
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ayman El-Menyar | -- | 5479 | 2023-09-25 10:52:47 | | | |
| 2 | Lindsay Dong | -1 word(s) | 5478 | 2023-09-26 10:53:58 | | |
Video Upload Options
Thyroid storm (TS) is a rare and fatal endocrine emergency that occurs due to undiagnosed and inadequately treated hyperthyroidism after stressful conditions in patients with thyroid disorders. The myocardial involvement in terms of injury, dysrhythmia, cardiomyopathy, failure, and cardiogenic shock (CS) during TS and the modalities of treatment and their efficiency, including pharmacological, mechanical, and surgical options are explored.
1. Introduction
2. Thyroid Storm Complicated with Myocardial Involvement and Shock
Precipitating factors: TS can be triggered by various factors; some may spark underlying thyroid dysfunction, and others exacerbate existing thyroid diseases, particularly Graves’ disease [16]. These precipitating factors include noncompliance with ATD, surgery (thyroid and non-thyroid), trauma, acute illness with infection, childbirth, hydatidiform mole, acute stress, drugs, and excess iodine intake [2][16][19][20][21][22]. As 99% of thyroid hormones are in a bound form, stressors may decrease the hormone-binding capacity and thus abruptly increase the concentration of the free hormones, leading to TS (2191). Even microtrauma in stable thyroid disorders, such as fine-needle aspiration (FNA), can cause TS [23].
Noncompliance with medications: Galindo et al. [6] reported non-compliance in 15.4% of TS cases in comparison with 6% in thyrotoxicosis without storm. Moreover, insurance and side effects of ATDs interfere with the continuity of therapy [24][25][26].
Drug-induced TS: A few cases of amiodarone-induced TS that led to cardiomyopathy and CS [27][28][29] were found. Amiodarone is a class III antiarrhythmic drug with iodine content used to treat tachyarrhythmia, in around 14–18% of patients; it may result in amiodarone-induced thyrotoxicosis (AIT) [30]. AIT can occur in up to 10% of patients [30]. The risk of developing AIT can be seen after 18 months to 3 years; even so, it may occur after withdrawal, as the drug can remain in the tissues for a prolonged time. There are two types of AIT; type 1 generally occurs in patients with clinical thyroid disease [31].
Acute illness and infection: The presence of infection can aggravate thyrotoxicosis in patients with TS, especially if unresolved or missed. Acute illnesses such as myocardial infarction, stroke, diabetic ketoacidosis (DKA), or hypoglycemia can lead to TS [24][32][33][34][35][36][37]. Das et al. [38] reported a case of TS with acute decompensated heart failure, possibly triggered by SARS-CoV-2 infection, in a 16-year-old female. This patient had pre-existing Graves’ disease and dilated cardiomyopathy; however, it was stable prior to COVID-19 infection. The overaction of the T helper cell response and elevation in interleukin-6 due to COVID-19 infection resulted in a change in thyroid gland functionality [38]. Prasankati et al. [39] described TS and COVID-19 in a patient without a history of heart or thyroid disease who presented with SVT.
| Measures of Treatment | Strength of Recommendation | Quality of Evidence |
|---|---|---|
| Antithyroid drugs (ATDs) | High | Low |
| Inorganic iodide | High | Moderate |
| Corticosteroids | High | Moderate |
| Cooling with acetaminophen and mechanical cooling | High | Low |
| Therapeutic plasmapheresis | Weak | Low |
| Central nervous system manifestations treatment | Strong | Low |
| Tachycardia treatment | High | Low |
| Atrial fibrillation treatment | High | Low |
| Acute congestive heart failure | High | Low |
| Treatment Modalities | N of Cases | Doses | Mechanism of Action/Indications | Side Effects and Contraindications |
|---|---|---|---|---|
| Anti-thyroid drugs (ATD) Carbimazole (CBZ) Methimazole (MMI) Propylthiouracil (PTU) |
228 | - MMI and CBZ oral 20–30 mg/day every 6–4 h. - PTU: 200 mg every 4 h. |
First line of treatment to control TS.
|
Agranulocytosis.
Rash. Thrombocytopenia (CBZ may be switched to PTU). Antineutrophilic cytoplasmic antibody vasculitis (PTU). Antithyroid arthritis syndrome (CBZ/MMI). |
| Inorganic iodide Saturated solution of Potassium iodide (SSKI) Lugol iodine |
111 | SKKI: 200 mg/day. Lugol Iodine: 5–10 drops orally once in 6–8 h. |
Wolff–Chaikoff effect
- Decreases blood flow to thyroid gland and so can be given prior to thyroidectomy. |
Hyperkalemia (potassium iodide). Due to the transient action:
|
| Cholestyramine | 33 | A total of 4 g oral intake 2–4 times a day. | - Elimination of thyroid hormone in enterohepatic circulation by binding to iodothyronines. - Indications:
|
|
| Corticosteroids Hydrocortisone/Dexamethasone prednisone |
172 | -IV/IM hydrocortisone: 150. mg/day every 6 h. -IV dexamethasone; 2 mg every 6 h. |
- When given in high doses, it inhibits thyroid hormone release, T4 and T3 conversion inhibition, and prevents adrenal insufficiency related to the hypermetabolic state of TS. - Increases vasomotor stability. - Given until TS resolves. |
|
| Beta Blockers Propranolol (NCBB) Metoprolol Esmolol (SC) Bisoprolol Landiolol (USC) Sotalol |
191 | -Propranolol: 1. oral or NGT 60–80 mg, 2. IV: 0.5–1 mg over 10 min followed by 1–2 mg over 10 every few hours. -Short-acting (Esmolol): a loading dose of 250–500 mcg/kg, followed by 50–100 mcg/kg infusion. |
|
Cardiogenic shock
Circulatory collapse. Hypotension. Refractory hypotension - Bronchoconstriction with bisoprolol. |
| Calcium channel blockers Verapamil Diltiazem |
30 | IV diltiazem push: 20 mg. | - Inhibit Ca2+ into excitable cells, resulting in smooth muscle dilation. - Negative inotropes in cardiac cells. - Indications:
- Was given for AF prior to TS diagnosis then discontinued when diagnosis made. |
- Cardiogenic shock. - Asystole. |
| Digoxin | 25 | IV: 0.125–0.25 mg. | Increases cardiac contractility as it binds and inhibits the Na/K-ATPase pump within cardiac myocytes. Positive inotropic effect:
|
Avoid in case of renal dysfunction as it increases renal clearance. - Worsening hypotension. |
| Inotropes (Vasopressors) Dopamine Dobutamine Epinephrine Levosimendan Noraderanline Milrinone |
81 | Dobutamine: infusion 2 (ug/kg/min) Noradrenaline. |
Dobutamine/dopamine: Inotrope with high affinity to B1 adrenergic receptors.
Milrinone:
|
|
| Amiodarone | 19 | IV: 125 mg over 10 min followed by a 0.8 mg infusion for 6 h. | - An iodine-rich class III antiarrhythmic - Blocks 5′mono-deiodination of t4 in peripheral tissues as the liver and pituitary gland.
- Most common antiarrhythmic in ICU due to stable properties.
|
- Hyperthyroid activity and thyrotoxic precipitant (Jod- Basedow phenomenon). - Amiodarone-induced thyrotoxicosis. - Hepatotoxicity; worsened ischemic hepatic failure. - Worsening hypotension |
Beta-blocker associated with circulatory collapse: BBs therapy has been described as a double-edged sword in TS [77]. Patients with prior clinical thyrotoxic cardiomyopathy or subclinical disease, especially in a setting of low-output heart failure, are prone to circulatory collapse when administered propranolol [63][78]. This may be attributed to NCBBs averting the compensatory hyperadrenergic state caused by thyrotoxicosis, and thus, a sharp decrease in the cardiac output in events as a TS leads to circulatory collapse [78]. Approximately 25.8% of cases reported hemodynamic instability and circulatory collapse, possibly due to BBs. In these cases, some were administered concomitantly with BBs and CCBs. Both agents are known to have negative inotropic effects [79]. Propranolol was the BB used in 46% of cases. Patients administered propranolol or atenolol tend to require extensive resuscitation [80]. Evidently, in conditions with signs of heart failure and low ejection fraction during thyrotoxic crisis, other agents are recommended [10][32][81][82]. Such agents are cardioselective BBs with shorting-acting properties, such as landiolol and esmolol, because titration and cessation are attainable [10][80][83][84]. Moreover, Voll et al. highlighted that even when dobutamine was administered during circulatory collapse alongside a high dose of propranolol, it was deemed less effective [25].
- (a)
-
Extracorporeal Membrane Oxygenation (VA-ECMO): In 2021, Lim et al. [77] reported that there were 27 cases in the literature at the time of thyrotoxic crisis requiring ECMO, and 85% of these patients survived. In severe cases, first-line pharmacotherapy may not be sufficient to restore cardiovascular function to normal levels after TS development. When faced with this, extracorporeal modalities are implemented. Among the 256 cases, the use of ECMO was reported in 16.3% of cases; hence, it was the most used mechanical support. ECMO bypasses the heart and lungs and provides gas exchange through the external membrane [77]. This process supports the heart by temporarily relieving the heart of its functions to allow it to heal, while thyroid hormones normalize, and the euthyroid state is restored [86].
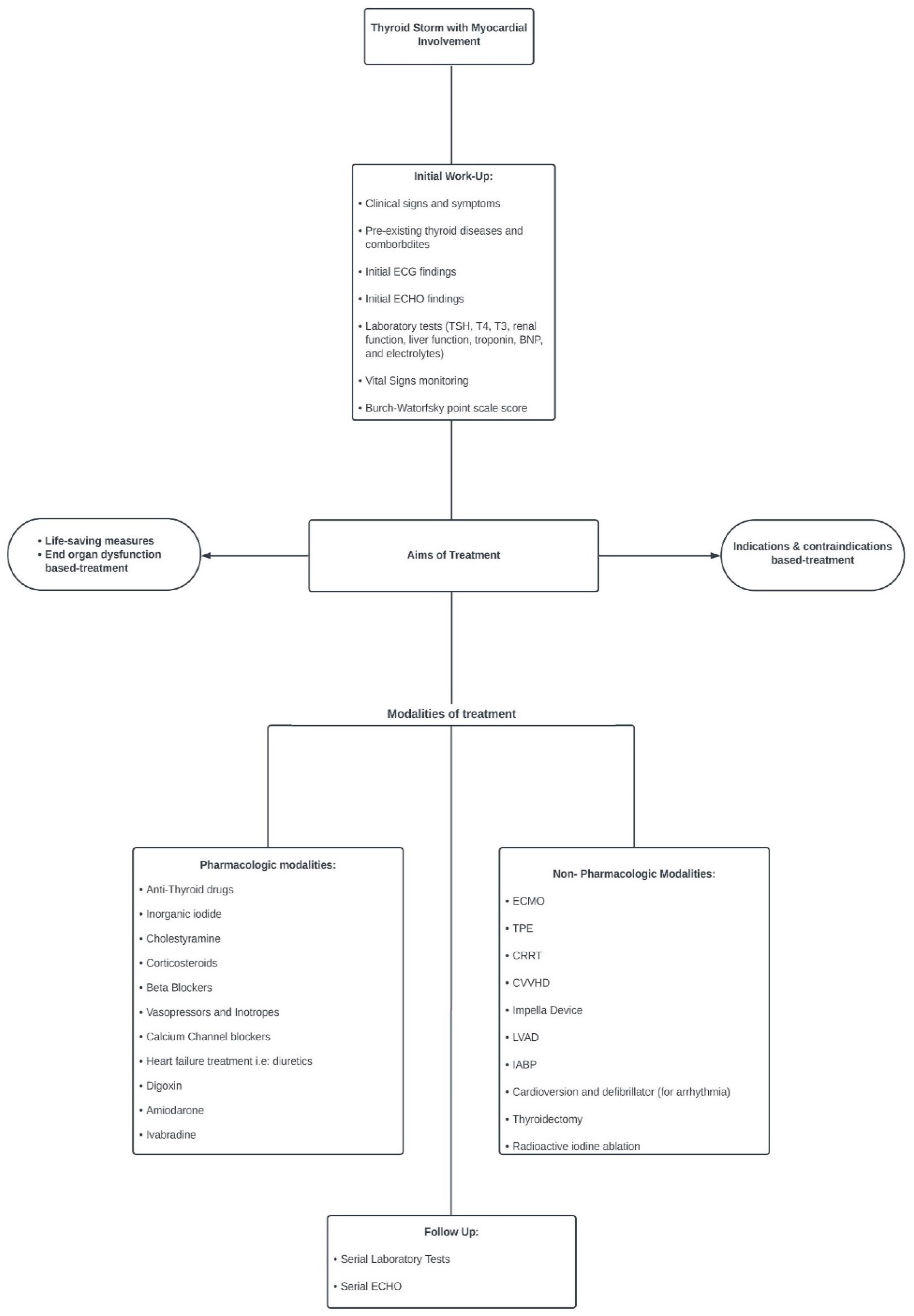
3. Conclusions
References
- Yamamoto, H.; Monno, S.; Ohta-Ogo, K.; Ishibashi-Ueda, H.; Hashimoto, T. Delayed diagnosis of dilated thyrotoxic cardiomyopathy with coexistent multifocal atrial tachycardia: A case report. BMC Cardiovasc. Disord. 2021, 21, 124.
- Chiha, M.; Samarasinghe, S.; Kabaker, A.S. Thyroid storm: An updated review. J. Intensive Care Med. 2015, 30, 131–140.
- Akamizu, T.; Satoh, T.; Isozaki, O.; Suzuki, A.; Wakino, S.; Iburi, T.; Tsuboi, K.; Monden, T.; Kouki, T.; Otani, H.; et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid 2012, 22, 661–679.
- Nayak, B.; Burman, K. Thyrotoxicosis and thyroid storm. Endocrinol. Metab. Clin. N. Am. 2005, 35, 661–679.
- Kornelius, E.; Chang, K.L.; Yang, Y.S.; Huang, J.Y.; Ku, M.S.; Lee, K.Y.; Ho, S.W. Epidemiology and factors associated with mortality of thyroid storm in Taiwan: A nationwide population-based study. Intern. Emerg. Med. 2021, 16, 601–607.
- Galindo, R.J.; Hurtado, C.R.; Pasquel, F.J.; García Tome, R.; Peng, L.; Umpierrez, G.E. National Trends in Incidence, Mortality, and Clinical Outcomes of Patients Hospitalized for Thyrotoxicosis with and without Thyroid Storm in the United States, 2004–2013. Thyroid 2019, 29, 36–43.
- Sarlis, N.J.; Gourgiotis, L. Thyroid emergencies. Rev. Endocr. Metab. Disord. 2003, 4, 129–136.
- Silva, J.E.; Bianco, S.D. Thyroid-adrenergic interactions: Physiological and clinical implications. Thyroid 2008, 18, 157–165.
- Lorlowhakarn, K.; Kitphati, S.; Songngerndee, V.; Tanathaipakdee, C.; Sinphurmsukskul, S.; Siwamogsatham, S.; Puwanant, S.; Ariyachaipanich, A. Thyrotoxicosis-Induced Cardiomyopathy Complicated by Refractory Cardiogenic Shock Rescued by Extracorporeal Membrane Oxygenation. Am. J. Case Rep. 2022, 23, e935029.
- Sourial, K.; Borgan, S.M.; Mosquera, J.E.; Abdelghani, L.; Javaid, A. Thyroid Storm-induced Severe Dilated Cardiomyopathy and Ventricular Tachycardia. Cureus 2019, 11, e5079.
- Abera, B.T.; Abera, M.A.; Berhe, G.; Abreha, G.; Gebru, H.T.; Abraha, H.E.; Ebrahim, M.M. Thyrotoxicosis and dilated cardiomyopathy in developing countries. BMC Endocr. Disord. 2021, 21, 132.
- Soomro, R.; Campbell, N.; Campbell, S.; Lesniak, C.; Sullivan, M.; Ong, R.; Cheng, J.; Hossain, M.A. Thyroid storm complicated by multisystem organ failure requiring plasmapheresis to bridge to thyroidectomy: A case report and literature review. Clin. Case Rep. Rev. 2019, 5, 1–4.
- Burch, H.B.; Wartofsky, L. Life-threatening thyrotoxicosis. Thyroid Storm. Endocrinol. Metab. Clin. N. Am. 1993, 22, 263–277.
- Clark, O.H.; Duh, Q.; Kebebew, E. Textbook of Endocrine Surgery, 2nd ed.; Elsevier Saunders: Philadelphia, PA, USA, 2005; pp. 216–219.
- Sakaan, R.A.; Poole, M.A.; Long, B. Diltiazem-Induced Reversible Cardiogenic Shock in Thyroid Storm. Cureus 2021, 13, e19261.
- Arai, M.; Asaumi, Y.; Murata, S.; Matama, H.; Honda, S.; Otsuka, F.; Tahara, Y.; Kataoka, Y.; Nishimura, K.; Noguchi, T. Thyroid Storm Patients with Elevated Brain Natriuretic Peptide Levels and Associated Left Ventricular Dilatation May Require Percutaneous Mechanical Support. Crit. Care Explor. 2021, 3, e0599.
- Pandey, R.; Kumar, S.; Kotwal, N. Thyroid Storm: Clinical Manifestation, Pathophysiology, and Treatment; IntechOpen: London, UK, 2020.
- Carroll, R.; Matfin, G. Endocrine and Metabolic Emergencies: Thyroid Storm. Ther. Adv. Endocrinol. Metab. 2010, 1, 139–145.
- Jiménez-Labaig, P.; Mañe, J.M.; Rivero, M.P.; Lombardero, L.; Sancho, A.; López-Vivanco, G. Just an Acute Pulmonary Edema? Paraneoplastic Thyroid Storm Due to Invasive Mole. Case Rep. Oncol. 2022, 15, 566–572.
- Jayasuriya, A.; Muthukuda, D.; Dissanayake, P.; Subasinghe, S. Recurrent Thyroid Storm Caused by a Complete Hydatidiform Mole in a Perimenopausal Woman. Case Rep. Endocrinol. 2020, 2020, 8842987.
- Samra, T.; Kaur, R.; Sharma, N.; Chaudhary, L. Peri-operative concerns in a patient with thyroid storm secondary to molar pregnancy. Indian J. Anaesth. 2015, 59, 739–742.
- Kofinas, J.D.; Kruczek, A.; Sample, J.; Eglinton, G.S. Thyroid storm-induced multi-organ failure in the setting of gestational trophoblastic disease. J. Emerg. Med. 2015, 48, 35–38.
- Idowu, A.O.; Adesegun, O.A.; Osibowale, B.; Ajiro, T.; Ezuduemoih, D.; Osonuga, A. A case of thyroxine (T4) toxicosis complicated by thyroid storm with an unusual precipitant. Casp. J. Intern. Med. 2020, 11, 231–234.
- Kinoshita, H.; Sugino, H.; Oka, T.; Ichikawa, O.; Shimonaga, T.; Sumimoto, Y.; Kashiwabara, A.; Sakai, T. A case in which SGLT2 inhibitor is a contributing factor to takotsubo cardiomyopathy and heart failure. J. Cardiol. Cases 2020, 22, 177–180.
- Voll, M.; Øystese, K.A.; Høiskar, E.; Johansen, O.; Nyvold, C.; Norheim, I.; von Lueder, T.G.; Andersen, G. Case report: A patient with thyroid storm, refractory cardiogenic shock, and cardiac arrest treated with Lugol’s iodine solution and veno-arterial extracorporeal membrane oxygenation support. Eur. Heart J. 2021, 5, ytab017.
- Matsubara, M.; Tanaka, T.; Wakamiya, A.; Tamanaha, T.; Makino, H.; Tanei, T.; Aiba, T.; Kusano, K.; Hosoda, K. First Case Report of Arrhythmogenic Right Ventricular Cardiomyopathy Showing Refractory Ventricular Tachycardia Induced by Thyroid Storm due to Graves’ Disease. Case Rep. Endocrinol. 2022, 2022, 6078148.
- Pong, V.; Yeung, C.-Y.; Ko, R.L.-Y.; Tse, H.-F.; Siu, C.-W. Extracorporeal Membrane Oxygenation in Hyperthyroidism-Related Cardiomyopathy: Two Case Reports. J. Endocrinol. Metab. 2013, 3, 24–28.
- Bou Chaaya, R.G.; Saint, L.M.; Ilonze, O.J. Thyroidectomy in Mechanical Circulatory Support—A Salvage Treatment for Thyrotoxicosis-Induced Cardiogenic Shock: Case Series. VAD J. 2021, 7, e2021710.
- Nakashima, Y.; Kenzaka, T.; Okayama, M.; Kajii, E. A Case of Thyroid Storm with Cardiac Arrest. Int. Med. Case Rep. J. 2019, 12, 413–416.
- Martino, E.; Bartalena, L.; BogazziI, F.; Braveman, L.E. The Effects of Amiodarone on the Thyroid. Endocr. Soc. 2001, 22, 240–254.
- Ozcan, E.E.; Dogdus, M.; Yilancioglu, R.Y.; Adiyman, S.C.; Turan, O.E. Invasive Heart Rate Control as a Salvage Therapy in Amiodarone-induced Thyroid Storm. Medeniyet Med. J. 2022, 37, 119–122.
- Wu, W.-T.; Hsu, P.-C.; Huang, H.-L.; Chen, Y.-C.; Chien, S.-C. A Case of Takotsubo Cardiomyopathy Precipitated by Thyroid Storm and Diabetic Ketoacidosis with Poor Prognosis. Acta Cardiol. Sin. 2014, 30, 574–577.
- Chang, C.H.; Lian, H.W.; Sung, Y.F. Cystic Encephalomalacia in a Young Woman After Cardiac Arrest Due to Diabetic Ketoacidosis and Thyroid Storm. Cureus 2022, 14, e23707.
- Ikeoka, T.; Otsuka, H.; Fujita, N.; Masuda, Y.; Maeda, S.; Horie, I.; Ando, T.; Abiru, N.; Kawakami, A. Thyroid Storm Precipitated by Diabetic Ketoacidosis and Influenza A: A Case Report and Literature Review. Intern. Med. 2017, 56, 181–185.
- Lin, C.H.; Chen, S.C.; Lee, C.C.; Ko, P.C.; Chen, W.J. Thyroid storm concealing diabetic ketoacidosis leading to cardiac arrest. Resuscitation 2004, 63, 345–347.
- Brown, J.; Cham, M.D.; Huang, G.S. Storm and STEMI: A case report of unexpected cardiac complications of thyrotoxicosis. Eur. Heart J. Case Rep. 2020, 4, 1–5.
- Abbasi, A.A.; Chandar, P.; Shankar, S.; Gupta, S.S.; Kupfer, Y. Thyrotoxic Periodic Paralysis and Cardiomyopathy in a Patient with Graves’ Disease. Cureus 2018, 10, e2837.
- Das, B.B.; Shakti, D.; Akam-Venkata, J.; Obi, O.; Weiland, M.D.; Moskowitz, W. SARS-CoV-2 infection induced thyroid storm and heart failure in an adolescent girl. Cardiol. Young 2022, 32, 988–992.
- Pranasakti, M.E.; Talirasa, N.; Rasena, H.A.; Purwanto, R.Y.; Anwar, S.L. Thyrotoxicosis occurrence in SARS-CoV-2 infection: A case report. Ann. Med. Surg. 2022, 78, 103700.
- Razvi, S.; Jabbar, A.; Pingitore, A.; Danzi, S.; Biondi, B.; Klein, I.; Peeters, R.P.; Zaman, A.; Iervasi, G. Thyroid Hormones and Cardiovascular Function and Diseases. J. Am. Coll. Cardiol. 2018, 71, 1781–1796.
- Albakri, A. Thyrotoxic heart failure: A review of clinical status and meta-analysis of electrocardiogram diagnosis and medical clinical management methods. Integr. Mol. Med. 2018, 5, 2–11.
- Waqar, Z.; Avula, S.; Shah, J.; Ali, S.S. Cardiovascular Events in Patients with Thyroid Storm. J. Endocr. Soc. 2021, 5, bvab040.
- Satoh, T.; Isozaki, O.; Suzuki, A.; Wakino, S.; Iburi, T.; Tsuboi, K.; Kanamoto, N.; Otani, H.; Furukawa, Y.; Teramukai, S.; et al. 2016 Guidelines for the management of thyroid storm from The Japan Thyroid Association and Japan Endocrine Society (First edition). Endocr. J. 2016, 63, 1025–1064.
- Zayour, M.; Yasmin, F.A.; Baydoun, A.; Tawk, M.; Sleiman, D.; Shatila, W.; Chamoun, C. Cardiac Arrest as First Presentation of Thyroid Storm. Cureus 2023, 15, e37057.
- Osuna, P.M.; Udovcic, M.; Sharma, M. DHyperthyroidism and the Heart. Methodist DeBakey Cardiovasc. J. 2017, 13, 60–63.
- Chao, A.; Wang, C.H.; You, H.C.; Chou, N.K.; Yu, H.Y.; Chi, N.H.; Huang, S.C.; Wu, I.H.; Tseng, L.J.; Lin, M.H.; et al. Highlighting Indication of extracorporeal membrane oxygenation in endocrine emergencies. Sci. Rep. 2015, 5, 13361.
- Ali, H.; Sarfraz, S.; Hassan, L.; Ali, H. Atrial Fibrillation as an Initial Presentation of Apathetic Thyroid Storm. Cureus 2021, 13, e17786.
- Naik, S.K.; Hallak, N.; Patel, R.; Esan, A.; Saleh, A.; Sung, A.; Raoof, S. Reversible thyrotoxic cardiomyopathy: Prompt. Chest 2009, 136, 37S.
- Sugiyama, Y.; Tanaka, R.; Yoshiyama, Y.; Ichino, T.; Hishinuma, N.; Shimizu, S.; Imai, N.; Mitsuzawa, K.; Kawamata, M. A case of sudden onset of thyroid storm just before cesarean section manifesting congestive heart failure and pulmonary edema. JA Clin. Rep. 2017, 3, 20.
- Ueno, A.; Yamamoto, T.; Sato, N.; Tanaka, K. Ventricular fibrillation associated with early repolarization in a patient with thyroid storm. J. Interv. Card. Electrophysiol. 2010, 29, 93–96.
- Omar, A.M.A.; Knott, K.; Saba, M.M.; Lim, P.O. Cardiac arrest in myocardial infarction with non-obstructive coronary artery (MINOCA) secondary to thyroid dysfunction. BMJ Case Rep. 2023, 16, e253500.
- Korte, A.K.; Derde, L.; van Wijk, J.; Tjan, D.H. Sudden cardiac arrest as a presentation of Brugada syndrome unmasked by thyroid storm. BMJ Case Rep. 2015, 2015, bcr2015212351.
- Chaker, L.; van den Berg, M.E.; Niemeijer, M.N.; Franco, O.H.; Dehghan, A.; Hofman, A.; Rijnbeek, P.R.; Deckers, J.W.; Eijgelsheim, M.; Stricker, B.H.; et al. Thyroid Function and Sudden Cardiac Death: A Prospective Population-Based Cohort Study. Circulation 2016, 134, 713–722.
- Jao, Y.T.F.N.; Chen, Y.; Lee, W.-H.; Tai, F.-T. Thyroid storm and ventricular tachycardia. South. Med. J. 2004, 97, 604–607.
- Lencu, C.; Alexescu, T.; Petrulea, M.; Lencu, M. Respiratory manifestations in endocrine diseases. Clujul Med. 2016, 89, 459–463.
- Klomp, M.; Siegelaar, S.E.; van de Hoef, T.P.; Beijk, M.A.M. A case report of myocardial infarction with non-obstructive coronary artery disease: Graves’ disease-induced coronary artery vasospasm. Eur. Heart J. Case Rep. 2020, 4, 1–5.
- El-Menyar, A.A. Drug-induced myocardial infarction secondary to coronary artery spasm in teenagers and young adults. J. Postgrad. Med. 2006, 52, 51–56.
- Zheng, W.; Zhang, Y.J.; Li, S.Y.; Liu, L.-L.; Sun, J. Painless thyroiditis-induced acute myocardial infarction with normal coronary arteries. Am. J. Emerg. Med. 2015, 33, 983.
- Kataoka, S.; Matsuno, K.; Sugano, K.; Takahashi, K. Thyroid storm induced by combined nivolumab and ipilimumab immunotherapy in advanced non-small cell lung cancer. BMJ Case Rep. 2022, 15, e250696.
- Hammond, H.K.; White, F.C.; Buxton, I.L.; Saltzstein, P.; Brunton, L.L.; Longhurst, J.C. Increased myocardial beta-receptors and adrenergic responses in hyperthyroid pigs. Am. J. Physiol. 1987, 252, H283–H290.
- Lassnig, E.; Berent, R.; Auer, J.; Eber, B. Cardiogenic shock due to myocardial infarction caused by coronary vasospasm associated with hyperthyroidism. Int. J. Cardiol. 2003, 90, 333–335.
- Allencherril, J.; Birnbaum, I. Heart Failure in Thyrotoxic Cardiomyopathy: Extracorporeal Membrane Oxygenation Treatment for Graves’ Disease. JECT 2015, 47, 231–232.
- Tolu-Akinnawo, O.Z.; Abiade, J.; Awosanya, T.; Okafor, H.E. Thyrotoxicosis-Induced Cardiogenic Shock: Acute Management Using a Multidisciplinary Approach. Cureus 2022, 14, e32841.
- Taylor, G.M.; Pop, A.M.C.; McDowell, E.L. A case report of thyroid storm presenting as hemodynamic instability and acute kidney injury. Oxford Med. Case Rep. 2019, 2019, 252–255.
- Dahl, P.; Danzi, S.; Klein, I. Thyrotoxic cardiac disease. Curr. Heart Fail. Rep. 2008, 5, 170–176.
- Alam, S.; Zaman, J. Case study of thyrotoxic cardiomyopathy. BMJ Case Rep. 2019, 12, e228896.
- Subahi, A.; Ibrahim, W.; Abugroun, A. Diltiazem-Associated Cardiogenic Shock in Thyrotoxic Crisis. Am. J. Ther. 2018, 25, 1075–1078.
- Chariyawong, P.; Rao, A.; Panikkath, D.; Panikkath, R. Hyperthyroidism-induced dilated cardiomyopathy. Southwest Respir. Crit. Care Chron. 2019, 7, 64–66.
- Witczak, J.K.; Ubaysekara, N.; Ravindran, R.; Rice, S.; Yousef, Z.; Premawardhana, L.D. Significant cardiac disease complicating Graves’ disease in previously healthy young adults. Endocrinol. Diabetes Metab. Case Rep. 2019, 2020, 19-0132.
- Rana, A.; Assad, S.; Abuzaid, M.; Farooqi, A.; Nolte, J. Thyrotoxicosis-Induced Cardiogenic Shock with Encephalopathy and Acute Respiratory Distress: A Case Report and Literature Review. Cureus 2020, 12, e8213.
- Rushing, M.W.; Rebolledo, M.A.; Lahoti, A.; Alemzadeh, R. Acute febrile illness in a teenage female with history of Graves’ disease. Oxf. Med. Case Rep. 2023, 2023, omad050.
- Manuel, L.; Fong, L.; Lahanas, A.; Grant, P. How to do it: Plasmapheresis via venoarterial extracorporeal membrane oxygenation circuit for thyroid storm. Ann. Med. Surg. 2021, 67, 102485.
- Carhill, A.; Gutierrez, A.; Lakhia, R.; Nalini, R. Surviving the storm: Two cases of thyroid storm successfully treated with plasmapheresis. BMJ Case Rep. 2012, 2012, bcr2012006696.
- Amin, T.; Austin, C.P.; Udongwo, N.; Wiseman, K.; Parhar, A.S.; Chaughtai, S. Iodinated Contrast-Induced Thyroid Storm with Concomitant Cardiac Tamponade: A Case Report. Cureus 2022, 14, e28001.
- Bui, P.V.; Zaveri, S.N.; Pierce, J.R. Sanguinous pericardial effusion and cardiac tamponade in the setting of Graves’ disease: Report of a case and review of previously reported cases. Case Rep. Med. 2016, 2016, 9653412.
- Pokhrel, B.; Aiman, W.; Bhusal, K. Thyroid Storm. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Lim, S.L.; Wang, K.; Lui, P.L.; Ramanathan, K.; Yang, S.P. Crash Landing of Thyroid Storm: A Case Report and Review of the Role of Extra-Corporeal Systems. Front. Endocrinol. 2021, 12, 725559.
- Çiçek, V.; Çınar, T.; Selçuk, M.; Orhan, A.L. Acute Thyrotoxicosis Induced Reversible Cardiomyopathy in an Adult Patient. Hamidiye Med. J. 2021, 2, 138–140.
- Chao, J.; Cook, R.; Dhingra, V. Escaping a storm alive: A case report of a young woman’s acute presentation of thyroid storm leading to cardiac arrest salvaged by VA-ECMO. J. Clin. Anesth. Intensive Care 2021, 2, 26–30.
- Dalan, R.; Leow, M. Cardiovascular Collapse Associated with Beta Blockade in Thyroid Storm. Exp. Clin. Endocrinol. Diabetes 2007, 115, 392–396.
- Du, F.; Liu, S.W.; Yang, H.; Duan, R.X.; Ren, W.X. Thyrotoxicosis after a massive levothyroxine ingestion: A case report. World J. Clin. Cases 2022, 10, 3624–3629.
- Noh, K.W.; Seon, C.S.; Choi, J.W.; Cho, Y.B.; Park, J.Y.; Kim, H.J. Thyroid Storm and Reversible Thyrotoxic Cardiomyopathy After Ingestion of Seafood Stew Thought to Contain Marine Neurotoxin. Thyroid 2011, 21, 679–682.
- Yamashita, Y.; Iguchi, M.; Nakatani, R.; Usui, T.; Takagi, D.; Hamatani, Y.; Unoki, T.; Ishii, M.; Ogawa, H.; Masunaga, N.; et al. Thyroid Storm with Heart Failure Treated with a Short-acting Beta-adrenoreceptor Blocker, Landiolol Hydrochloride. Intern. Med. 2015, 54, 1633–1637.
- Misumi, K.; Kodera, S.; Nagura, F.; Kushida, S.; Shiojiri, T.; Kanda, J. Cardiac arrest caused by landiolol in a patient in thyroid crisis. J. Cardiol. Cases 2016, 14, 62–64.
- Frenkel, A.; Bichovsky, Y.; Arotsker, N.; Besser, L.; Joshua, B.Z.; Fraenkel, M.; Zahger, D.; Klein, M. Ivabradine for Uncontrolled Sinus Tachycardia in Thyrotoxic Cardiomyopathy–Case Report. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 992–995.
- Dyer, M.; Neal, M.D.; Rollins-Raval, M.A.; Raval, J.S. Simultaneous Extracorporeal membrane oxygenation and therapeutic plasma exchange procedures are tolerable in both pediatric and adult patients. Transfusion 2014, 54, 1158–1165.
- Cheah, J.M.; Ng, D.; Low, M.Y.; Foo, S.H. Weathering the Crisis: A Case of Thyroid Crisis with Propranolol-Induced Circulatory Collapse Successfully Treated with Therapeutic Plasma Exchange. ASEAN Fed. Endocr. Soc. 2019, 34, 206–209.
- Park, H.S.; Kwon, S.K.; Kim, Y.N. Successful Treatment of Thyroid Storm Presenting as Recurrent Cardiac Arrest and Subsequent Multiorgan Failure by Continuous Renal Replacement Therapy. Endocrinol. Diabetes Metab. 2017, 16, 0115.
- Tandukar, S.; Palevsky, P.M. Continuous Renal Replacement Therapy: Who, When, Why, and How. Chest 2019, 155, 626–638.
- Khan, T.M.; Siddiqui, A.H. Intra-Aortic Balloon Pump. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Glazier, J.J.; Kaki, A. The Impella Device: Historical Background, Clinical Applications and Future Directions. Int. J. Angiol. 2019, 28, 118–123.
- Fu, Y.; Ge, H.; Zhang, Y.; Li, Y.; Mu, B.; Shang, W.; Li, S.; Ma, Q. Targeted Temperature Management for In-hospital Cardiac Arrest Caused by Thyroid Storm: A Case Report. Front. Cardiovasc. Med. 2021, 8, 634987.
- Biello, A.; Kinberg, E.C.; Wirtz, E.D. Thyroidectomy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Klubo-Gwiezdzinska, J.; Wartofsky, L. Thyroid emergencies. Med. Clin. N. Am. 2012, 96, 385–403.




