Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zaneta Kimber-Trojnar | -- | 2480 | 2023-09-22 20:26:07 | | | |
| 2 | Rita Xu | + 1 word(s) | 2481 | 2023-09-25 04:14:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Majsterek, M.; Wierzchowska-Opoka, M.; Makosz, I.; Kreczyńska, L.; Kimber-Trojnar, �.; Leszczyńska-Gorzelak, B. Bile Acids in Intrahepatic Cholestasis of Pregnancy. Encyclopedia. Available online: https://encyclopedia.pub/entry/49544 (accessed on 15 January 2026).
Majsterek M, Wierzchowska-Opoka M, Makosz I, Kreczyńska L, Kimber-Trojnar �, Leszczyńska-Gorzelak B. Bile Acids in Intrahepatic Cholestasis of Pregnancy. Encyclopedia. Available at: https://encyclopedia.pub/entry/49544. Accessed January 15, 2026.
Majsterek, Maciej, Magdalena Wierzchowska-Opoka, Inga Makosz, Lena Kreczyńska, Żaneta Kimber-Trojnar, Bożena Leszczyńska-Gorzelak. "Bile Acids in Intrahepatic Cholestasis of Pregnancy" Encyclopedia, https://encyclopedia.pub/entry/49544 (accessed January 15, 2026).
Majsterek, M., Wierzchowska-Opoka, M., Makosz, I., Kreczyńska, L., Kimber-Trojnar, �., & Leszczyńska-Gorzelak, B. (2023, September 22). Bile Acids in Intrahepatic Cholestasis of Pregnancy. In Encyclopedia. https://encyclopedia.pub/entry/49544
Majsterek, Maciej, et al. "Bile Acids in Intrahepatic Cholestasis of Pregnancy." Encyclopedia. Web. 22 September, 2023.
Copy Citation
Intrahepatic cholestasis of pregnancy (ICP) is the most common, reversible, and closely related to pregnancy condition characterized by elevated levels of bile acids (BAs) in blood serum and an increased risk of adverse perinatal outcomes. Due to the complex interactions between the mother and the fetus in metabolism and transplacental BAs transport, ICP is classified as a fetal-maternal disease. The disease is usually mild in pregnant women, but it can be fatal to the fetus, leading to numerous complications, including intrauterine death. The pathophysiology of the disease is based on inflammatory mechanisms caused by elevated BA levels.
bile acids
intrahepatic cholestasis of pregnancy
pregnancy
1. Introduction
Intrahepatic cholestasis of pregnancy (ICP) is the most common liver disease that develops during pregnancy [1][2][3][4]. It is a condition specific for the period of pregnancy, which resolves relatively quickly and spontaneously after its completion, but may recur in subsequent pregnancies (up to 90% of subsequent pregnancies), often with a more intense course [3][5].
ICP is diagnosed on the basis of typical clinical symptoms, laboratory abnormalities, and differential diagnosis excluding other causes of skin pruritus and liver dysfunction in the pregnant woman. The disease is not associated with abnormalities detected in imaging owing to the fact that biliary ducts are not dilated and hepatic parenchyma appears normal. Its dominant symptoms are skin pruritus and increased levels of bile acids (BAs) in the blood serum of the pregnant woman [6]. An increase in serum total BA concentration is the key laboratory finding, which allows for identification of the disease as it is present in over 90% of affected pregnancies. The physical examination does not reveal any primary skin lesions, but traces of scratching and prurigo nodules secondary to scratching may be visible. Jaundice occurs within one month of the onset of pruritus in 14–25% of patients [7]. Nevertheless, jaundice as the only symptom prompts the search for other causes.
2. Pathophysiology, Etiology, and Complications of ICP
ICP is a disorder that is potentially harmful to the fetus. A clear relationship between elevated BA levels in maternal serum and fetal disorders has been confirmed in clinical practice, but the underlying mechanisms remain uncertain. One of these mechanisms is undoubtedly the inflammatory mechanism that underlies the pathophysiology of ICP [8][9][10][11]. Elevated levels of BAs induce the production of pro-inflammatory mediators in hepatocytes, attracting immune cells and initiating inflammation in the liver, eventually leading to cholestatic liver damage [12]. Furthermore, besides direct cytotoxic liver damage from BAs, oxidative stress and BA-induced mitochondrial damage lead to an inflammatory cascade [10][13]. The NLR family pyrin domain containing 3 (NLRP3) inflammasome in hepatic stellate cells and Kupffer cells is activated by BAs, causing inflammation or fibrosis [13]. The level of intracellular γ-glutamyl-L-cysteinyl-glycine (GSH), which protects cells against oxidative stress, cell proliferation, and division, is significantly influenced by inflammation and oxidative stress related to cholestasis [14]. Activation of the NF-κB pathway through the G protein-coupled BA receptor 1 (GPBAR1) is induced by BAs, resulting in elevated levels of inflammatory genes in trophoblasts, abnormal leukocyte infiltration, and placental inflammation [9]. ICP, by altering the metabolism of BAs and the fetal intestinal microflora, may increase the offspring’s susceptibility to inflammation [8]. Lin et al. [8] suggested that supplementation with Lactobacillus rhamnosus LRX01 may improve intestinal immunity in ICP offspring by inhibiting the expression of farnesoid X receptor (FXR) in the ileum.
ICP is a disease that is characteristic of the second half of pregnancy, especially the late second and entire third trimester of pregnancy. Despite this, cases of ICP already developing in the first trimester of pregnancy have been described in the literature [15][16][17][18][19][20]. The reported cases were associated with the hyperstimulation of the ovaries after in vitro fertilization.
The disease is usually mild in pregnant women, but it can be fatal to the fetus, leading to numerous complications, including intrauterine death [21]. The main complications related to this disease are presented in Figure 1. They include, but are not limited to, neonatal respiratory distress syndrome (associated with the presence of BA in the lungs), meconium-stained amniotic fluid, preterm birth, and an increased risk of stillbirth [22][23][24][25][26][27][28][29].
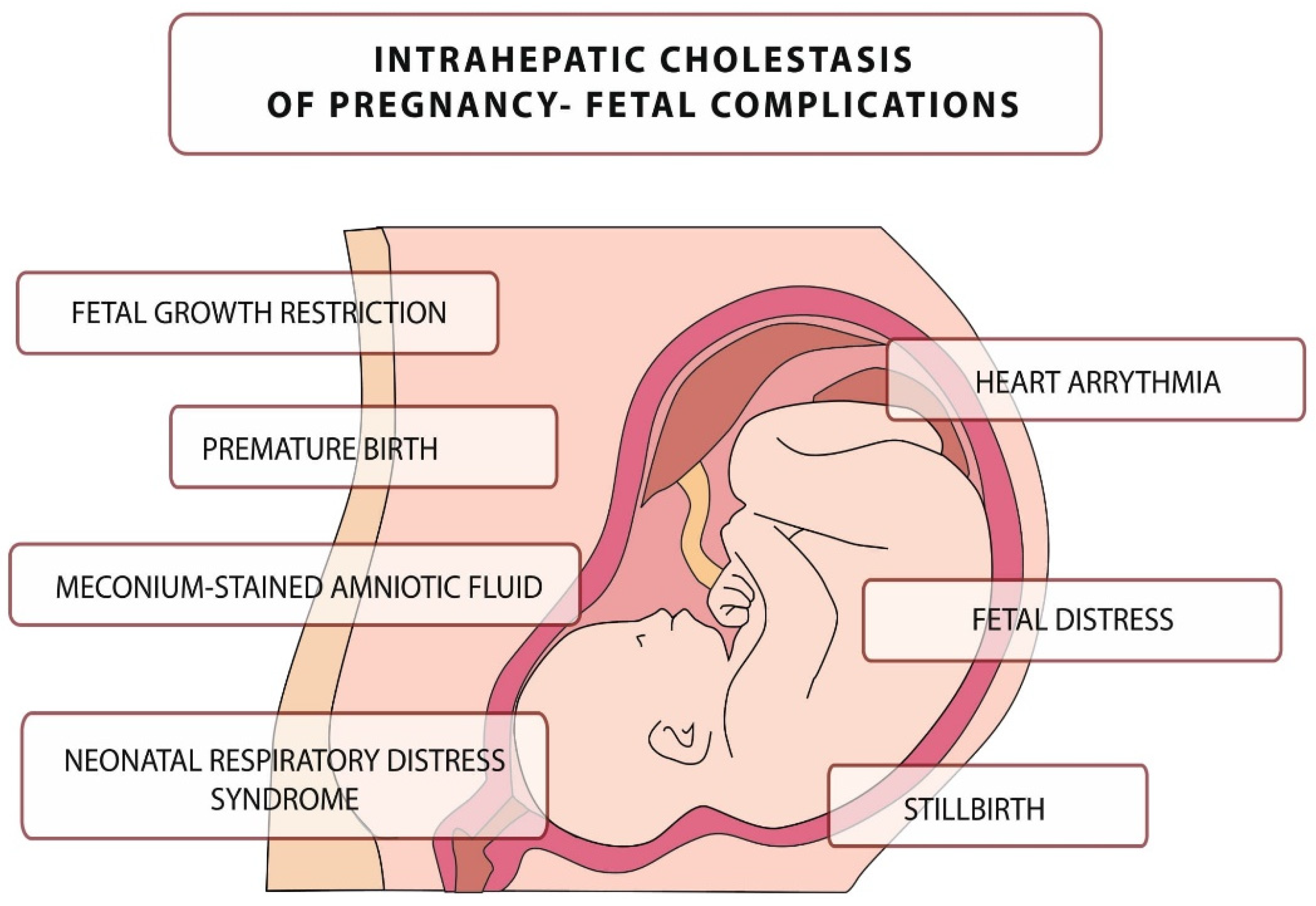
Figure 1. Intrahepatic cholestasis of pregnancy–fetal complications.
Fetal death in ICP may be caused by sudden vasoconstriction of the placental surface vessels or the development of arrhythmias induced by elevated BA levels, although pathophysiology has not been confirmed [24][25][26].
3. Bile Acids
3.1. Synthesis and Enterohepatic Circulation of Bile Acids
BAs are synthesized in the liver and constitute the major end product of cholesterol catabolism which proceeds by a multi-enzymatic pathway involving at least 17 different enzymes. In an adult human, approximately 500 mg of cholesterol is converted into BAs per day [30][31]. The rate of BA biosynthesis in the initial stage is partially limited by cholesterol 7α-hydroxylase, an enzyme from the cytochrome P450 family. The expression of the CYP7A1 gene encoding cholesterol 7α-hydroxylase, as well as the activity of the enzyme itself, are subject to strict, multifactorial regulation, including such factors as: BAs concentration, hormones (including insulin, glucagon, glucocorticosteroids), cholesterol (oxysterols), cytokines, and the daily cycle [32][33][34][35]. During the synthesis process, primary BAs, CA, and CDCA are first formed, which in the final stage are coupled with the amino acids: glycine or taurine (in a ratio of approximately 3:1). As a result of the conjugation process, primary BAs lose their ability to cross cell membranes. The process of bile formation is a process that requires the participation of energy from the breakdown of ATP (adenosine-5′-triphosphate) and consists of the transport of bile components through cell membranes, from hepatocytes to the bile ducts, against their concentration gradient. BAs are cleared into the bile ducts by a specific ATP-dependent transporter, bile salt export pump (BSEP), a product of the ABCB11 gene, which is highly specific and only transports conjugated BAs. BAs are the main component of bile, and their concentration in bile can be up to 1000 times higher than in the interior of the hepatocyte [36]. The second major component of bile is phosphatidylcholine (PC), which is excreted into the biliary tract by the ATP-dependent multidrug resistance protein (MRP) 3 transporter, a product of the ABCB4 gene. Phosphatidylcholine plays an important, protective role in the interstitial space. During bile formation, BAs transported by BSEP form mixed micelles with phosphatidylcholine. These complexes have a protective effect on the epithelium lining the bile ducts against the toxic and detergent effects of bile salts and thus allow their secretion without damaging the surrounding cells. Phosphatidylcholine secretion, in parallel with bile salts, is necessary to maintain adequate bile flow [37].
Bile produced in the liver is accumulated in the gallbladder until it is released into the gastrointestinal tract under the influence of postprandial cholecystokinin release—a peptide hormone that initiates postprandial contraction of the gallbladder [38]. In the small intestine, BAs emulsify dietary fats, fat-soluble vitamins, and other lipids. Under the influence of the anaerobic bacterial flora in the intestine, a number of primary BA transformations occur, including deconjugation and dehydroxylation, which lead to the formation of secondary BAs, i.e., DCA and LCA [39][40][41][42][43]. As a result of further changes, under the influence of both hepatic and intestinal mechanisms, substances of minor importance are formed, i.e., tertiary BAs: HDCA and UDCA. Through specific protein transport systems in enterocytes, bile salts are reabsorbed and reach the liver via the portal vein and are taken up by hepatocytes within the sinusoidal membrane [44][45].
Enterohepatic circulation is extremely efficient; the liver takes up about 95% of BAs and the remaining 5% is excreted in the feces. This loss is replaced by de novo synthesis of BAs in the liver [30][46].
3.2. The Biological Role of Bile Acids
The basic function of BAs in the human body is above all participation in digestive processes, and additionally participation in other physiological processes occurring in the human body [46]. These processes include the emulsification and absorption of lipids and lipophilic vitamins contained in food, and the absorption of calcium. In combination with phospholipids, they form complexes that facilitate the dissolution of cholesterol and other lipids in bile. BAs affect the secretion of pancreatic enzymes and cholecystokinins. The osmotic pressure gradient that arises during the secretion of BAs into the bile ducts is one of the most important factors ensuring the proper flow of bile through the liver [32][37]. Due to their detergent properties, BAs can damage the cell membranes of the biliary epithelium and, consequently, damage the liver parenchyma [47][48][49][50].
Originally, four major functions of BAs were identified:
-
constitute the main important mechanism for the elimination of excess cholesterol through their synthesis and subsequent fecal excretion.
-
BAs and phospholipids prevent cholesterol from precipitating in the gallbladder by dissolving cholesterol in the bile.
-
they act as emulsifiers, increasing the availability of fats for pancreatic lipases, facilitating the digestion of triacylglycerols in the diet.
After the characterization and isolation of FXR, for which bile acids are physiological ligands, the functions of Bas in the regulation of glucose and lipid homeostasis were confirmed. As indicated above, BAs binding to FXR impair the expression of genes that participate in overall BA homeostasis (e.g., FGF19). However, genes that participate in BA metabolism are not the only ones that are controlled by FXR action as an effect of binding BA [51][52][53].
FXR controls genes that metabolize in the liver lipids (e.g., SREBP-1c), lipoproteins (e.g., apoC-II), glucose (e.g., PEPCK), and that are involved in hepatoprotection (e.g., CYP3A4, which is nifedipine oxidase).
3.3. Bile Acids in the Fetus
During intrauterine life, due to the anatomical and functional immaturity of the developing fetus’ liver, there are some functional and structural differences in the transformation and elimination of harmful substances. The main organ responsible for metabolism and elimination of metabolic products during fetal intrauterine life is the placenta [54][55][56][57]. The processes in the placenta are significantly similar to those in the liver of an adult human.
In utero, hepatic biosynthesis of BAs and bilirubin begins relatively early, and BAs themselves reach relatively high concentrations [58][59]. The dominant BA present in fetal serum is CDCA, while CA is present in lower concentrations. It has been observed that already in the 12th week of pregnancy, the CA/CDCA bile acid concentration ratio is 0.85, around the date of delivery (38–40 weeks of pregnancy) it is 1.9, in the neonatal period it is 2.5, and in an adult 1.6 [60]. In addition, more BAs unbound or bound to glycine than to taurine are observed in fetal serum compared to adult serum. The above differences in the composition and concentration of BAs in the fetus, compared to adults, are caused by the immaturity of the enzyme systems involved in the metabolism of BAs in the fetus and their selective, transplacental exchange.
The process of BA synthesis in the fetus develops before the fetus has developed mechanisms that enable the effective secretion of BAs into bile. As a result, most of the synthesized BAs pass into the fetal blood serum, then a small fraction is excreted via the fetal kidneys into the amniotic fluid, and the remainder, the greater part, is eliminated through the placenta into the mother’s body. Most of the fetal pool of BAs is eliminated from the mother’s body via the gastrointestinal tract [61][62]. The identified factors influencing the maintenance of BA balance in the maternal-fetal circulation include: activity of hepatic metabolic pathways involved in the biosynthesis and transformation of BAs in both the mother and the fetus, the rate of BA elimination from the maternal organism, and the transport properties of the placenta [58][63].
3.4. Bile Acids in Physiological Pregnancy
In physiological pregnancy, the transplacental flow of BAs is supported by a gradient of BAs and bicarbonate concentrations and proceeds from the fetus to the mother [6][64]. This process depends on the protein transporters from the organic anion transporting polypeptides (OATP) family and ATP-dependent protein carriers from the ATP—binding cassette (ABC) family [44][65]. Simple diffusion, despite the possible free two-way flow of BAs through the placenta, does not appear to be the main mechanism responsible for the transport of BAs.
In physiologically running pregnancy, a slight increase in the total concentration of BAs in the blood serum is observed with the advancement of pregnancy. In studies that assessed the level of individual BAs, it was found that the concentration of secondary BAs did not change significantly, while the level of CDCA doubled around the time of delivery [66]. The data on CA levels are inconclusive as some studies have shown a significant increase in CA levels in the third trimester compared to the first trimester, while others have not shown changes in CA levels [66][67].
4. Bile Acids in Pregnancy Complicated by ICP
The most important role in the pathomechanism of ICP is played by the increased concentration of BAs and their detergent properties, which accumulate in hepatocytes, causing damage to cell membranes and the release of aminotransferases, bilirubin, γ-glutamyl transpeptidases (GGTP), and alkaline phosphatase into the blood serum.
Most guidelines agree that typical clinical symptoms include pruritus of the skin, which is frequently generalized, but commonly begins and predominates on the palms and soles. The itching sensation strengthens at night, oftentimes involves the right upper quadrant pain and may be accompanied by nausea, poor appetite, sleep deprivation, or steatorrhea. Abnormalities in the biochemical functions of the liver should be identified in the absence of diseases with similar clinical symptoms and laboratory abnormalities [68]. However, the type and reference values of laboratory markers of liver dysfunction that should be considered diagnostic for the diagnosis of ICP may differ significantly between societies [68][69][70][71][72][73][74][75][76].
Pregnant skin pruritus is accompanied by elevated levels of hepatic function tests or BAs, not caused by other diseases, which normalize after delivery. In addition, the Royal College of Obstetricians and Gynaecologists (RCOG) guidelines emphasize that in the presence of typical clinical symptoms and abnormal levels of hepatic function tests, an increased concentration of BAs is not necessary for the diagnosis of ICP, and the definitive confirmation of the diagnosis is the resolution of clinical symptoms and normalization of laboratory markers of liver function after delivery [72]. It is important to repeat the laboratory tests every week when the initial level of total aminotransferase and BAs are normal due to the fact that pruritus may precede an increase in serum BAs by several weeks. However, if UDCA becomes empirical, aminotransferases and BAs may never increase. Nevertheless 23% of pregnancies are affected by pruritus, but only a minority are caused by ICP.
An altered BA profile is observed in women with ICP. CA remains the major BA and its concentration is significantly higher than that of CDCA, resulting in an increase in the CA/CDCA ratio and a decrease in the percentage of CDCA in the total pool of BAs. There is also an increase in the level of secondary BAs, mainly DCA, less than that of the primary BAs, which may indicate an impairment of their enterohepatic circulation [77]. In addition, ICP shows an increase in taurine-conjugated BAs and a decrease in glycine-conjugated BAs, resulting in a lowered glycine/taurine ratio. Changes in BAs in pregnancy complicated by ICP are shown in Figure 2.

Figure 2. Bile acids in pregnancy complicated by ICP.
References
- Shan, D.; Dong, R.; Hu, Y. Current understanding of autophagy in intrahepatic cholestasis of pregnancy. Placenta 2021, 115, 53–59.
- Westbrook, R.H.; Dusheiko, G.; Williamson, C. Pregnancy and liver disease. J. Hepatol. 2016, 64, 933–945.
- Piechota, J.; Jelski, W. Intrahepatic Cholestasis in Pregnancy: Review of the Literature. J. Clin. Med. 2020, 9, 1361.
- Marciniak, B.; Kimber-Trojnar, Ż.; Leszczyńska-Gorzelak, B.; Patro-Małysza, J.; Trojnar, M.; Oleszczuk, J. Treatment of obstetric cholestasis with polyunsaturated phosphatidylcholine and ursodeoxycholic acid. Ginekol. Pol. 2011, 82, 26–31.
- Panaitescu, A.M.; Popescu, M.R.; Ciobanu, A.M.; Gica, N.; Cimpoca-Raptis, B.A. Pregnancy Complications Can Foreshadow Future Disease—Long-Term Outcomes of a Complicated Pregnancy. Medicina 2021, 57, 1320.
- Xiao, J.; Li, Z.; Song, Y.; Sun, Y.; Shi, H.; Chen, D.; Zhang, Y. Molecular Pathogenesis of Intrahepatic Cholestasis of Pregnancy. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6679322.
- Kondrackiene, J.; Kupcinskas, L. Intrahepatic cholestasis of pregnancy-current achievements and unsolved problems. World J. Gastroenterol. 2008, 14, 5781.
- Lin, Q.X.; Huang, W.W.; Shen, W.; Deng, X.S.; Tang, Z.Y.; Chen, Z.H.; Zhao, W.; Fan, H.Y. Intrahepatic Cholestasis of Pregnancy Increases Inflammatory Susceptibility in Neonatal Offspring by Modulating Gut Microbiota. Front. Immunol. 2022, 13, 889646.
- Zhang, Y.; Pan, Y.; Lin, C.; Zheng, Y.; Sun, H.; Zhang, H.; Wang, J.; Yuan, M.; Duan, T.; Du, Q.; et al. Bile acids evoke placental inflammation by activating Gpbar1/NF-κB pathway in intrahepatic cholestasis of pregnancy. J. Mol. Cell. Biol. 2016, 8, 530–541.
- Shah, P.A.; Nishio, A.; Hasan, S.; Wu, L.; Chie, L.; Rehermann, B.; T-Y Lau, D. A rare case of recurrent intrahepatic cholestasis of pregnancy with prolonged postpartum hepatic inflammation despite normalization of bile acid levels. Gastro Hep Adv. 2023, 2, 46–48.
- Biberoglu, E.; Kirbas, A.; Daglar, K.; Kara, O.; Karabulut, E.; Yakut, H.I.; Danisman, N. Role of inflammation in intrahepatic cholestasis of pregnancy. J. Obstet. Gynaecol. Res. 2016, 42, 252–257.
- Evangelakos, I.; Heeren, J.; Verkade, E.; Kuipers, F. Role of bile acids in inflammatory liver diseases. Semin. Immunopathol. 2021, 43, 577–590.
- Holtmann, T.M.; Inzaugarat, M.E.; Knorr, J.; Geisler, L.; Schulz, M.; Bieghs, V.; Frissen, M.; Feldstein, A.E.; Tacke, F.; Trautwein, C.; et al. Bile Acids Activate NLRP3 Inflammasome, Promoting Murine Liver Inflammation or Fibrosis in a Cell Type-Specific Manner. Cells 2021, 10, 2618.
- Kawase, A.; Hatanaka, M.; Matsuda, N.; Shimada, H.; Iwaki, M. Slc25a39 and Slc25a40 Expression in Mice with Bile Duct Ligation or Lipopolysaccharide Treatment. Int. J. Mol. Sci. 2022, 23, 8573.
- Stulic, M.; Culafic, D.; Boricic, I.; Stojkovic Lalosevic, M.; Pejic, N.; Jankovic, G.; Milovanovic, T.; Culafic-Vojinovic, V.; Vlaisavljevic, Z.; Culafic, M. Intrahepatic Cholestasis of Pregnancy: A Case Study of the Rare Onset in the First Trimester. Medicina 2019, 55, 454.
- Wongjarupong, N.; Bharmal, S.; Lim, N. Never Too Soon: An Unusual Case of Intrahepatic Cholestasis of Pregnancy at Five Weeks Gestation. Cureus 2020, 12, e10540.
- Koh, K.; Kathirvel, R.; Mathur, M. Rare case of obstetric cholestasis presenting in the first trimester following in vitro fertilisation. BMJ Case Rep. 2021, 14, e244254.
- Salame, A.A.; Jaffal, M.J.; Mouanness, M.A.; Nasser Eddin, A.R.; Ghulmiyyah, L.M. Unexplained First Trimester Intrahepatic Cholestasis of Pregnancy: A Case Report and Literature Review. Case Rep. Obstet. Gynecol. 2019, 2019, 4980610.
- Hubschmann, A.G.; Orzechowski, K.M.; Berghella, V. Severe First Trimester Recurrent Intrahepatic Cholestasis of Pregnancy: A Case Report and Literature Review. AJP Rep. 2016, 6, 38–41.
- Mutlu, M.F.; Aslan, K.; Guler, I.; Mutlu, I.; Erdem, M.; Bozkurt, N.; Erdem, A. Two cases of first onset intrahepatic cholestasis of pregnancy associated with moderate ovarian hyperstimulation syndrome after IVF treatment and review of the literature. J. Obstet. Gynaecol. 2017, 37, 547.
- Ovadia, C.; Seed, P.T.; Sklavounos, A.; Geenes, V.; Di Ilio, C.; Chambers, J.; Kohari, K.; Bacq, Y.; Bozkurt, N.; Brun-Furrer, R.; et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: Results of aggregate and individual patient data meta-analyses. Lancet 2019, 393, 899–909.
- Wang, J.; Lun, W.; Shi, W. Effects of elevated bile acid levels on fetal myocardium in intrahepatic cholestasis of pregnancy, a retrospective study from a neonatal perspective. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 102013.
- Çelik, S.; Çalışkan, C.S.; Çelik, H.; Güçlü, M.; Başbuğ, A. Predictors of adverse perinatal outcomes in intrahepatic cholestasis of pregnancy. Ginekol. Pol. 2019, 90, 217–222.
- Di Mascio, D.; Quist-Nelson, J.; Riegel, M.; George, B.; Saccone, G.; Brun, R.; Haslinger, C.; Herrera, C.; Kawakita, T.; Lee, R.H.; et al. Perinatal death by bile acid levels in intrahepatic cholestasis of pregnancy: A systematic review. J. Matern. Fetal Neonatal Med. 2021, 34, 3614–3622.
- Rodriguez, M.; Bombin, M.; Ahumada, H.; Bachmann, M.; Egaña-Ugrinovic, G.; Sepúlveda-Martínez, A. Fetal cardiac dysfunction in pregnancies affected by intrahepatic cholestasis of pregnancy: A cohort study. J. Obstet. Gynaecol. Res. 2022, 48, 1658–1667.
- Sarker, M.; Zamudio, A.R.; DeBolt, C.; Ferrara, L. Beyond stillbirth: Association of intrahepatic cholestasis of pregnancy severity and adverse outcomes. Am. J. Obstet. Gynecol. 2022, 227, 517.e1–517.e517.
- Devalla, A.; Srivastava, K. Unfolding newer concepts in placental pathology of obstetric cholestasis-a cause for prematurity. Int. J. Physiol. Pathophysiol. Pharmacol. 2022, 14, 16–23.
- Blumenfeld, J.; Koo, K. Navigating Uncertainty: A Case Study of Intrahepatic Cholestasis of Pregnancy. J. Midwifery Womens Health 2022, 67, 398–402.
- Huri, M.; Seravalli, V.; Lippi, C.; Tofani, L.; Galli, A.; Petraglia, F.; Di Tommaso, M. Intrahepatic cholestasis of pregnancy-Time to redefine the reference range of total serum bile acids: A cross-sectional study. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 1887–1896.
- Zhang, B.; Kuipers, F.; de Boer, J.F.; Kuivenhoven, J.A. Modulation of Bile Acid Metabolism to Improve Plasma Lipid and Lipoprotein Profiles. J. Clin. Med. 2022, 11, 4.
- Grant, S.M.; DeMorrow, S. Bile Acid Signaling in Neurodegenerative and Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 5982.
- Shulpekova, Y.; Shirokova, E.; Zharkova, M.; Tkachenko, P.; Tikhonov, I.; Stepanov, A.; Sinitsyna, A.; Izotov, A.; Butkova, T.; Shulpekova, N.; et al. A Recent Ten-Year Perspective: Bile Acid Metabolism and Signaling. Molecules 2022, 27, 1983.
- Chiang, J.Y.L.; Ferrell, J.M. Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 2020, 4, 47–63.
- Tagawa, R.; Kobayashi, M.; Sakurai, M.; Yoshida, M.; Kaneko, H.; Mizunoe, Y.; Nozaki, Y.; Okita, N.; Sudo, Y.; Higami, Y. Long-Term Dietary Taurine Lowers Plasma Levels of Cholesterol and Bile Acids. Int. J. Mol. Sci. 2022, 23, 1793.
- Phelps, T.; Snyder, E.; Rodriguez, E.; Child, H.; Harvey, P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol. Sex Differ. 2019, 10, 52.
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212.
- Boyer, J.L.; Soroka, C.J. Bile formation and secretion: An update. J. Hepatol. 2021, 75, 190–201.
- Wang, H.H.; Portincasa, P.; Wang, D.Q. Update on the Molecular Mechanisms Underlying the Effect of Cholecystokinin and Cholecystokinin-1 Receptor on the Formation of Cholesterol Gallstones. Curr. Med. Chem. 2019, 26, 3407–3423.
- Poland, J.C.; Flynn, C.R. Bile Acids, Their Receptors, and the Gut Microbiota. Physiology 2021, 36, 235–245.
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128.
- An, C.; Chon, H.; Ku, W.; Eom, S.; Seok, M.; Kim, S.; Lee, J.; Kim, D.; Lee, S.; Koo, H.; et al. Bile Acids: Major Regulator of the Gut Microbiome. Microorganisms 2022, 10, 1792.
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300.
- Grüner, N.; Mattner, J. Bile Acids and Microbiota: Multifaceted and Versatile Regulators of the Liver–Gut Axis. Int. J. Mol. Sci. 2021, 22, 1397.
- Durník, R.; Šindlerová, L.; Babica, P.; Jurček, O. Bile Acids Transporters of Enterohepatic Circulation for Targeted Drug Delivery. Molecules 2022, 27, 2961.
- Slijepcevic, D.; van de Graaf, S.F. Bile Acid Uptake Transporters as Targets for Therapy. Dig. Dis. 2017, 35, 251–258.
- Mertens, K.L.; Kalsbeek, A.; Soeters, M.R.; Eggink, H.M. Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front. Neurosci. 2017, 11, 617.
- Shulpekova, Y.; Zharkova, M.; Tkachenko, P.; Tikhonov, I.; Stepanov, A.; Synitsyna, A.; Izotov, A.; Butkova, T.; Shulpekova, N.; Lapina, N.; et al. The Role of Bile Acids in the Human Body and in the Development of Diseases. Molecules 2022, 27, 3401.
- Režen, T.; Rozman, D.; Kovács, T.; Kovács, P.; Sipos, A.; Bai, P.; Mikó, E. The role of bile acids in carcinogenesis. Cell. Mol. Life Sci. 2022, 79, 243.
- di Gregorio, M.C.; Cautela, J.; Galantini, L. Physiology and Physical Chemistry of Bile Acids. Int. J. Mol. Sci. 2021, 22, 1780.
- Caliceti, C.; Punzo, A.; Silla, A.; Simoni, P.; Roda, G.; Hrelia, S. New Insights into Bile Acids Related Signaling Pathways in the Onset of Colorectal Cancer. Nutrients 2022, 14, 2964.
- Zhao, L.; Xuan, Z.; Song, W.; Zhang, S.; Li, Z.; Song, G.; Zhu, X.; Xie, H.; Zheng, S.; Song, P. A novel role for farnesoid X receptor in the bile acid-mediated intestinal glucose homeostasis. J. Cell. Mol. Med. 2020, 24, 12848–12861.
- Massafra, V.; van Mil, S.W.C. Farnesoid X receptor: A "homeostat" for hepatic nutrient metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 45–59.
- Stofan, M.; Guo, G.L. Bile Acids and FXR: Novel Targets for Liver Diseases. Front. Med. 2020, 7, 544.
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.; et al. The Pivotal Role of the Placenta in Normal and Pathological Pregnancies: A Focus on Preeclampsia, Fetal Growth Restriction, and Maternal Chronic Venous Disease. Cells 2022, 11, 568.
- Wojczakowski, W.; Kimber-Trojnar, Ż.; Dziwisz, F.; Słodzińska, M.; Słodziński, H.; Leszczyńska-Gorzelak, B. Preeclampsia and Cardiovascular Risk for Offspring. J. Clin. Med. 2021, 10, 3154.
- Yañez, M.J.; Leiva, A. Human Placental Intracellular Cholesterol Transport: A Focus on Lysosomal and Mitochondrial Dysfunction and Oxidative Stress. Antioxidants 2022, 11, 500.
- Nikolov, A.; Popovski, N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021, 11, 480.
- Wang, P.; Song, Y.; Zhong, H.; Lin, S.; Zhang, X.; Li, J.; Che, L.; Feng, B.; Lin, Y.; Xu, S.; et al. Transcriptome Profiling of Placenta through Pregnancy Reveals Dysregulation of Bile Acids Transport and Detoxification Function. Int. J. Mol. Sci. 2019, 20, 4099.
- Gagnon, M.; Trottier, J.; Weisnagel, S.J.; Gagnon, C.; Carreau, A.M.; Barbier, O.; Morisset, A.S. Bile acids during pregnancy: Trimester variations and associations with glucose homeostasis. Health Sci. Rep. 2021, 4, e243.
- Ronin-Walknowska, E. Cholestaza ciężarnych-choroba niedoceniona. Przegląd piśmiennictwa. Perinatol. Neonatol. Ginekol. 2010, 3, 165–174.
- Fan, H.M.; Mitchell, A.L.; Williamson, C. ENDOCRINOLOGY in PREGNANCY: Metabolic impact of bile acids in gestation. Eur. J. Endocrinol. 2021, 184, R69–R83.
- Pataia, V.; Dixon, P.H.; Williamson, C. Pregnancy and bile acid disorders. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G1–G6.
- Ontsouka, E.; Epstein, A.; Kallol, S.; Zaugg, J.; Baumann, M.; Schneider, H.; Albrecht, C. Placental Expression of Bile Acid Transporters in Intrahepatic Cholestasis of Pregnancy. Int. J. Mol. Sci. 2021, 22, 10434.
- McIlvride, S.; Dixon, P.H.; Williamson, C. Bile acids and gestation. Mol. Aspects Med. 2017, 56, 90–100.
- Kroll, T.; Prescher, M.; Smits, S.H.J.; Schmitt, L. Structure and Function of Hepatobiliary ATP Binding Cassette Transporters. Chem. Rev. 2021, 121, 5240–5288.
- Heikkinen, J.; Mäentausta, O.; Ylöstalo, P.; Jänne, O. Changes in serum bile acid concentrations during normal pregnancy, in patients with intrahepatic cholestasis of pregnancy and in pregnant women with itching. Br. J. Obstet. Gynaecol. 1981, 88, 240–245.
- Lunzer, M.; Barnes, P.; Byth, K.; O’Halloran, M. Serum bile acid concentrations during pregnancy and their relationship to obstetric cholestasis. Gastroenterology 1986, 91, 825–829.
- Bicocca, M.J.; Sperling, J.D.; Chauhan, S.P. Intrahepatic cholestasis of pregnancy: Review of six national and regional guidelines. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 180–187.
- Geenes, V.; Chappell, L.C.; Seed, P.T.; Steer, P.J.; Knight, M.; Williamson, C. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: A prospective population-based case-control study. Hepatology 2014, 59, 1482–1491.
- Wood, A.M.; Livingston, E.G.; Hughes, B.L.; Kuller, J.A. Intrahepatic cholestasis of pregnancy: A review of diagnosis and management. Obstet. Gynecol. Surv. 2018, 73, 103–109.
- Jurate, K.; Rimantas, Z.; Jolanta, S.; Vladas, G.; Limas, K. Sensitivity and specificity of biochemical tests for diagnosis of intrahepatic cholestasis of pregnancy. Ann. Hepatol. 2017, 16, 569–573.
- Girling, J.; Knight, C.L.; Chappell, L. Royal College of Obstetricians and Gynaecologists. Intrahepatic cholestasis of pregnancy: Green-top Guideline No. 43 June 2022. BJOG Int. J. Obstet. Gynaecol. 2022.
- Society for Maternal-Fetal Medicine (SMFM); Lee, R.H.; Greenberg, M.; Metz, T.D.; Pettker, C.M. Society for Maternal-Fetal Medicine Consult Series #53: Intrahepatic cholestasis of pregnancy: Replaces Consult #13, April 2011. Am. J. Obstet. Gynecol. 2021, 224, B2–B9.
- South Australian Perinatal Practice Guideline. Intrahepatic Cholestasis of Pregnancy. Available online: https://www.sahealth.sa.gov.au/wps/wcm/connect/f91fbf004ee530b2a5ebadd150ce4f37/Intrahepatic+cholestasis+of+pregnancy_PPG_v4_0.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-f91fbf004ee530b2a5ebadd150ce4f37-ocQJ9qe (accessed on 4 November 2022).
- Leszczyńska-Gorzelak, B.; Oleszczuk, J.; Maciniak, B.; Poręba, R.; Oszukowski, P.; Wielgoś, M.; Czajkowski, K.; Zespoł Ekspertów Polskiego Towarzystwa Ginekologicznego. Clinical practice guidelines of the Team of Experts of the Polish Gynecological Society: Management of the intrahepatic cholestasis of pregnancy. Ginekol. Pol. 2012, 83, 713–717.
- Lu, L.; Chinese Society of Hepatology and Chinese Medical Association. Guidelines for the Management of Cholestatic Liver Diseases (2021). J. Clin. Transl. Hepatol. 2022, 10, 757–769.
- Heikkinen, J. Serum bile acids in the early diagnosis of intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 1983, 61, 581–587.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
902
Revisions:
2 times
(View History)
Update Date:
25 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




