
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kazutaka Akagi | -- | 2998 | 2023-09-25 07:08:37 | | | |
| 2 | Lindsay Dong | Meta information modification | 2998 | 2023-09-27 02:49:11 | | |
Video Upload Options
Aging is the slowest process in a living organism. During this process, mortality rate increases exponentially due to the accumulation of damage at the cellular level. Cellular senescence is a well-established hallmark of aging, as well as a promising target for preventing aging and age-related diseases. Given that the appearance of senescent cells is considered to be a cell fate transition from the proliferative state to the non-proliferative state, similar to the critical transitions that occur during cell differentiation and symptom onset, it can be detectable by the dynamical network biomarkers (DNB) theory, which detects early warning signals just before bifurcation points, such as “the pre-disease state”.
1. Introduction
2. Dynamical Network Biomarkers Theory
2.1. The Concept of DNB Theory
2.2. The Applications of DNB Theory
| Models | Cell Types or Species | Datasets | References |
|---|---|---|---|
| Influenza A (H3N2) infection | Human | Microarray of the blood samples | [21][23] |
| COVID-19 infection | Human | Case reports in five different countries and regions | [22] |
| Hepatocellular carcinoma | Xenograft mouse model of HCCLM3 cells | Microarray of the liver samples | [24] |
| Breast cancer | Human breast adenocarcinoma MCF-7 cell line | RNA-seq of MCF-7 cells | [25] |
| Skin photodamage | The LSE model (3D skin model consisting of normal human keratinocyte and melanocyte) | RNA-seq of the LSE model | [26] |
| Lung cancer | KrasLSL-G12D/+; Lkb1flox/flox (KL) mice | RNA-seq of the KL lung samples | [27] |
| Hematopoietic stem cell differentiation | Mouse hematopoietic stem cells (mHSCs) | scRNA-seq of mHSCs | [28] |
| Embryonic stem cell differentiation | Human embryonic stem cells (hESCs) | scRNA-seq of hESCs | [29] |
| Immune cell differentiation | T cells from DO11.10 TCR mice | Raman imaging | [30] |
| Metabolic syndrome | Metabolic syndrome model mouse (TSOD mice) | Microarray of the adipose tissues | [31][32] |
| Type 2 diabetes | Diabetes model rat (GK rats) | Microarray of the adipose tissues | [33] |
2.3. Cancer and Cellular Senescence
3. Senolytics and Senomorphics
The emerging therapeutic strategies for targeting senescent cells are called senotherapies, which include senolytics and senomorphics. Senolytics is the selective elimination of senescent cells by small molecules, while senomorphics is the inhibition of pathological SASPs to cause senostasis (senescent cells stay there but are less harmful) [42]. The majority of the senolytics identified to date promote the apoptosis of senescent cells by targeting the key enzymes involved in cellular pro-survival and anti-apoptotic mechanisms, such as SRC kinases, BCL-2 family proteins, HSP90, PI3K-AKT, p53-FOXO4, GLS1, and others.
Senomorphics, on the other hand, is considered to be a safer alternative to senolytics, as it suppresses the unwanted SASP expressions from senescent cells rather than directly removing them. Senomorphics can directly or indirectly attenuate the SASP of senescent cells by inhibiting mTOR, NF-κB, SIRT1, p38MAPK, JAK-STAT, and other signaling pathways. The safety concern associated with senomorphics is the potential suppression of the growth-promoting functions induced by the SASP, similar to those seen in senolytics.
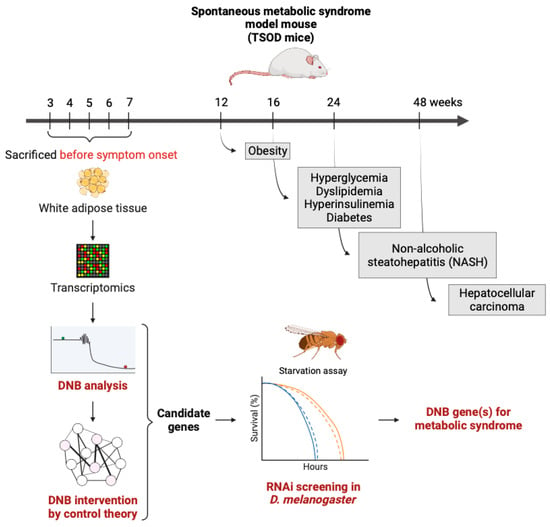
4. DNB Analysis in Metabolism
4.1. Identification of DNB Genes
4.2. Verification of DNB Genes Using a Drosophila Model
5. Homeostasis and Allostasis in Aging
6. Conclusions
References
- Beard, J.R.; Si, Y.; Liu, Z.; Chenoweth, L.; Hanewald, K.; Lipsitz, L. Intrinsic Capacity: Validation of a New WHO Concept for Healthy Aging in a Longitudinal Chinese Study. J. Gerontol. Ser. A 2022, 77, 94–100.
- Kirkland, J.L.; Stout, M.B.; Sierra, F. Resilience in Aging Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1407–1414.
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2022, 186, 243–278.
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713.
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic inflammation and the hallmarks of aging. Mol. Metab. 2023, 74, 101755.
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827.
- Roninson, I.B.; Broude, E.V.; Chang, B.D. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist. Updat. 2001, 4, 303–313.
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453.
- Gurkar, A.U.; Gerencser, A.A.; Mora, A.L.; Nelson, A.C.; Zhang, A.R.; Lagnado, A.B.; Enninful, A.; Benz, C.; Furman, D.; Beaulieu, D.; et al. Spatial mapping of cellular senescence: Emerging challenges and opportunities. Nat. Aging 2023, 3, 776–790.
- Sterling, P. Allostasis: A New Paradigm to Explain Arousal Pathology. In Handbook of Life Stress, Cognition and Health; Wiley: Hoboken, NJ, USA, 1988.
- Scheffer, M.; Bascompte, J.; Brock, W.A.; Brovkin, V.; Carpenter, S.R.; Dakos, V.; Held, H.; van Nes, E.H.; Rietkerk, M.; Sugihara, G. Early-warning signals for critical transitions. Nature 2009, 461, 53–59.
- Scheffer, M.; Carpenter, S.R.; Lenton, T.M.; Bascompte, J.; Brock, W.; Dakos, V.; van de Koppel, J.; van de Leemput, I.A.; Levin, S.A.; van Nes, E.H.; et al. Anticipating critical transitions. Science 2012, 338, 344–348.
- Tytgat, H.L.P.; Nobrega, F.L.; van der Oost, J.; de Vos, W.M. Bowel Biofilms: Tipping Points between a Healthy and Compromised Gut? Trends Microbiol. 2019, 27, 17–25.
- Carpenter, S.R.; Brock, W.A. Rising variance: A leading indicator of ecological transition. Ecol. Lett. 2006, 9, 311–318.
- Dakos, V.; Scheffer, M.; van Nes, E.H.; Brovkin, V.; Petoukhov, V.; Held, H. Slowing down as an early warning signal for abrupt climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 14308–14312.
- Moon, H.; Lu, T.C. Network catastrophe: Self-organized patterns reveal both the instability and the structure of complex networks. Sci. Rep. 2015, 5, 9450.
- Veraart, A.J.; Faassen, E.J.; Dakos, V.; van Nes, E.H.; Lurling, M.; Scheffer, M. Recovery rates reflect distance to a tipping point in a living system. Nature 2011, 481, 357–359.
- Wissel, C. A universal law of the characteristic return time near thresholds. Oecologia 1984, 65, 101–107.
- Raj, A.; van Oudenaarden, A. Nature, nurture, or chance: Stochastic gene expression and its consequences. Cell 2008, 135, 216–226.
- Liu, X.; Chang, X.; Leng, S.; Tang, H.; Aihara, K.; Chen, L. Detection for disease tipping points by landscape dynamic network biomarkers. Natl. Sci. Rev. 2019, 6, 775–785.
- Liu, R.; Zhong, J.; Hong, R.; Chen, E.; Aihara, K.; Chen, P.; Chen, L. Predicting local COVID-19 outbreaks and infectious disease epidemics based on landscape network entropy. Sci. Bull. 2021, 66, 2265–2270.
- Gao, R.; Yan, J.; Li, P.; Chen, L. Detecting the critical states during disease development based on temporal network flow entropy. Brief. Bioinform. 2022, 23, bbac164.
- Yang, B.; Li, M.; Tang, W.; Liu, W.; Zhang, S.; Chen, L.; Xia, J. Dynamic network biomarker indicates pulmonary metastasis at the tipping point of hepatocellular carcinoma. Nat. Commun. 2018, 9, 678.
- Liu, R.; Wang, J.; Ukai, M.; Sewon, K.; Chen, P.; Suzuki, Y.; Wang, H.; Aihara, K.; Okada-Hatakeyama, M.; Chen, L. Hunt for the tipping point during endocrine resistance process in breast cancer by dynamic network biomarkers. J. Mol. Cell Biol. 2019, 11, 649–664.
- Zhang, C.; Zhang, H.; Ge, J.; Mi, T.; Cui, X.; Tu, F.; Gu, X.; Zeng, T.; Chen, L. Landscape dynamic network biomarker analysis reveals the tipping point of transcriptome reprogramming to prevent skin photodamage. J. Mol. Cell Biol. 2022, 13, 822–833.
- Fang, Z.; Han, X.; Chen, Y.; Tong, X.; Xue, Y.; Yao, S.; Tang, S.; Pan, Y.; Sun, Y.; Wang, X.; et al. Oxidative stress-triggered Wnt signaling perturbation characterizes the tipping point of lung adeno-to-squamous transdifferentiation. Signal Transduct. Target. Ther. 2023, 8, 16.
- Freedman, S.L.; Xu, B.; Goyal, S.; Mani, M. A dynamical systems treatment of transcriptomic trajectories in hematopoiesis. Development 2023, 150, dev201280.
- Li, L.; Xu, Y.; Yan, L.; Li, X.; Li, F.; Liu, Z.; Zhang, C.; Lou, Y.; Gao, D.; Cheng, X.; et al. Dynamic network biomarker factors orchestrate cell-fate determination at tipping points during hESC differentiation. Innovation 2023, 4, 100364.
- Haruki, T.; Yonezawa, S.; Koizumi, K.; Yoshida, Y.; Watanabe, T.M.; Fujita, H.; Oshima, Y.; Oku, M.; Taketani, A.; Yamazaki, M.; et al. Application of the Dynamical Network Biomarker Theory to Raman Spectra. Biomolecules 2022, 12, 1730.
- Koizumi, K.; Oku, M.; Hayashi, S.; Inujima, A.; Shibahara, N.; Chen, L.; Igarashi, Y.; Tobe, K.; Saito, S.; Kadowaki, M.; et al. Identifying pre-disease signals before metabolic syndrome in mice by dynamical network biomarkers. Sci. Rep. 2019, 9, 8767.
- Koizumi, K.; Oku, M.; Hayashi, S.; Inujima, A.; Shibahara, N.; Chen, L.; Igarashi, Y.; Tobe, K.; Saito, S.; Kadowaki, M.; et al. Suppression of Dynamical Network Biomarker Signals at the Predisease State (Mibyou) before Metabolic Syndrome in Mice by a Traditional Japanese Medicine (Kampo Formula) Bofutsushosan. Evid. Based Complement. Altern. Med. 2020, 2020, 9129134.
- Yang, Y.; Tian, Z.; Song, M.; Ma, C.; Ge, Z.; Li, P. Detecting the Critical States of Type 2 Diabetes Mellitus Based on Degree Matrix Network Entropy by Cross-Tissue Analysis. Entropy 2022, 24, 1249.
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602.
- Schmitt, C.A.; Fridman, J.S.; Yang, M.; Lee, S.; Baranov, E.; Hoffman, R.M.; Lowe, S.W. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 2002, 109, 335–346.
- Saleh, T.; Bloukh, S.; Carpenter, V.J.; Alwohoush, E.; Bakeer, J.; Darwish, S.; Azab, B.; Gewirtz, D.A. Therapy-Induced Senescence: An “Old” Friend Becomes the Enemy. Cancers 2020, 12, 822.
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Dabritz, J.H.M.; Zhao, Z.; Yu, Y.; Dorr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100.
- Lee, S.; Schmitt, C.A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019, 21, 94–101.
- Erenpreisa, J.; Salmina, K.; Anatskaya, O.; Cragg, M.S. Paradoxes of cancer: Survival at the brink. Semin. Cancer Biol. 2022, 81, 119–131.
- Jackson, T.R.; Salmina, K.; Huna, A.; Inashkina, I.; Jankevics, E.; Riekstina, U.; Kalnina, Z.; Ivanov, A.; Townsend, P.A.; Cragg, M.S.; et al. DNA damage causes TP53-dependent coupling of self-renewal and senescence pathways in embryonal carcinoma cells. Cell Cycle 2013, 12, 430–441.
- Huna, A.; Salmina, K.; Erenpreisa, J.; Vazquez-Martin, A.; Krigerts, J.; Inashkina, I.; Gerashchenko, B.I.; Townsend, P.A.; Cragg, M.S.; Jackson, T.R. Role of stress-activated OCT4A in the cell fate decisions of embryonal carcinoma cells treated with etoposide. Cell Cycle 2015, 14, 2969–2984.
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383.
- Paramos-de-Carvalho, D.; Jacinto, A.; Saúde, L. The right time for senescence. eLife 2021, 10, e72449.
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733.
- Suzuki, W.; Iizuka, S.; Tabuchi, M.; Funo, S.; Yanagisawa, T.; Kimura, M.; Sato, T.; Endo, T.; Kawamura, H. A new mouse model of spontaneous diabetes derived from ddY strain. Exp. Anim. 1999, 48, 181–189.
- Zitzmann, M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat. Rev. Endocrinol. 2009, 5, 673–681.
- Emont, M.P.; Jacobs, C.; Essene, A.L.; Pant, D.; Tenen, D.; Colleluori, G.; Di Vincenzo, A.; Jorgensen, A.M.; Dashti, H.; Stefek, A.; et al. A single-cell atlas of human and mouse white adipose tissue. Nature 2022, 603, 926–933.
- Cannon, W.B. Organization for Physiological Homeostasis. Physiol. Rev. 1929, 9, 399–431.
- McEwen, B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998, 338, 171–179.
- Kemoun, P.; Ader, I.; Planat-Benard, V.; Dray, C.; Fazilleau, N.; Monsarrat, P.; Cousin, B.; Paupert, J.; Ousset, M.; Lorsignol, A.; et al. A gerophysiology perspective on healthy ageing. Ageing Res. Rev. 2022, 73, 101537.
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199.
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80.
- Ferrucci, L.; Gonzalez-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; de Cabo, R. Measuring biological aging in humans: A quest. Aging Cell 2020, 19, e13080.
- Chen, Z.; Raj, A.; Prateek, G.V.; Di Francesco, A.; Liu, J.; Keyes, B.E.; Kolumam, G.; Jojic, V.; Freund, A. Automated, high-dimensional evaluation of physiological aging and resilience in outbred mice. Elife 2022, 11, e72664.
- Scheffer, M.; Bolhuis, J.E.; Borsboom, D.; Buchman, T.G.; Gijzel, S.M.W.; Goulson, D.; Kammenga, J.E.; Kemp, B.; van de Leemput, I.A.; Levin, S.; et al. Quantifying resilience of humans and other animals. Proc. Natl. Acad. Sci. USA 2018, 115, 11883–11890.
- Olde Rikkert, M.G.; Dakos, V.; Buchman, T.G.; Boer, R.; Glass, L.; Cramer, A.O.; Levin, S.; van Nes, E.; Sugihara, G.; Ferrari, M.D.; et al. Slowing Down of Recovery as Generic Risk Marker for Acute Severity Transitions in Chronic Diseases. Crit. Care Med. 2016, 44, 601–606.
- Tanaka, T.; Biancotto, A.; Moaddel, R.; Moore, A.Z.; Gonzalez-Freire, M.; Aon, M.A.; Candia, J.; Zhang, P.; Cheung, F.; Fantoni, G.; et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018, 17, e12799.
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging as Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960.
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9.
- Fabbri, E.; An, Y.; Zoli, M.; Simonsick, E.M.; Guralnik, J.M.; Bandinelli, S.; Boyd, C.M.; Ferrucci, L. Aging and the burden of multimorbidity: Associations with inflammatory and anabolic hormonal biomarkers. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 63–70.
- Kallen, V.; Tahir, M.; Bedard, A.; Bongers, B.; van Riel, N.; van Meeteren, N. Aging and Allostasis: Using Bayesian Network Analytics to Explore and Evaluate Allostatic Markers in the Context of Aging. Diagnostics 2021, 11, 157.
- Poganik, J.R.; Zhang, B.; Baht, G.S.; Tyshkovskiy, A.; Deik, A.; Kerepesi, C.; Yim, S.H.; Lu, A.T.; Haghani, A.; Gong, T.; et al. Biological age is increased by stress and restored upon recovery. Cell Metab. 2023, 35, 807–820.e805.
- Avchaciov, K.; Antoch, M.P.; Andrianova, E.L.; Tarkhov, A.E.; Menshikov, L.I.; Burmistrova, O.; Gudkov, A.V.; Fedichev, P.O. Unsupervised learning of aging principles from longitudinal data. Nat. Commun. 2022, 13, 6529.
- Pyrkov, T.V.; Avchaciov, K.; Tarkhov, A.E.; Menshikov, L.I.; Gudkov, A.V.; Fedichev, P.O. Longitudinal analysis of blood markers reveals progressive loss of resilience and predicts human lifespan limit. Nat. Commun. 2021, 12, 2765.




