
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sunil J. Wimalawansa | -- | 3174 | 2023-09-20 17:00:40 | | | |
| 2 | Rita Xu | -6 word(s) | 3168 | 2023-09-21 04:33:10 | | | | |
| 3 | Rita Xu | -3 word(s) | 3165 | 2023-09-21 04:43:09 | | |
Video Upload Options
Musculoskeletal benefits of vitamin D include calcium homeostasis, bone mineralization, etc., through its hormonal actions. This requires serum 25(OH)D less than 20 ng/mL. In contrast, many other tissues require above 30 or 40 ng/mL steady-state concentrations. To reduce infections, autoimmune diseases, cancer, and all-cause mortality require a minimum level of 50 ng/mL. Vitamin D is an economical and widely available (generic) nutrient obtained over the counter without a prescription. At the recommended doses, vitamin D does not cause any adverse effects. Disease prevention and minimizing complications and premature deaths can be achieved by maintaining serum 25(OH)D concentrations between 50 and 80 ng/mL. This costs less than 0.01% of the cost of one day of hospitalization.
1. Introduction
Most people know the musculoskeletal benefits of vitamin D. This includes calcium homeostasis—intestinal absorption and phosphate and mineral conservation, skeletal calcification, and its effects on the muscular system [1][2]. The bone formation, resorption, and mineralization involved the hormonal form of calcitriol with parathyroid hormone (PTH) [3]: the latter is a crucial hormone influencing renal tubular calcium and phosphate handling [4].
In renal tubular, parathyroid, fat, and musculoskeletal cells, an in-built active system transports steroidal molecules, especially vitamin D and 25(OH)D—megalin-cubilin endocytotic system [5]. Because of this energy-dependent system, these cells can internalize such molecules against a concentration gradient [5]. Consequently, even when the serum 25(OH)D and vitamin D concentrations are between 12 and 20 ng/mL, renal tubular cells continue to extract these molecules from the circulation. This is why, despite such low levels (i.e., by definition, vitamin D deficiency), kidneys can generate the hormonal calcitriol and maintain most of the above-mentioned musculoskeletal functions of vitamin D, such as preventing rickets in children and osteomalacia in adults.
Most steroid hormones enter cells via diffusion and endocytosis via the membrane-based, megalin-cubilin system, as in the kidney and parathyroid gland, muscle, and fat cells [5]. In addition, this mechanism of active cellular entry is essential for generating the hormonal form of calcitriol in renal tubules and parathyroid glands—for vitamin D’s endocrine functions [3][5]. However, unlike the cells mentioned above, other peripheral target cells, like immune cells, do not have an active vitamin D megalin-cubilin transportation system [6]. Thus, in addition to some endocytosis, these cells mainly depend on a concentration-dependent gradient for diffusions of vitamin D and 25(OH)D (mostly bound to VDBP) into them [7].
2. Extra-Skeletal Benefits of Vitamin D
The biological activity of calcitriol in most extra-musculoskeletal tissues is activated following the generation of calcitriol within peripheral target cells—not via the circulatory hormonal form. In addition to genomic functions in these cells, it acts as a local cytokine and signaling molecule. The genomic functions include controlling the proliferation and maturity of cells, preventing cancer cell growth, brain development, respiratory and reproductive functions, and mitochondrial energy generation [8][9][10][11]. Vitamin D maintains a robust immune system, which helps to overcome infections, including COVID-19 [12][13][14], and prevents autoimmunity [15][16]. Calcitriol’s primary life-saving extra-skeletal role is keeping a person healthy [17][18].
Extra Musculoskeletal Benefits of Vitamin D—Dissemination of Information
Large emerging data sets support multiple physiological vitamin D functions occurring via calcitriol. These data suggest vitamin D should considered for preventative and adjunct therapy for many disorders, including sepsis and COVID-19 infection [19][20][21][22]. With a handful of exceptions [23], vitamin D is almost never included in clinical protocols or guidelines. No leading health authorities or governments advised their fellow citizens to keep them healthy by providing proper advice on micronutrients, especially vitamin D [11]. What they have provided is grossly outdated [24][25][26][27][28].
In addition, recommendations from medical and scientific societies are confusing, contradictory [29][30], and out-of-date [31][32]. Despite this negative publicity, public awareness of vitamin D and its beneficial effects on the immune system has improved since the COVID-19 pandemic. This is primarily due to relentless positive work by individuals and small groups of scientists, although the negative publicity by big pharma. In contrast, clinical guidelines from the Front-Line COVID-19 Critical Care Alliance [23] and affirmative Substack articles provided reliable data to the public [33].
3. Mechanisms and Clinical Relevance
Sufficient calcitriol synthesis within immune cells prevents chronic diseases, autoimmunity, inflammation, and infections [34][35]. These physiological actions manifest by several mechanisms, including suppressing inflammatory cytokines and increasing anti-inflammatory cytokines and anti-oxidative compounds [21][36]. Chronic diseases are associated with chronic inflammation, which maintains and gradually worsens the disease process [34]. In addition, calcitriol enhances the production and release of antimicrobial peptides, cathelicidin, and beta-defensin via its autocrine and paracrine actions. These antimicrobial peptides stimulate white blood cells, macrophages, and natural killer cells and direct the circulating viruses to macrophages to destroy them [37].
3.1. Mechanisms of Action of Calcitriol
Vitamin D signaling plays a crucial role in intrinsic defense against intracellular microorganisms via generating antimicrobial proteins like cathelicidin [35]. In addition, calcitriol stabilizes tight junctions of epithelial cells of the respiratory tract and cardiovascular system, protecting them from fluid leakage and viral dissemination into soft tissues [38][39]. Figure 1 illustrates the generation of calcitriol and its broader actions. Notably, it demonstrates the critical difference between the actions of the hormonal form vs. the non-hormonal form of calcitriol (the bottom half).
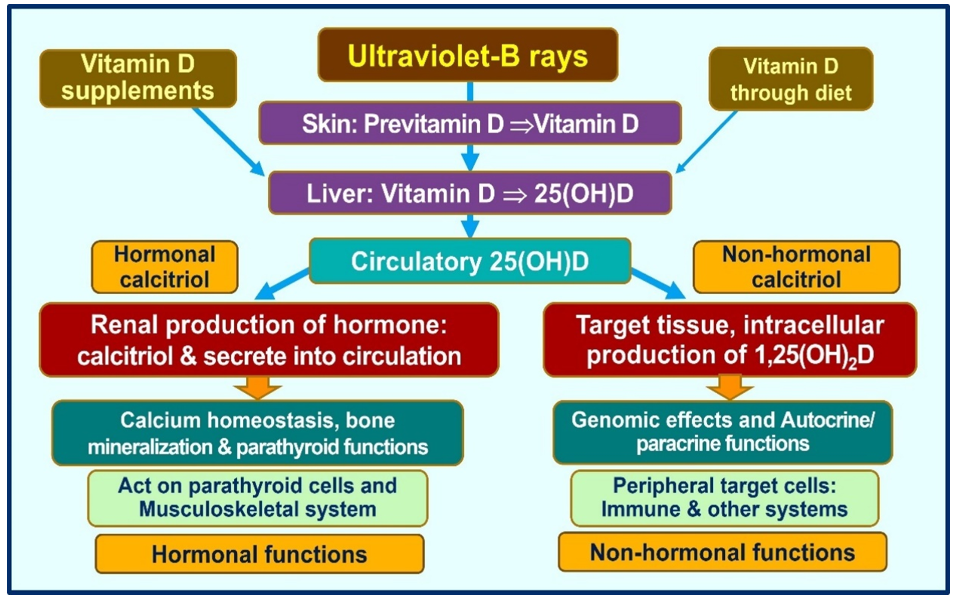
Figure 1. Vitamin D is expected to be generated predominantly following exposure to ultraviolet-B (UCB) rays. The amounts of vitamin D obtained via diet are small supplements. Therefore, those not exposed to sufficient UVB exposure depend on vitamin D supplements for their health. The figure illustrated the main differences between the circulatory hormonal form of calcitriol (generated in renal tubular cells) vs. the intracellularly generated calcitriol in peripheral target cells (as in immune cells).
3.2. Importance of Circulatory Vitamin D and 25(OH)D for Target Cell Generation of Calcitriol
The past few decades have focused on cholecalciferol (D3) in preventing musculoskeletal disorders [40]. However, in the past 15 years, several fundamental advances were made in researchers in understanding the biology and physiology of calcifediol and calcitriol. These delineate how and when to use them properly as therapies. Yet, as described above, the doses recommended are grossly inadequate, and no attempts were made to update them. Emerging evidence has provided more value in recent years, highlighting the importance of different vitamin D compounds in human biology and clinical immunology [5]. While the musculoskeletal system functions maintained with smaller doses, of between 800 and 1,000 IU/day, higher amounts, like 5,000 to 10,000 IU per day or 50,000 IU once a week are necessary for a non-obese 70 kg adult, to maintain serum 25(OH)D concentrations above 50 ng/mL—that needed to overcome infections [6][13] and overcome cancers [41][42].
Those who are obese, taking medications that increase catabolic activity of vitamin D (e.g., anti-epileptic and retroviral agents), or have significant fat malabsorption require severalfold higher doses than those mentioned above. Even with such amounts, unless a loading dose is administered [43][44], a vitamin D-deficient person takes several months to increase their serum 25(OH)D to therapeutic levels of over 50 ng/mL [6]. Using the mentioned doses of vitamin D, even in a vitamin D-sufficient person to reach and maintain a serum 25(OH)D concentration of above 40 ng/mL (as guidelines for community-dwelling persons) would take a few weeks to raise serum 25(OH)D concentration above 50 ng/mL [13]. Therefore, such doses could be insufficient and ineffective to achieve the desired target serum 25(OH)D concentration in emergencies. Consequently, even moderately high daily doses without administering an upfront (one-time) loading dose are unlikely to significantly benefit a person in overcoming critical disorders like infections, sepsis, and cancer.
4. Doses of Vitamin D Needed to Overcome Disorders
Serum 25(OH)D concentrations are reduced in chronic diseases like metabolic disorders, obesity, cancer, infections, and all-cause mortality [45][46][47][48][49]. Less frequent administration—intervals of less than once a month—(i.e., intermittent bolus dosing) and even repeat administration of higher doses, like 300,000 once in six months, do not generate the intended clinical outcomes and thus should be avoided. This is because the half-life of vitamin D is about one day, and 25(OH)D is between two to three weeks, depending on the vitamin D status. No matter the dose, the serum 25(OH)D concentration would not remain high enough for more than three months [50][51][52]. In addition, infrequent administrations lead to unphysiological fluctuation of serum and tissue levels of vitamin D metabolites and could stimulate catabolic enzymes, like 24-hydroxylase (see below).
4.1. Clinical Study Outcomes Using Higher Doses of Vitamin D
Meta-analyses of RCTs concerning vitamin D supplementation reported a significant reduction in the incidence and severity of respiratory tract infections. Daily vitamin D supplements provide better clinical outcomes than with infrequent administration. In contrast, when vitamin D is administered at longer intervals than once a month, benefits are less, and the outcomes are not satisfactory [53][54].
Using higher doses of vitamin D consistently has been reported to have better clinical outcomes than the government-recommended doses of 800 IU/day, which has no tangible effect on any disease other than muscular skeletal disorders [26][55]. For example, adequate supplementation with vitamin D reduces cancer [49][56], regress prostate cancer [57], lowers blood pressure (especially in African Americans) [58], and reduces insulin resistance [59][60], including in obese children [61], and prevent multiple sclerosis [62][63].
However, those studies that used pediatric doses of vitamin D in adults based on outdated recommendations (i.e., using 280 IU/day or less than 1,000 IU/day) [64][65], as with the Women’s Health Initiative study of cancer prevention and infrequent administration of 100,000 IU vitamin D3 quarterly [66], failed to prevent cancer and other disorders. Based on vitamin D biology and physiology, this is not surprising. Most clinical studies reported an inverse association between vitamin D status and mortality [48][67], and the relation is curvilinear [31].
4.2. Entry of D and 25(OH)D into Peripheral Target Cells
In peripheral target cells as immune cells, genomic action follows binding to vitamin D/calcitonin receptors, and non-genomic functions, like intracrine/autocrine and paracrine signaling/functions of calcitriol, are driven by calcitriol synthesized within these cells. In these peripheral target cells, calcitriol is synthesized by 1a-hydroxylase enzyme, transcribed by the CYP27B1 gene. This hydroxylation of 1a-position, however, is dependent on the ability to diffuse enough vitamin D and 25(OH)D from the circulation [6][13]. This mainly occurs via the diffusion of these two molecules across the cell membranes, which is crucial for all immune cell activities. This is the prime reason why, in contrast to musculoskeletal tissues, peripheral target cells (tissues) need higher circulatory 25(OH)D concentrations.
In addition to diffusion, a smaller proportion of VDBP-bound D and 25(OH)D enters these cells via endocytosis [68]. Since the affinity of vitamin D to VDBP is less than 25(OH)D, given the same concentration in the blood, more D is likely to enter immune cells. However, since the half-life of vitamin D is only one day, the total entry of D is still less than 25(OH)D. Figure 2 illustrates the mode of vitamin D and 25(OH)D access in peripheral target cells, like immune cells [13]. This entry of vitamin D and 25(OH)D from the circulation into immune cells allows the generation of calcitriol intracellularly [31], which is crucial for both genomic, autocrine, and paracrine functions of immune cells and other peripheral target cells [14][69][70][71].
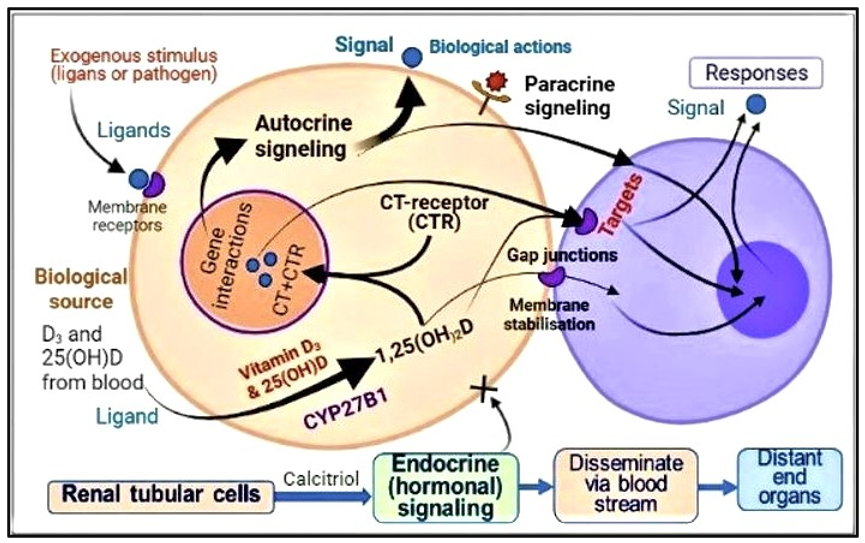
Figure 2. Pathways and mechanisms of actions of calcitriol activating immune cell functions: Activation of D and 25(OH)D into calcitriol [1,25(OH)2D] intracellularly leads to genomic actions, autocrine (activation of functions within the same cells) and paracrine (indicating cell to effector cells) signaling.
When vitamin D is taken daily, the circulatory vitamin D concentrations are more stable and likely higher than 25(OH)D concentrations than when the same dose is taken once in two weeks or monthly [31]. Therefore, more vitamin D is likely to diffuse into peripheral target cells because of the higher concentration gradient of D with daily doses than 25(OH)D. When this is the case, the measurement of serum 25(OH)D alone, as done in routine clinical practice today, may not provide the correct information about vitamin D adequacy or guide the replacement requirements for physiological functions, including maintaining a robust immune system. The opposite happens when the same dose of vitamin is consumed infrequently; a higher concentration of 25(OH)D is present in the circulation than in vitamin D.
4.3. Vitamin D, Epithelial Barriers, and Gap Junction Stability
D3 enhances epithelial and endothelial stability, independent of canonical pathways through calcitriol/CTR-derived genomic outcome [72]. Disruption of endothelial stability and an enhancement of vascular leak is prevented by D3 supplementation. These rapid membrane-related actions of vitamin D, 25(OH)D, and 1,25(OH)2D, are at a similar potency.
The deficiency of D3 and its metabolites impairs endothelial barriers, leading to vascular fluid leakage into soft tissues [72]. Similarly, weakening gap junctions and epithelial barriers lead to viral infiltration and propagation of infections, as seen in sepsis and viral infections like SARS-CoV-2 [73]. These non-transcriptional (non-genomic) mechanisms are essential in controlling inflammation and preventing endothelial and epithelial cell destabilization.
5. Novel Information Related to Clinical Aspects of Vitamin D
5.1. Amounts of Daily Vitamin D Doses Needed to Maintain Clinically Effective Serum 25(OH)D Concentrations Cover 99.5% of Disorders
Different dosing schedules have varied effects on serum vitamin D and 25(OH)D concentrations—daily doses (but not infrequent doses) maintain a stable circulating concentration [74]. In contrast, ingesting vitamin D longer than monthly intervals results in significant circulatory 25(OH)D concentration fluctuations, which is not physiological and may not benefit [53][75][76]. Schedules recommended below for vitamin D supplementation as prophylactic and longer-term RCTs in hypovitaminosis D will significantly increase (at least double) the serum D and 25(OH)D concentrations, thus profoundly affecting intended beneficial clinical outcomes. A simplified formula is illustrated below for calculating the vitamin D dose for an individual based on BMI (body weight and fat mass) for different body weight groups [6][13].
Not obese (average wt.: BMI, <29): 70-90 IU/kg BW
Moderately obese (BMI, 30-39): 100-130 IU/kg BW
Morbid obesity (BMI, over 40): 140-180 IU/kg BW
5.2. What has Changed Over the Years Related to Vitamin D?
A century ago, it was observed that exposure to sun rays (vitamin D) reversed rickets in children, and it was effective against tuberculosis. Since then, much scientific evidence has demonstrated that vitamin D is central to disease prevention, complications, and deaths [77]. Previously, it was believed that exposure to sufficient UVB rays generated about 3,000 IU/day. However, recent data confirmed a person with a lighter skin color could generate up to 10,000 IU of vitamin D3 within one hour following exposure of a third of the upper body to sunlight [78][79][80].
Maintaining a steady state of D and 25(OH)D in circulation is helpful for physiological functions. In contrast, marked fluctuating serum 25(OHD concentrations from intermittent administration of high doses of vitamin D is unphysiological. Such could over-express the catabolic enzyme, 24-hydroxylase enzyme (via CYP24A1). Based on the circulatory half-life, the frequency of administration of vitamin D must not exceed once a month, preferably not more than two-week intervals [76]. This would avoid significant fluctuations in serum 25(OH)D concentration [53][75].
Most of the positive respiratory tract infections-related RCTs are conducted in children [42][43][57][58][59], using daily doses of vitamin D [46][47][81]. Meta-analysis of RCTs on vitamin D in respiratory tract infections reported that vitamin D is more effective as a treatment when administered in daily doses than intermittently [82]. Chronic diseases are most common among older people partly due to longer-term vitamin D deficiency [83] and are associated with an increased rate of deaths [67][77]. They also have multiple co-morbidities associated with hypovitaminosis D and low circulating ACE-2 receptors, increasing the vulnerability to infections and other pathological ailments (Figure 3).
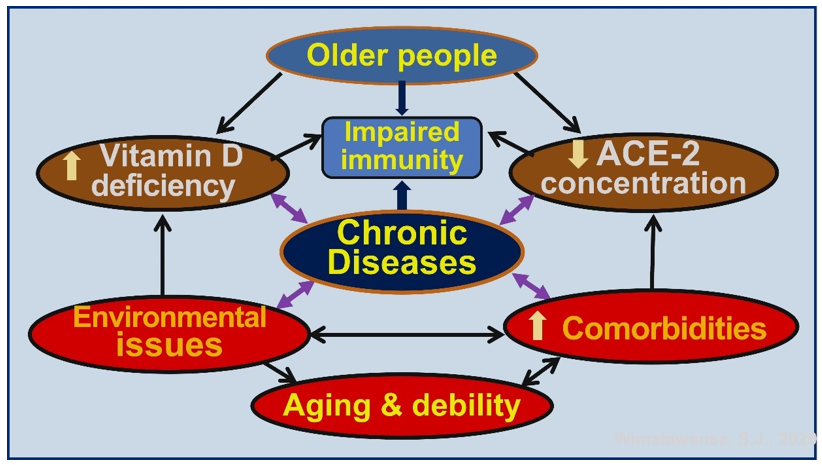
Figure 3. Schematic representation of how chronic diseases increase morbidity and mortality in older people. These are exacerbated by hypovitaminosis D, low angiotensin converting enzyme-2 (ACE-2) concentrations, environmental issues/pollution, and co-morbidities.
6. Discussion
The current paradigms related to vitamin D are primarily based on retrospective analyses and epidemiological studies (cohort, cross-sectional, observational, prospective, and ecological studies) [5][31]. Many have used false concepts and assumptions of doses and serum 25(OH)D concentration needed to improve outcomes based on outdated information [31][84]. In contrast, recent reports overwhelmingly support the positive effects of vitamin D in extra-musculoskeletal disorders, including chronic diseases and infections.
During the past decade, many advances were made in understanding the physiology and biology of vitamin D and its receptor ecology. The knowledge of the physiology of D3 and vitamin D–VDR has advanced the understanding of the biology, metabolism, and effects of gene polymorphisms on the vitamin D axis. Data pointed towards the need for a minimum serum 25(OH)D concentration of 50 ng/mL for extra-musculoskeletal target cell physiological activity. It will take time to incorporate such into vitamin guidelines and recommendations.
Evidence supports strong physiological associations of vitamin D with disease risk reduction and improved physical and mental functions. Together, these data have facilitated the understanding of new rationale to prevent and treat diseases cost-efficiently. Overall evidence suggests that vitamin D deficiency, as determined by maintaining serum 25(OH)D concentrations of more than 40 ng/mL, is associated with increased risks of many illnesses and disorders and higher all-cause mortality, even among otherwise healthy individuals. The proper functioning of the vitamin D endocrine, paracrine, and autocrine systems is essential for many physiological activities and maintaining good health.
Recent data from epidemiological, cross-sectional, and longitudinal studies support that having physiological serum concentrations of 25(OH)D, levels greater than 40 ng/mL, significantly reduces the incidence of extra-musculoskeletal disorders. The latter includes diabetes, MS, rheumatoid arthritis, osteoporosis, autoimmune diseases, and certain types of cancer [49], as well as reducing all-cause mortality.
The dosages of vitamin D prescribed for non-obese deficient persons of average weight of 70 kg should be between 4000 and 7000 IU/day, 20,000 IU twice a week, or 50,000 IU once a week or once in 10 days. Such doses would allow approximately 97.5% of people to maintain their serum 25(OH)D concentrations above 40 ng/mL [5][30]. However, intermittent doses at intervals longer than once a month are unphysiological and thus ineffective. Daily vitamin D supplements are more beneficial than supplementation administered less frequently.
Furthermore, some medications, environmental pollutants, and physical activities/ lifestyles influence vitamin D metabolism and actions, modulating the balance between energy intake and expenditure. However, using vitamin D analogs is inappropriate for alleviating hypovitaminosis D or treating osteoporosis. In the absence of adequate exposure to sunlight, average-weight non-obese individuals require daily vitamin D intake (food plus supplements) of between 5000 and 7000 IU to maintain serum 25(OH)D concentrations above 50 ng/mL (125 nmol/L). Longer-term maintenance of a steady state of the serum 25(OH)D concentration is necessary to have a meaningful impact on reducing disease incidences and all-cause mortality.
Clinical practice recommendations should be geared toward healthcare professionals and the public, patient education, and informing the public regarding appropriate actions for avoiding micronutrient deficiency. However, most countries neither have policies or guidance on sun exposure and vitamin D intake nor cost-effective public health interventions, especially for micronutrients. They should consider embracing cost-effective measures to prevent diseases, significantly reducing healthcare costs.
Maintaining serum 25(OH)D concentrations above 50 ng/mL improves overall health and reduces the severity of chronic diseases, infection and autoimmunity, and all-cause mortality. Furthermore, it minimizes infection-related complications, including COVID-19-related hospitalizations and deaths. Vitamin D sufficiency is the most cost-effective way to reduce illnesses, infections, and healthcare costs. It should be a part of routine public health and clinical care.
References
- Vieth, R. Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol 2004, 89-90, 575-579, doi:10.1016/j.jsbmb.2004.03.038.
- Wimalawansa, S.J. Vitamin D in the new millennium. Curr Osteoporos Rep 2012, 10, 4-15, doi:10.1007/s11914-011-0094-8.
- Marzolo, M.P.; Farfan, P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol Res 2011, 44, 89-105, doi:10.4067/S0716-97602011000100012.
- Wimalawansa, S.J. Biology of vitamin D. J steroids Horm Sci 2019, 10, 1-8, doi:10.24105/2157-7536.10.198.
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999, 96, 507-515, doi:10.1016/s0092-8674(00)80655-8.
- Wimalawansa, S.J. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections-Sepsis and COVID-19. Nutrients 2022, 14, doi:10.3390/nu14142997.
- Hollis, B.W.; Wagner, C.L. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab 2013, 98, 4619-4628, doi:10.1210/jc.2013-2653.
- Oliver, S.M. The immune system and new therapies for inflammatory joint disease. Musculoskeletal Care 2003, 1, 44-57, doi:10.1002/msc.38.
- Wimalawansa, S.J. Global epidemic of coronavirus—COVID-19: What can we do to minimize risks? European J. Biomed & Pharma Sci. 2020, 7, 432-438, doi:https://storage.googleapis.com/journal-uploads/ejbps/article_issue/volume_7_march_issue_3/1584436192.pdf.
- Wimalawansa, S.J.; Razzaque, M.S.; Al-Daghri, N.M. Calcium and vitamin D in human health: Hype or real? J Steroid Biochem Mol Biol 2018, 180, 4-14, doi:10.1016/j.jsbmb.2017.12.009.
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J Steroid Biochem Mol Biol 2018, 175, 60-81, doi:10.1016/j.jsbmb.2016.09.016.
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; McCarthy, C.M.; Bhan, I.; Camargo, C.A., Jr. Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit Care Med 2014, 42, 1365-1371, doi:10.1097/CCM.0000000000000210.
- Wimalawansa, S. Overcoming infections including COVID-19, by maintaining circulating 25(OH)D concentrations above 50 ng/mL. Pathology & Lab. Medicine Int. 2022, 14, 37–60.
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of T(H)1 cells. Nat Immunol 2022, 23, 62-74, doi:10.1038/s41590-021-01080-3.
- Sun, L.; Arbesman, J.; Piliang, M. Vitamin D, autoimmunity and immune-related adverse events of immune checkpoint inhibitors. Arch Dermatol Res 2021, 313, 1-10, doi:10.1007/s00403-020-02094-x.
- Johnson, C.R.; Thacher, T.D. Vitamin D: immune function, inflammation, infections and auto-immunity. Paediatr Int Child Health 2023, 1-11, doi:10.1080/20469047.2023.2171759.
- Soltani-Zangbar, M.S.; Mahmoodpoor, A.; Dolati, S.; Shamekh, A.; Valizadeh, S.; Yousefi, M.; Sanaie, S. Serum levels of vitamin D and immune system function in patients with COVID-19 admitted to intensive care unit. Gene Rep 2022, 26, 101509, doi:10.1016/j.genrep.2022.101509.
- Arora, J.; Wang, J.; Weaver, V.; Zhang, Y.; Cantorna, M.T. Novel insight into the role of the vitamin D receptor in the development and function of the immune system. J Steroid Biochem Mol Biol 2022, 219, 106084, doi:10.1016/j.jsbmb.2022.106084.
- McCartney, D.M.; Byrne, D.G. Optimisation of vitamin D status for enhanced immuno-protection against COVID-19. Ir Med J 2020, 113, 58.
- Zhou, Y.F.; Luo, B.A.; Qin, L.L. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine (Baltimore) 2019, 98, e17252, doi:10.1097/MD.0000000000017252.
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 1626, doi:10.3390/nu12040988.
- Tsujino, I.; Ushikoshi-Nakayama, R.; Yamazaki, T.; Matsumoto, N.; Saito, I. Pulmonary activation of vitamin D(3) and preventive effect against interstitial pneumonia. J Clin Biochem Nutr 2019, 65, 245-251, doi:10.3164/jcbn.19-48.
- FLCCC. Critical treatment protocols. Available online: https://covid19criticalcare.com/understanding-vitamin-d/ (accessed on May 13, 2023).
- Ross, A., Taylor, CL, Yaktine, AL, Del Valle, HB. Dietary Reference Intakes for Calcium and Vitamin D; Institue of Medicine: Washington (DC) 2011.
- Institute of Medicine. Scientific Evaluation of Dietary reference intakes for calcium and vitamin D. Available online: http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D.aspx.
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011, 96, 53-58, doi:10.1210/jc.2010-2704.
- Gov.UK. Guidance: Vitamin D deficiency: migrant health guide; London, UK, 2022.
- Australia.gov. Australian health Advice: Vitamin D and your health; Australia.
- NICE. NICE (National Institute for Health and Care Excellence). Vitamin D: supplement use in specific population groups. Available online: https://www.nice.org.uk/guidance/ph56 (accessed on August 3, 2021).
- Annonymus. Vitamin D: Fact Sheet for Consumers. Available online: https://ods.od.nih.gov/factsheets/vitamind-healthprofessional/ (accessed on May 30).
- Wimalawansa, S.J. Physiological basis for using vitamin D to improve health. Biomedicines 2023, 11, doi:10.3390/biomedicines11061542.
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011, 96, 1911-1930, doi:10.1210/jc.2011-0385.
- Anonymus. Vitamin D for COVID-19: real-time analysis of all 300 studies: https://c19early.org/d. Available online: https://c19vitamind.com/ (accessed on January 25, 2023).
- Mangin, M.; Sinha, R.; Fincher, K. Inflammation and vitamin D: the infection connection. Inflamm Res 2014, 63, 803-819, doi:10.1007/s00011-014-0755-z.
- Chung, M.K.; Karnik, S.; Saef, J.; Bergmann, C.; Barnard, J.; Lederman, M.M.; Tilton, J.; Cheng, F.; Harding, C.V.; Young, J.B.; et al. SARS-CoV-2 and ACE2: The biology and clinical data settling the ARB and ACEI controversy. EBioMedicine 2020, 58, 102907, doi:10.1016/j.ebiom.2020.102907.
- Adams, J.S., Modlin, R.L, Diz, MM, Barnes, P.F. Potentiation of the macrophage 25-hydroxyvitamin D-1-hydroxylation reaction by human tuberculous pleural effusion fluid. J Clin Endocrinol Metab 1989, 69, 457-460, doi:10.1210/jcem-69-2-457.
- Antal, A.S.; Dombrowski, Y.; Koglin, S.; Ruzicka, T.; Schauber, J. Impact of vitamin D3 on cutaneous immunity and antimicrobial peptide expression. Dermatoendocrinol 2011, 3, 18-22, doi:10.4161/derm.3.1.14616.
- Aloia, J.F.; Li-Ng, M. Re: epidemic influenza and vitamin D. Epidemiol Infect 2007, 135, 1095-1096; author reply 1097-1098, doi:10.1017/S0950268807008308.
- Fleming, D.M.; Elliot, A.J. Epidemic influenza and vitamin D. Epidemiol Infect 2007, 135, 1091-1092; author reply 1092-1095, doi:10.1017/S0950268807008291.
- Ali, M.; Uddin, Z. Factors associated with vitamin D deficiency among patients with musculoskeletal disorders seeking physiotherapy intervention: a hospital-based observational study. BMC Musculoskelet Disord 2022, 23, 817, doi:10.1186/s12891-022-05774-z.
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations >/=60 vs <20 ng/ml (150 vs 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS One 2018, 13, e0199265, doi:10.1371/journal.pone.0199265.
- Granato, T.; Manganaro, L.; Petri, L.; Porpora, M.G.; Viggiani, V.; Angeloni, A.; Anastasi, E. Low 25-OH vitamin D levels at time of diagnosis and recurrence of ovarian cancer. Tumour Biol 2016, 37, 2177-2181, doi:10.1007/s13277-015-4055-1.
- Grant, W.B.; Boucher, B.J.; Pludowski, P.; Wimalawansa, S.J. The emerging evidence for non-skeletal health benefits of vitamin D supplementation in adults. Nat Rev Endocrinol 2022, 18, 323, doi:10.1038/s41574-022-00646-x.
- Wimalawansa, S.J.; Whittle, R. Vitamin D: A single initial dose is not bogus if followed by an appropriate maintenance intake. JBMR Plus 2022, 6, e10606, doi:10.1002/jbm4.10606.
- Dawodu, A., Saadi, H.F, Bekdache, G, Javed, Y, Altaye, M, Hollis, B.W. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J Clin Endocrinol Metab 2013, 98, 2337-2346, doi:10.1210/jc.2013-1154.
- Camargo, C.A., Jr.; Ganmaa, D.; Frazier, A.L.; Kirchberg, F.F.; Stuart, J.J.; Kleinman, K.; Sumberzul, N.; Rich-Edwards, J.W. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012, 130, e561-567, doi:10.1542/peds.2011-3029.
- Bergman, P.; Norlin, A.C.; Hansen, S.; Rekha, R.S.; Agerberth, B.; Bjorkhem-Bergman, L.; Ekstrom, L.; Lindh, J.D.; Andersson, J. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open 2012, 2, doi:10.1136/bmjopen-2012-001663.
- Hutchinson, M.S.; Grimnes, G.; Joakimsen, R.M.; Figenschau, Y.; Jorde, R. Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population: the Tromso study. Eur J Endocrinol 2010, 162, 935-942, doi:10.1530/EJE-09-1041.
- Arayici, M.E.; Basbinar, Y.; Ellidokuz, H. Vitamin D intake, serum 25-hydroxyvitamin-D (25(OH)D) levels, and cancer Risk: A comprehensive meta-meta-analysis including meta-analyses of randomized controlled Trials and observational epidemiological studies. Nutrients 2023, 15, doi:10.3390/nu15122722.
- Murdoch, D.R.; Slow, S.; Chambers, S.T.; Jennings, L.C.; Stewart, A.W.; Priest, P.C.; Florkowski, C.M.; Livesey, J.H.; Camargo, C.A.; Scragg, R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA 2012, 308, 1333-1339, doi:10.1001/jama.2012.12505.
- Manaseki-Holland, S., Maroof, Z, Bruce, J, Mughal, M.Z, ; Masher, M.I., Bhutta, ZA, Walraven, G, Chandramohan, D. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 2012, 379, 1419-1427, doi:10.1016/S0140-6736(11)61650-4.
- Martineau, A.R.; Timms, P.M.; Bothamley, G.H.; Hanifa, Y.; Islam, K.; Claxton, A.P.; Packe, G.E.; Moore-Gillon, J.C.; Darmalingam, M.; Davidson, R.N.; et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet 2011, 377, 242-250, doi:10.1016/S0140-6736(10)61889-2.
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010, 303, 1815-1822, doi:10.1001/jama.2010.594.
- Nowson, C.A.; McGrath, J.J.; Ebeling, P.R.; Haikerwal, A.; Daly, R.M.; Sanders, K.M.; Seibel, M.J.; Mason, R.S.; Working Group of, A.; New Zealand, B.; et al. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust 2012, 196, 686-687, doi:10.5694/mja11.10301.
- Schwalfenberg, G.K.; Whiting, S.J. A Canadian response to the 2010 Institute of Medicine vitamin D and calcium guidelines. Public Health Nutr 2011, 14, 746-748, doi:10.1017/S1368980011000292.
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007, 85, 1586-1591, doi:10.1093/ajcn/85.6.1586.
- Marshall, D.T.; Savage, S.J.; Garrett-Mayer, E.; Keane, T.E.; Hollis, B.W.; Horst, R.L.; Ambrose, L.H.; Kindy, M.S.; Gattoni-Celli, S. Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J Clin Endocrinol Metab 2012, 97, 2315-2324, doi:10.1210/jc.2012-1451.
- Forman, J.P.; Scott, J.B.; Ng, K.; Drake, B.F.; Suarez, E.G.; Hayden, D.L.; Bennett, G.G.; Chandler, P.D.; Hollis, B.W.; Emmons, K.M.; et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension 2013, 61, 779-785, doi:10.1161/HYPERTENSIONAHA.111.00659.
- Mitri, J., Dawson-Hughes, B, Hu, FB, Pittas, A.G. Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr 2011, 94, 486-494, doi:10.3945/ajcn.111.011684.
- von Hurst, P.R.; Stonehouse, W.; Coad, J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr 2010, 103, 549-555, doi:10.1017/S0007114509992017.
- Belenchia, A.M.; Tosh, A.K.; Hillman, L.S.; Peterson, C.A. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr 2013, 97, 774-781, doi:10.3945/ajcn.112.050013.
- Derakhshandi, H.; Etemadifar, M.; Feizi, A.; Abtahi, S.H.; Minagar, A.; Abtahi, M.A.; Abtahi, Z.A.; Dehghani, A.; Sajjadi, S.; Tabrizi, N. Preventive effect of vitamin D3 supplementation on conversion of optic neuritis to clinically definite multiple sclerosis: a double blind, randomized, placebo-controlled pilot clinical trial. Acta neurologica Belgica 2013, 113, 257-263, doi:10.1007/s13760-012-0166-2.
- Kimball, S.V., R, Dosch, HM, Bar-Or, A, et al.
- Heaney, R.P.; Davies, K.M.; Chen, T.C.; Holick, M.F.; Barger-Lux, M.J. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003, 77, 204-210, doi:10.1093/ajcn/77.1.204.
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr 2004, 80, 1752S-1758S, doi:10.1093/ajcn/80.6.1752S.
- Trivedi, D., Doll, R, Khaw, KTT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003, 326, 469, doi:10.1136/bmj.326.7387.469.
- Johansson, H.; Oden, A.; Kanis, J.; McCloskey, E.; Lorentzon, M.; Ljunggren, O.; Karlsson, M.K.; Thorsby, P.M.; Tivesten, A.; Barrett-Connor, E.; et al. Low serum vitamin D is associated with increased mortality in elderly men: MrOS Sweden. Osteoporos Int 2012, 23, 991-999, doi:10.1007/s00198-011-1809-5.
- Kissmeyer, A.; Mathiasen, I.S.; Latini, S.; Binderup, L. Pharmacokinetic studies of vitamin D analogues: relationship to vitamin D binding protein (DBP). Endocrine 1995, 3, 263-266, doi:10.1007/BF03021403.
- Morris, H.A.; Anderson, P.H. Autocrine and paracrine actions of vitamin d. Clin Biochem Rev 2010, 31, 129-138.
- Atkinson, R.L. Viruses as an etiology of obesity. Mayo Clin Proc 2007, 82, 1192-1198, doi:10.4065/82.10.1192.
- McGregor, E., Kazemian M, Afzali, B, et al. An autocrine Vitamin D-driven Th1 shutdown program can be exploited for COVID-19. https://www.biorxiv.org/content/10.1101/2020.07.18.210161v1 2020.
- Gibson, C.C.; Davis, C.T.; Zhu, W.; Bowman-Kirigin, J.A.; Walker, A.E.; Tai, Z.; Thomas, K.R.; Donato, A.J.; Lesniewski, L.A.; Li, D.Y. Dietary Vitamin D and Its Metabolites Non-Genomically Stabilize the Endothelium. PLoS One 2015, 10, e0140370, doi:10.1371/journal.pone.0140370.
- Moromizato, T.; Litonjua, A.A.; Braun, A.B.; Gibbons, F.K.; Giovannucci, E.; Christopher, K.B. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med 2014, 42, 97-107, doi:10.1097/CCM.0b013e31829eb7af.
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011, 26, 2341-2357, doi:10.1002/jbmr.463.
- Hollis, B.W. Short-term and long-term consequences and concerns regarding valid assessment of vitamin D deficiency: comparison of recent food supplementation and clinical guidance reports. Curr Opin Clin Nutr Metab Care 2011, 14, 598-604, doi:10.1097/MCO.0b013e32834be798.
- Heaney, R.; Armas, L.; Shary, J.; Bell, N.; Binkley, N.; Hollis, B. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr 2008, 87, 1738-1742, doi:10.1093/ajcn/87.6.1738.
- Zittermann, A.; Iodice, S.; Pilz, S.; Grant, W.B.; Bagnardi, V.; Gandini, S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr 2012, 95, 91-100, doi:10.3945/ajcn.111.014779.
- Tolppanen, A.M.; Fraser, A.; Fraser, W.D.; Lawlor, D.A. Risk factors for variation in 25-hydroxyvitamin D(3) and D(2) concentrations and vitamin D deficiency in children. J Clin Endocrinol Metab 2012, 97, 1202-1210, doi:10.1210/jc.2011-2516.
- Bae, J.H.; Choe, H.J.; Holick, M.F.; Lim, S. Association of vitamin D status with COVID-19 and its severity : Vitamin D and COVID-19: a narrative review. Rev Endocr Metab Disord 2022, 23, 579-599, doi:10.1007/s11154-021-09705-6.
- Holick, M.F. Sunlight, UV Radiation, Vitamin D, and Skin Cancer: How Much Sunlight Do We Need? Adv Exp Med Biol 2020, 1268, 19-36, doi:10.1007/978-3-030-46227-7_2.
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 2010, 91, 1255-1260, doi:10.3945/ajcn.2009.29094.
- Bergman, P.; Lindh, A.U.; Bjorkhem-Bergman, L.; Lindh, J.D. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One 2013, 8, e65835, doi:10.1371/journal.pone.0065835.
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: state of the art. Critical reviews in food science and nutrition 2015, 55, 1193-1205, doi:10.1080/10408398.2012.688897.
- Wimalawansa, S.J. Controlling chronic diseases and acute infections with vitamin D sufficiency. Nutrients 2023, 15, doi:10.3390/nu15163623.




