeve gastrectomy fistulas. Endosc. Int. Open 2018, 6, E745–E750.
- Chon, S.H.; Scherdel, J.; Rieck, I.; Lorenz, F.; Dratsch, T.; Kleinert, R.; Gebauer, F.; Fuchs, H.F.; Goeser, T.; Bruns, C.J. A new hybrid stent using endoscopic vacuum therapy in treating esophageal leaks: A prospective single-center experience of its safety and feasibility with mid-term follow-up. Dis. Esophagus 2022, 35, doab067.
- Chon, S.H.; Töx, U.; Lorenz, F.; Rieck, I.; Wagner, B.J.; Kleinert, R.; Fuchs, H.F.; Goeser, T.; Quaas, A.; Bruns, C.J. A Novel Hybrid Stent with Endoscopic Vacuum Therapy for Treating Leaks of the Upper Gastrointestinal Tract. Visc. Med. 2021, 37, 403–409.
- Pattynama, L.M.D.; Eshuis, W.J.; Van Berge Henegouwen, M.I.; Bergman, J.; Pouw, R.E. Vacuum-stent: A combination of endoscopic vacuum therapy and an intraluminal stent for treatment of esophageal transmural defects. Front. Surg. 2023, 10, 1145984.
- Mahmood, Z.; Mcmahon, B.P.; Arfin, Q.; Byrne, P.J.; Reynolds, J.V.; Murphy, E.M.; Weir, D.G. Endocinch therapy for gastro-oesophageal reflux disease: A one year prospective follow up. Gut 2003, 52, 34–39.
- Schiefke, I.; Neumann, S.; Zabel-Langhennig, A.; Moessner, J.; Caca, K. Use of an Endoscopic Suturing Device (the ”ESD”) to Treat Patients with Gastroesophageal Reflux Disease, After Unsuccessful EndoCinch Endoluminal Gastroplication: Another Failure. Endoscopy 2005, 37, 700–705.
- Moran, E.A.; Gostout, C.J.; Bingener, J. Preliminary performance of a flexible cap and catheter-based endoscopic suturing system. Gastrointest. Endosc. 2009, 69, 1375–1383.
- Muniraj, T.; Harry, R. The use of OverStitchTM for the treatment of intestinal perforation, fistulas and leaks. Int. J. Gastrointest. Interv. 2017, 6, 151–156.
- Zhang, L.Y.; Bejjani, M.; Ghandour, B.; Khashab, M.A. Endoscopic through-the-scope suturing. VideoGIE 2022, 7, 46–51.
- Bi, D.; Zhang, L.Y.; Alqaisieh, M.; Shrigiriwar, A.; Farha, J.; Mahmoud, T.; Akiki, K.; Almario Jose, A.; Shah-Khan, S.M.; Gordon, S.R.; et al. Novel Through-the-Scope Suture Closure of Colonic Endoscopic Mucosal Resection Defects. Gastrointest. Endosc. 2023, 98, 122–129.
- Chon, S.H.; Toex, U.; Plum, P.S.; Kleinert, R.; Bruns, C.J.; Goeser, T.; Berlth, F. Efficacy and feasibility of OverStitch suturing of leaks in the upper gastrointestinal tract. Surg. Endosc. 2020, 34, 3861–3869.
- Mohan, B.; Khan, S.R.; Madhu, D.; Kassab, L.; Jonnadula, S.; Chandan, S.; Facciorusso, A.; Adler, D. Clinical Outcomes of Endoscopic Suturing in Gastrointestinal Fistulas, Leaks, Perforations and Mucosal Defects: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2021, 116, S470–S471.
- Granata, A.; Amata, M.; Ligresti, D.; Martino, A.; Tarantino, I.; Barresi, L.; Traina, M. Endoscopic management of post-surgical GI wall defects with the overstitch endosuturing system: A single-center experience. Surg. Endosc. 2020, 34, 3805–3817.
- Ge, P.S.; Thompson, C.C. The Use of the Overstitch to Close Perforations and Fistulas. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 147–161.
- Pang, M.; Mousa, O.; Werlang, M.; Brahmbhatt, B.; Woodward, T. A hybrid endoscopic technique to close tracheoesophageal fistula. VideoGIE 2018, 3, 15–16.
- Nachira, D.; Trivisonno, A.; Costamagna, G.; Toietta, G.; Margaritora, S.; Pontecorvi, V.; Punzo, G.; Porziella, V.; Boskoski, I. Successful Therapy of Esophageal Fistulas by Endoscopic Injection of Emulsified Adipose Tissue Stromal Vascular Fraction. Gastroenterology 2021, 160, 1026–1028.
- Porziella, V.; Nachira, D.; Boškoski, I.; Trivisonno, A.; Costamagna, G.; Margaritora, S. Emulsified stromal vascular fraction tissue grafting: A new frontier in the treatment of esophageal fistulas. Gastrointest. Endosc. 2020, 92, 1262–1263.
- Mongardini, F.M.; Cacciatore, C.; Catauro, A.; Maglione, F.; Picardi, F.; Lauro, A.; Gambardella, C.; Allaria, A.; Docimo, L. Stemming the Leak: A Novel Treatment for Gastro-Bronchial Fistula. Dig. Dis. Sci. 2022, 67, 5425–5432.
- Shogan, B.D.; Belogortseva, N.; Luong, P.M.; Zaborin, A.; Lax, S.; Bethel, C.; Ward, M.; Muldoon, J.P.; Singer, M.; An, G.; et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci. Transl. Med. 2015, 7, 286ra68.
- Olivas, A.D.; Shogan, B.D.; Valuckaite, V.; Zaborin, A.; Belogortseva, N.; Musch, M.; Meyer, F.; Trimble, W.L.; An, G.; Gilbert, J.; et al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: Possible role in anastomotic leak. PLoS ONE 2012, 7, e44326.
- Guyton, K.; Belogortseva, N.; Levine, Z.; Kaiser, B.D.; Sangwan, N.; Hyman, N.; Shogan, B.D.; Zaborina, O.; Alverdy, J.C. Patient Acceptance of Routine Serial Postoperative Endoscopy Following Low Anterior Resection (LAR) and Its Ability to Detect Biomarkers in Anastomotic Lavage Fluid. World J. Surg. 2021, 45, 2227–2234.
- Kotzampassi, K. Why Give My Surgical Patients Probiotics. Nutrients 2022, 14, 4389.
- Kotzampassi, K. What Surgeon Should Know about Probiotics. Nutrients 2022, 14, 4374.
- Tarapatzi, G.; Filidou, E.; Kandilogiannakis, L.; Spathakis, M.; Gaitanidou, M.; Arvanitidis, K.; Drygiannakis, I.; Valatas, V.; Kotzampassi, K.; Manolopoulos, V.G.; et al. The Probiotic Strains Bifidοbacterium lactis, Lactobacillus acidophilus, Lactiplantibacillus plantarum and Saccharomyces boulardii Regulate Wound Healing and Chemokine Responses in Human Intestinal Subepithelial Myofibroblasts. Pharmaceuticals 2022, 15, 1293.
- Filidou, E.; Kolios, G. Probiotics in Intestinal Mucosal Healing: A New Therapy or an Old Friend? Pharmaceuticals 2021, 14, 1181.
- Williamson, A.J.; Alverdy, J.C. Influence of the Microbiome on Anastomotic Leak. Clin. Colon Rectal Surg. 2021, 34, 439–446.
- Moysidis, M.; Stavrou, G.; Cheva, A.; Abba Deka, I.; Tsetis, J.K.; Birba, V.; Kapoukranidou, D.; Ioannidis, A.; Tsaousi, G.; Kotzampassi, K. The 3-D configuration of excisional skin wound healing after topical probiotic application. Injury 2022, 53, 1385–1393.
- Kelm, M.; Anger, F. Mucosa and microbiota—The role of intrinsic parameters on intestinal wound healing. Front. Surg. 2022, 9, 905049.
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 2015, 39, 2776–2783.
