Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fatima tuz Zahra | -- | 1854 | 2023-09-27 04:36:51 | | | |
| 2 | Camila Xu | Meta information modification | 1854 | 2023-09-27 09:41:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zahra, F.T.; Quick, Q.; Mu, R. Electrospinning for Drug Delivery Systems. Encyclopedia. Available online: https://encyclopedia.pub/entry/49692 (accessed on 06 March 2026).
Zahra FT, Quick Q, Mu R. Electrospinning for Drug Delivery Systems. Encyclopedia. Available at: https://encyclopedia.pub/entry/49692. Accessed March 06, 2026.
Zahra, Fatima T., Quincy Quick, Richard Mu. "Electrospinning for Drug Delivery Systems" Encyclopedia, https://encyclopedia.pub/entry/49692 (accessed March 06, 2026).
Zahra, F.T., Quick, Q., & Mu, R. (2023, September 27). Electrospinning for Drug Delivery Systems. In Encyclopedia. https://encyclopedia.pub/entry/49692
Zahra, Fatima T., et al. "Electrospinning for Drug Delivery Systems." Encyclopedia. Web. 27 September, 2023.
Copy Citation
The term “drug delivery” refers to administrating the therapeutic effect of a pharmaceutical compound to humans or animals. Progress in the field of disease exploration is widely acknowledged, leading to ongoing research and development of advanced techniques for advanced drug delivery systems (DDSs). Electrospinning is a cost-effective and simple tool that is used for the preparation of drug delivery systems using natural, synthetic, and blended polymers.
electrospinning
polyvinyl alcohol (PVA)
nanofibers
drug delivery
wound dressing
1. Introduction
The term “drug delivery” refers to administrating the therapeutic effect of a pharmaceutical compound to humans or animals. Progress in the field of disease exploration is widely acknowledged, leading to ongoing research and development of advanced techniques for advanced drug delivery systems (DDSs). Some of the key factors in developing an effective and controlled drug delivery system include its inertness, non-toxicity, biocompatibility, high mechanical strength, patient comfort, and high drug-loading capability. To streamline and optimize the performance of this domain, an extensive array of polymers has been researched and effectively employed. The polymeric drug delivery systems have various categories such as microparticles, micelles, hydrogels/transplants, nanoparticles, and drug conjugates [1][2][3][4][5][6][7]. These systems are employed to improve controlled drug release, targeted drug delivery, and solubility. Generally, polymers are categorized as natural or synthetic polymers. Figure 1 illustrates the most frequently used polymers in both categories for the development of DDSs. Natural polymers are sometimes blended with synthetic compounds to chemically modify their functional groups, resulting in what is known as semi-synthetic polymers. Chitosan (CS) is one of the most commonly explored natural polymers and various studies have been reported on its potential in developing advanced drug delivery systems [8][9][10][11][12]. Synthetic polymers are sub-divided into hydrophobic and hydrophilic groups which are utilized in DDSs as core–shell nanoparticles depending on the required criteria. The drug release and drug loading efficiency of hydrophilic polymers is believed to be higher than those of hydrophobic polymers [13]. Combining hydrophobic and hydrophilic synthetic polymers in various proportions has also been employed for the development of drug nanocarriers. For instance, the combination of poly(ethylene glycol) (PEG) and polycaprolactone (PCL) has been utilized to investigate the endocytosis pathways into cells [14]. Polymeric capsules have been developed for cancer therapy by incorporating targeting ligands, thereby mitigating the accompanying side effects and toxicity [15]. Polymers are commonly used to develop transdermal DDSs, wound dressings, cancer therapy, tissue engineering, and oral DDSs. The disadvantages associated with commonly used synthetic/natural polymers such as PCL, PLGA (poly(lactic-co-glycolic acid)), and PLA (poly(lactic acid)) include the slow degradation and biocompatibility issues due to the formation of acidic degradation products [16][17].
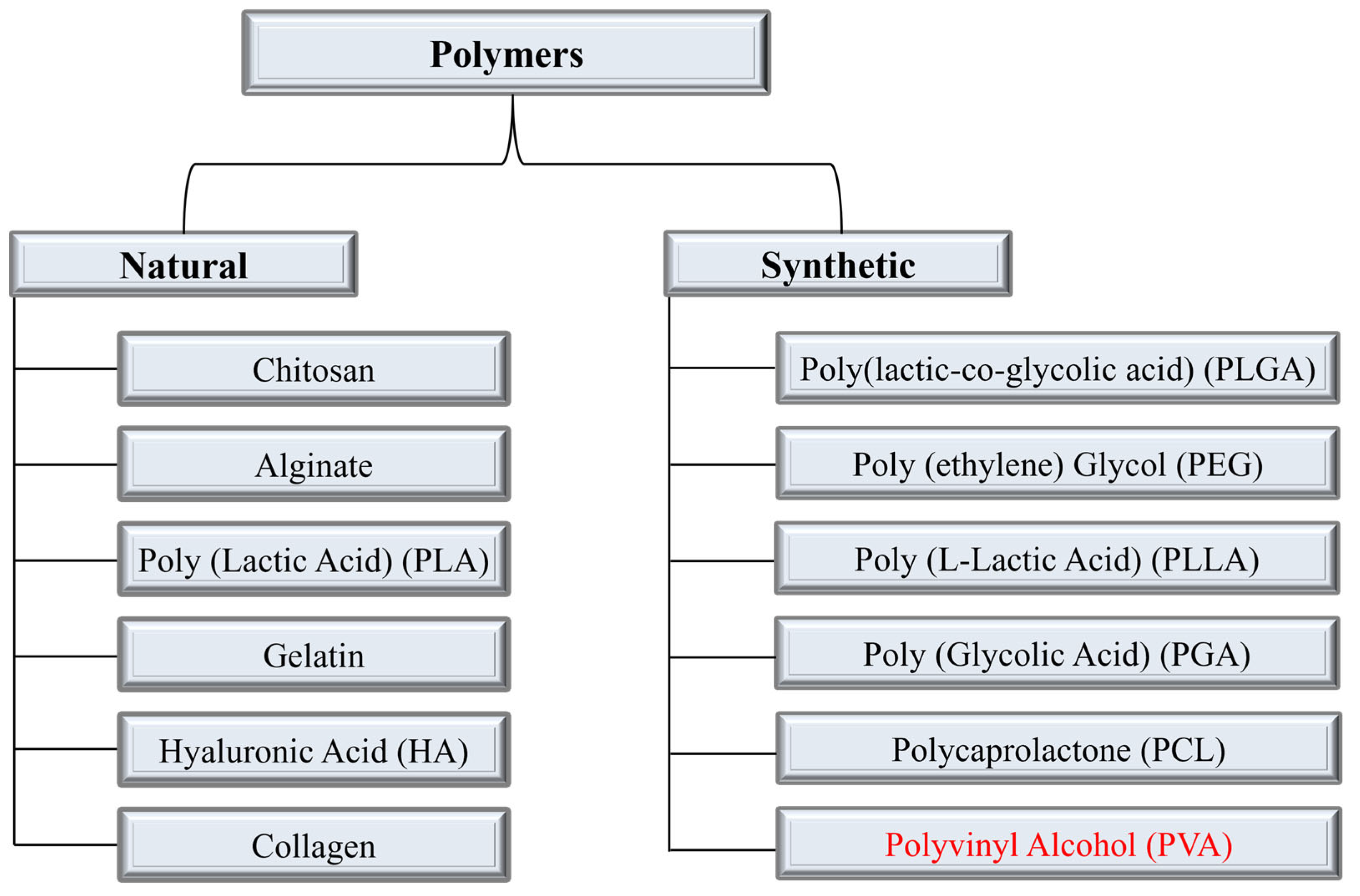
Figure 1. Categories of polymers and the most used polymers in each category for DDSs. The polymer under discussion Polyvinyl Alcohol (PVA) is highlighted in synthetic polymers category.
Polyvinyl alcohol (PVA) is a synthetic biopolymer that has been used for decades as a blending polymer with natural and synthetic polymers to improve the efficacy of DDSs. It is synthesized by the hydrolysis of polyvinyl acetate (PVAc) [18]. Depending on the degree of hydrolysis, which varies between 80 and 99%, PVA [CH2CH(OH)]n is subdivided into two categories, i.e., partially hydrolyzed and fully hydrolyzed. Figure 2 shows the molecular structure of both categories.
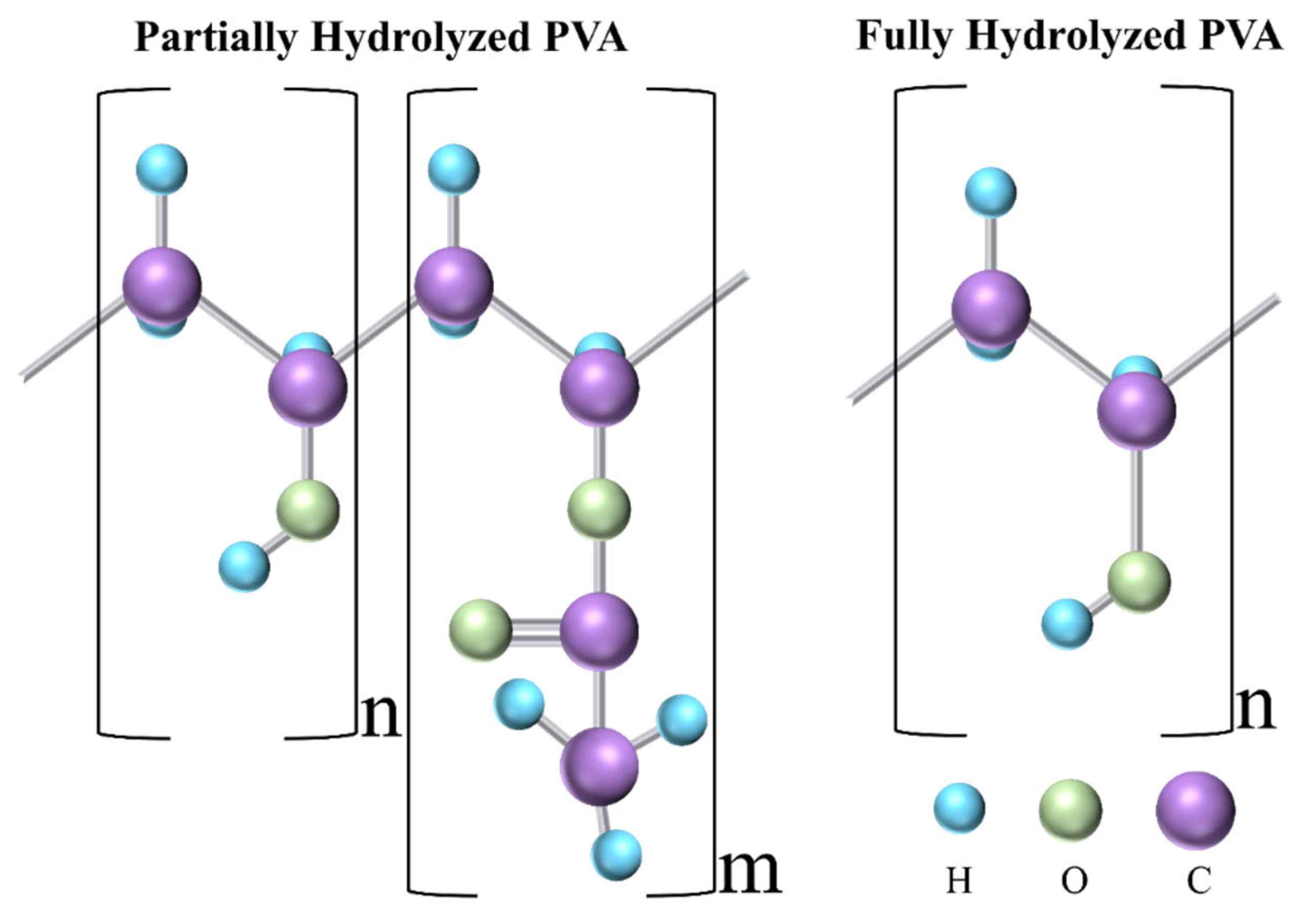
Figure 2. Molecular structure of partially and fully hydrolyzed PVA. C, O, and H refer to carbon, oxygen, and hydrogen, respectively.
PVA possesses a semicrystalline solid structure and is not only biocompatible, biodegradable, non-toxic, and hydrophilic, but also has a good spinning capability, and thus holds significant potential in electrospinning-based DDSs. There have been a few review papers that have examined the use of PVA in drug delivery systems for specific applications, such as tissue engineering and cancer therapy [19][20].
2. Electrospinning for DDSs
Electrospinning is a cost-effective and simple tool that is used for the preparation of drug delivery systems using natural, synthetic, and blended polymers. Figure 3 shows the schematic of the electrospinning process. In the electrospinning process, generally, the flow pump, spinneret, collector (e.g., a plane conducting sheet, rotating drum), and high voltage power supply are the main components. The spinneret is connected to a high-voltage source while the collector is grounded. The flow rate is set using the flow pump and it depends mainly on the viscosity of the polymer solution. Once the voltage is applied, the positively charged solution travels from the needle tip toward the grounded collector. The formation of the Taylor cone on the tip of the needle is an important factor without which the fibers may not be formed. Electrospinning has excessively been used for the development of different drug delivery systems and the process parameters mentioned above significantly affect the fiber morphology. For example, a higher flow rate can lead to high bead density, higher viscosity leads to an increase in diameter, lower viscosity results in bead formation, and longer needle-to-collector distance can result in instability of the electric field and hence negatively affect the fiber formation [21]. Therefore, it can be concluded that optimization of the processing parameters is an important phase in obtaining fine and bead-free fibers and surface morphology. Natural and synthetic polymers that have been most widely used in electrospun drug delivery systems are listed in Table 1.
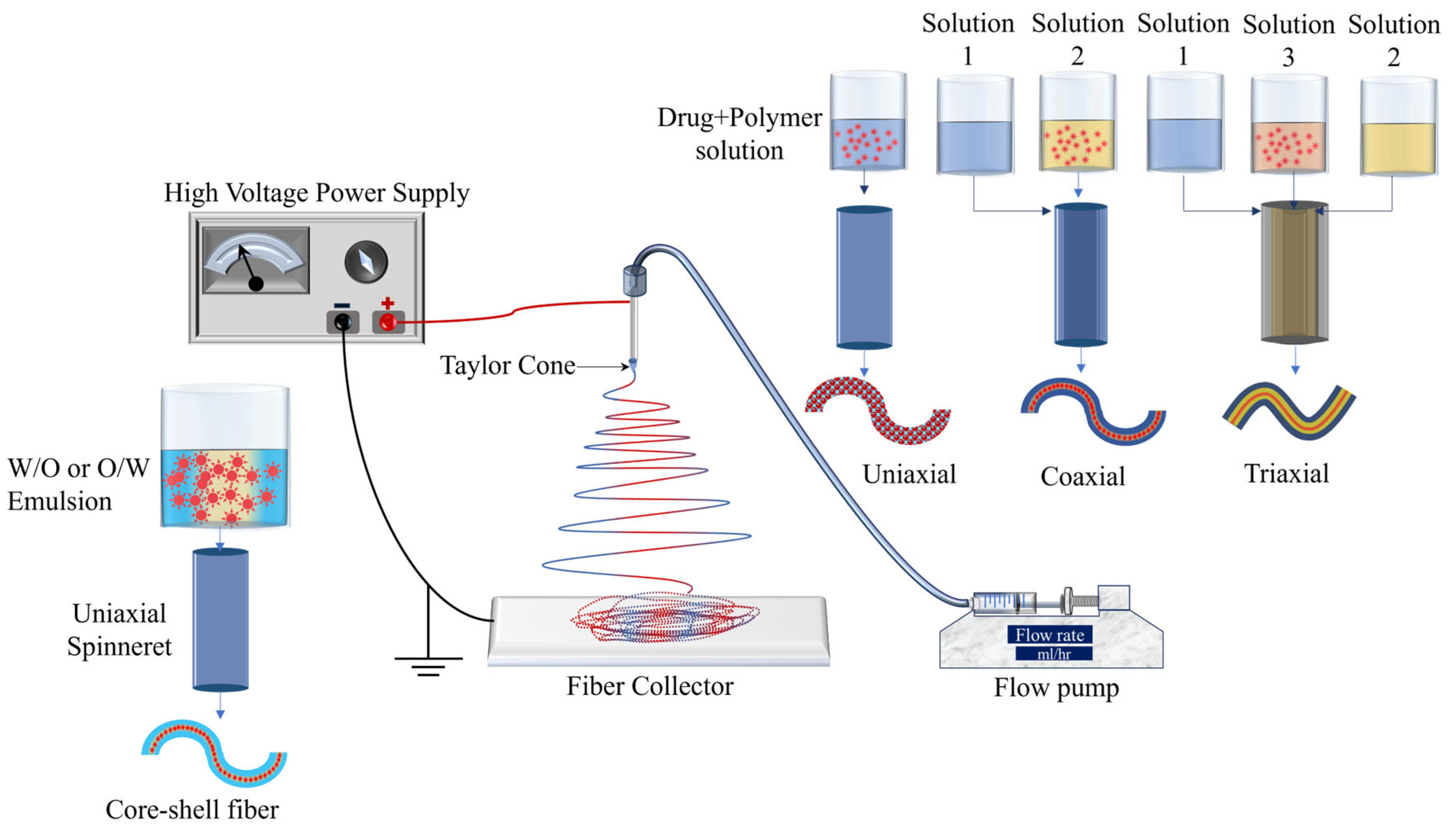
Figure 3. Schematic of electrospinning and its types.
Table 1. Limitations and advantages of the most commonly used natural and synthetic polymers for electrospun DDSs.
| Polymer, Type of Polymer |
Limitations | Advantages | Ref. |
|---|---|---|---|
| Chitosan Natural |
Extremely hydrophilic which leads to loss of nanofibrous structure, high degradation rate, and poor mechanical strength | Non-toxic, and biodegradable qualities make it biocompatible with a wide range of organs, tissues, and cells. | [9][22] |
| Gelatin Natural |
Rapid degradation, poor mechanical strength, and complete dissolution | Intrinsic bioactivity, high biocompatibility, cell adhesion, biodegradability, low immunogenicity | [23][24] |
| Hyaluronic Acid Natural |
High viscosity, high surface tension, low evaporability, high electrical conductivity that may lead to electrospinning circuit failure | Biocompatibility, non-immunogenicity, biodegradability, excellent tumor-targeting ability | [25][26][27][28] |
| Collagen Natural |
Variability in enzymatic degradation rate (depending on enzyme concentration), difficult to maintain its dimension in vivo due to swelling, poor mechanical strength, in vivo (not suitable for load-bearing tissues) | Biocompatible, non-antigenic, non-toxic, biodegradable (degradation can be regulated via crosslinking), compatible with synthetic polymers, promotes blood coagulation | [29][30] |
| Alginate Natural |
Low solubility, high viscosity due to high MW, high density of hydrogen bonding, polyelectrolyte nature of aqueous solution, and lack of appropriate organic solvent. | High water content, nontoxicity, soft consistency, biocompatibility, biodegradability, low immunogenicity | [31][32] |
| PLA Natural |
Poor mechanical strength, low cell adhesion because of its hydrophobicity, biological inertness, acidic degradation products, inflammation in vivo | Biocompatible, biodegradable by hydrolysis and enzymatic activity, low immunogenicity | [33][34] |
| PLGA Synthetic |
Poor hydrophilicity, poor cell adhesion, higher viscosity, production of acids upon degradation | Strong biodegradability, suitable for controlled-release drug delivery of medicines, peptides, proteins, and other substances | [35][36] |
| PCL Synthetic |
High hydrophobicity, poor bioactivity, low mechanical strength, and higher amount of PCL reduces the swelling capability of DDS | Slower degradation rate, shorter in vivo adsorbable time, generation of a minimal acidic environment during degradation | [37][38] |
| PEG Synthetic |
Low molecular weight makes it challenging to electrospin | Non-toxic, non-immunogenicity, good biocompatibility, and anti-protein adsorption | [39][40] |
The bold and italic texts in Column 1 signify the name of the polymer and its category, respectively.
One of the primary benefits of utilizing electrospinning in drug delivery systems lies in its ability to regulate the rate of drug release. This control is achieved by manipulating the degradation rate of the fibers through factors such as molecular weight and distribution, porosity of the fibers, and composition. The volatility of the solvent is a crucial consideration, particularly in the development of drug delivery systems (DDSs), as it significantly influences the achievement of the desired porosity of the fibers. This porosity is a critical factor in obtaining the desired drug release characteristics. Non-volatile solvents result in the generation of fibers characterized by larger pore sizes and reduced surface area, which is unfavorable for drug delivery applications [41][42]. Alongside solution volatility, solution viscosity, surface tension, and conductivity are noteworthy attributes that impact the morphology of the fibers. The viscosity of the solution is directly influenced by the concentration of the polymer employed, and it serves as a crucial parameter for controlling the spinnability of the solution. Lower viscosity is associated with the production of thin fibers that exhibit favorable mechanical strength. However, when the viscosity of the solution is very low, specifically below 1 poise, it results in the formation of beads and has a detrimental impact on the spinnability of the solution. Similarly, the presence of a significantly high viscosity, exceeding 20 poise, impedes the flow of the polymer, thereby preventing the successful fabrication of fibers. It is widely believed that in order to achieve optimal fiber morphology, the recommended viscosity range is between 1 and 20 poise, with a corresponding surface tension range of 35–55 dyn/cm2 [43]. Additionally, the conductivity of the solution is a crucial parameter that primarily influences the formation of the Taylor cone. If the surface of the droplet does not possess sufficient charge, it can hinder the formation of the Taylor cone, thereby potentially impeding the process of fiber formation. Additionally, it has been observed that the diameter of the fibers decreases as the solution conductivity increases. This phenomenon can be attributed to the application of tensile force in the presence of an electric field [44][45][46].
As shown in Figure 3, uniaxial, coaxial, and triaxial electrospinning is utilized for the preparation of monolithic fibers, core–shell fibers, and triaxial fibers, respectively, for the development of DDSs. In the case of uniaxial fiber fabrication, a single spinneret is used with a solution containing a combination of a drug and a polymer. For core–shell fibers, two spinnerets are utilized, each with its own solution: one for the core (typically a drug) and another for the shell (a polymer). Lastly, three spinnerets are employed for triaxial fibers, with one for the shell (a polymer), another for an intermediate layer, and a third for the core material [21]. Encapsulation of drugs in the electrospun fibers has been utilized for the development of controlled-release therapeutic elements in a timely manner to avoid potential side effects [47]. Moreover, electrospun fiber scaffolds have also been utilized for the restoration as well as repair of tissues/organs [48].
Emulsion electrospinning (Figure 3) is a type of uniaxial (needle-less (rotating electrode) or with a needle) electrospinning technique that is used to prepare core–shell fibrous structures using water-in-oil (W/O) or oil-in-water (O/W) emulsions to encapsulate hydrophilic or hydrophobic compounds, respectively. The emulsion consists of two liquid phases: an aqueous phase (hydrophilic) and an oil phase (hydrophobic). The drug is dissolved in the aqueous phase and then dispersed in the organic polymer solution (oil phase). The oil phase, characterized by a higher evaporation rate, exhibits increased viscosity. During the emulsion electrospinning process, the aqueous phase, containing the drug, merges into the core of the fiber structure, while the oil phase tends to migrate towards the periphery. This technique has demonstrated efficacy in achieving prolonged drug release.
Figure 4 shows the publication data until 2022 for keywords “electrospinning” and “drug delivery” extracted from Web of Science. More than 200 papers in the same field have already been published in 2023. This clearly shows the high potential of electrospinning and the interest of researchers in electrospinning to develop drug delivery systems.
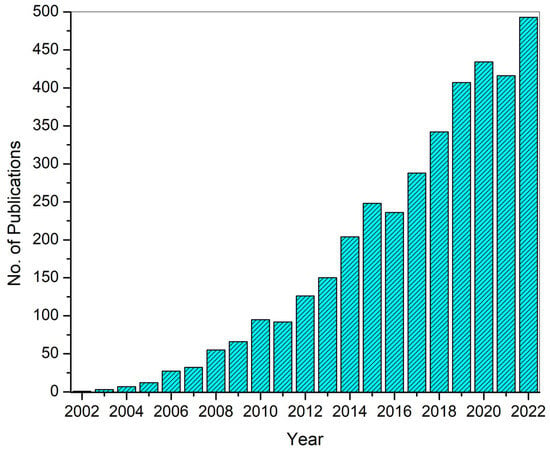
Figure 4. Number of publications until 2022 on electrospinning-based DDSs. The data were extracted from Web of Science (WoS).
References
- Guan, J.; Ferrell, N.; James Lee, L.; Hansford, D.J. Fabrication of Polymeric Microparticles for Drug Delivery by Soft Lithography. Biomaterials 2006, 27, 4034–4041.
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359.
- Croy, S.R.; Kwon, G.S. Polymeric Micelles for Drug Delivery. Curr. Pharm. Des. 2006, 12, 4669–4684.
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523.
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379.
- Nagati, V.; Tenugu, S.; Pasupulati, A.K. Chapter 4—Stability of Therapeutic Nano-Drugs during Storage and Transportation as Well as after Ingestion in the Human Body. In Nanotechnology in Biomedicine; Das Talukdar, A., Dey Sarker, S., Patra, J.K.B.T.-A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–102. ISBN 978-0-323-88450-1.
- Sharma, P.; Negi, P.; Mahindroo, N. Recent Advances in Polymeric Drug Delivery Carrier Systems. Adv. Polym. Biomed. Appl. 2018, 10, 369–388.
- Hadjianfar, M.; Semnani, D.; Varshosaz, J. Polycaprolactone/Chitosan Blend Nanofibers Loaded by 5-Fluorouracil: An Approach to Anticancer Drug Delivery System. Polym. Adv. Technol. 2018, 29, 2972–2981.
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313.
- Gouda, M.; Khalaf, M.M.; Shaaban, S.; Abd El-Lateef, H.M. Fabrication of Chitosan Nanofibers Containing Some Steroidal Compounds as a Drug Delivery System. Polymers 2022, 14, 2094.
- Mendes, A.C.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybrid Electrospun Chitosan-Phospholipids Nanofibers for Transdermal Drug Delivery. Int. J. Pharm. 2016, 510, 48–56.
- Song, T.; Yao, C.; Li, X. Electrospinning of Zein/Chitosan Composite Fibrous Membranes. Chin. J. Polym. Sci. 2010, 28, 171–179.
- Torres-Martinez, E.J.; Cornejo Bravo, J.M.; Serrano Medina, A.; Pérez González, G.L.; Villarreal Gómez, L.J. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374.
- Zhang, Z.; Qu, Q.; Li, J.; Zhou, S. The Effect of the Hydrophilic/Hydrophobic Ratio of Polymeric Micelles on Their Endocytosis Pathways into Cells. Macromol. Biosci. 2013, 13, 789–798.
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298.
- Ma, S.; Feng, X.; Liu, F.; Wang, B.; Zhang, H.; Niu, X. The Pro-Inflammatory Response of Macrophages Regulated by Acid Degradation Products of Poly(Lactide-Co-Glycolide) Nanoparticles. Eng. Life Sci. 2021, 21, 709–720.
- Mishra, D.; Gade, S.; Pathak, V.; Vora, L.; Mcloughlin, K.; Medina, R.; Donnelly, R.; Raghu Raj Singh, T. Ocular Application of Electrospun Materials for Drug Delivery and Cellular Therapies. Drug Discov. Today 2023, 28, 103676.
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A.; McPhee, D.J. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847.
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol )-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2019, 12, 7.
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl Alcohol Based-Drug Delivery Systems for Cancer Treatment. Int. J. Pharm. 2021, 600, 120478.
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484.
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963.
- El-Seedi, H.R.; Said, N.S.; Yosri, N.; Hawash, H.B.; El-Sherif, D.M.; Abouzid, M.; Abdel-Daim, M.M.; Yaseen, M.; Omar, H.; Shou, Q.; et al. Gelatin Nanofibers: Recent Insights in Synthesis, Bio-Medical Applications and Limitations. Heliyon 2023, 9, e16228.
- Milano, F.; Masi, A.; Madaghiele, M.; Sannino, A.; Salvatore, L.; Gallo, N. Current Trends in Gelatin-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1499.
- Snetkov, P.; Morozkina, S.; Uspenskaya, M.; Olekhnovich, R. Hyaluronan-Based Nanofibers: Fabrication, Characterization and Application. Polymers 2019, 11, 2036.
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic Acid and Its Biomedical Applications: A Review. Eng. Regen. 2020, 1, 102–113.
- Humaira; Raza Bukhari, S.A.; Shakir, H.A.; Khan, M.; Saeed, S.; Ahmad, I.; Muzammil, K.; Franco, M.; Irfan, M.; Li, K. Hyaluronic Acid-Based Nanofibers: Electrospun Synthesis and Their Medical Applications; Recent Developments and Future Perspective. Front. Chem. 2022, 10, 1092123.
- Fu, C.P.; Cai, X.Y.; Chen, S.L.; Yu, H.W.; Fang, Y.; Feng, X.C.; Zhang, L.M.; Li, C.Y. Hyaluronic Acid-Based Nanocarriers for Anticancer Drug Delivery. Polymers 2023, 15, 2317.
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718.
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 1–22.
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication Challenges and Trends in Biomedical Applications of Alginate Electrospun Nanofibers. Carbohydr. Polym. 2020, 228, 115419.
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8.
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175.
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. Part B Eng. 2020, 199, 108238.
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-Co-Glycolic Acid) and Progress of Poly (Lactic-Co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454.
- Yao, J.; Wang, Y.; Ma, W.; Dong, W.; Zhang, M.; Sun, D. Dual-Drug-Loaded Silk Fibroin/PLGA Scaffolds for Potential Bone Regeneration Applications. J. Nanomater. 2019, 2019, 8050413.
- Homaeigohar, S.; Boccaccini, A.R. Nature-Derived and Synthetic Additives to Poly(ɛ-Caprolactone) Nanofibrous Systems for Biomedicine; an Updated Overview. Front. Chem. 2022, 9, 809676.
- Bhadran, A.; Shah, T.; Babanyinah, G.K.; Polara, H.; Taslimy, S.; Biewer, M.C.; Stefan, M.C. Recent Advances in Polycaprolactones for Anticancer Drug Delivery. Pharmaceutics 2023, 15, 19–21.
- Lobo, A.O.; Afewerki, S.; de Paula, M.M.M.; Ghannadian, P.; Marciano, F.R.; Zhang, Y.S.; Webster, T.J.; Khademhosseini, A. Electrospun Nanofiber Blend with Improved Mechanical and Biological Performance. Int. J. Nanomed. 2018, 13, 7891–7903.
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug Delivery Systems Based on Polyethylene Glycol Hydrogels for Enhanced Bone Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647.
- Williams, G.R.; Raimi-Abraham, B.T.; Luo, C.J. Nanofibres in Drug Delivery Applications. Fibers 2023, 11, 21.
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Electrospun Starch Nanofibers: Recent Advances, Challenges, and Strategies for Potential Pharmaceutical Applications. J. Control. Release 2017, 252, 95–107.
- Amariei, N.; Manea, L.R.; Bertea, A.P.; Bertea, A.; Popa, A. The Influence of Polymer Solution on the Properties of Electrospun 3D Nanostructures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 12092.
- Haider, A.; Haider, S.; Kang, I.K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188.
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in Three-Dimensional Nanofibrous Macrostructures via Electrospinning. Prog. Polym. Sci. 2014, 39, 862–890.
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117.
- Khalf, A.; Madihally, S.V. Recent Advances in Multiaxial Electrospinning for Drug Delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17.
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
767
Revisions:
2 times
(View History)
Update Date:
27 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





