Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daria Bortolotti | -- | 2106 | 2023-09-20 10:41:07 | | | |
| 2 | Catherine Yang | Meta information modification | 2106 | 2023-09-20 10:44:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Beltrami, S.; Rizzo, S.; Schiuma, G.; Speltri, G.; Di Luca, D.; Rizzo, R.; Bortolotti, D. Human Herpesviruses (HHVs). Encyclopedia. Available online: https://encyclopedia.pub/entry/49415 (accessed on 15 January 2026).
Beltrami S, Rizzo S, Schiuma G, Speltri G, Di Luca D, Rizzo R, et al. Human Herpesviruses (HHVs). Encyclopedia. Available at: https://encyclopedia.pub/entry/49415. Accessed January 15, 2026.
Beltrami, Silvia, Sabrina Rizzo, Giovanna Schiuma, Giorgia Speltri, Dario Di Luca, Roberta Rizzo, Daria Bortolotti. "Human Herpesviruses (HHVs)" Encyclopedia, https://encyclopedia.pub/entry/49415 (accessed January 15, 2026).
Beltrami, S., Rizzo, S., Schiuma, G., Speltri, G., Di Luca, D., Rizzo, R., & Bortolotti, D. (2023, September 20). Human Herpesviruses (HHVs). In Encyclopedia. https://encyclopedia.pub/entry/49415
Beltrami, Silvia, et al. "Human Herpesviruses (HHVs)." Encyclopedia. Web. 20 September, 2023.
Copy Citation
Human herpesviruses (HHVs) are highly widespread among humans and therefore are among the pathogens most responsible for gestational infections. HHVs are classified into three subfamilies (alpha-, beta- and gammaherpesvirinae), and they are able to establish permanent latency within the host in specific cells. The alphaherpesvirinae family includes herpes simplex type-1 (HSV-1 or HHV-1), herpes simplex type-2 (HSV-2 or HHV-2) and varicella zoster virus (VZV or HHV-3). The betaherpesvirinae family includes cytomegalovirus (CMV or HHV-5), HHV-6A/B and HHV-7. The gammaherpesvirinae family consists of Epstein–Barr virus (EBV or HHV-4) and Kaposi’s sarcoma-associated herpesvirus (KSHV or HHV-8).
viruses
immune system
DNA viruses
RNA viruses
1. HSV-1 and HSV-2
Normally, HSV-1 predominates in orofacial lesions and typically is found in the trigeminal ganglia, while HSV-2 is most found in the lumbosacral ganglia. The greatest risk of disease in the newborn is represented by the late-pregnancy infection of genitals in a previously unexposed woman, while recurrent infections are rarely associated with disseminated neonatal disease in immune-competent women.
However, a primary HSV infection of a pregnant woman leads to greater risks for both mother and child. Although HSV-infected pregnant women have rare or no clinical recurrences, there is still the risk of intrapartum transmission [1].
Women who already has antibodies to both HSV-1 and HSV-2 at the onset of pregnancy, which is the most common condition, have the least risk of perinatal transmission [2]. On the contrary, new-onset HSVs infection occurring late in pregnancy carries a 30% to 50% risk of neonatal infection, while early pregnancy infection carries a risk of less than 1% [3]. The possible explanation could be that when primary HSVs infection occurs during late pregnancy, the time for developing specific antibodies and suppressing viral replication before labor is not enough.
Again, while HSV-1 transmission from mother to newborn seems to be easier in the presence of both primary infection or recurrences [4], primary HSV-2 infection transmission to the fetus is less frequent [5], but it has been associated with a higher incidence of preterm birth [6] (Figure 1). The clinical manifestations of neonatal HSVs infection include encephalitis and disseminated disease, with a mortality rate of more than 50%. Survivors are, however, compromised, usually with significant neurologic deficits, blindness, seizures and learning disabilities (Figure 1). Different studies have also stated that HSVs infection can affect maternal immune responses, resulting in loss of HLA-G [7], cell death and reduced human chorionic gonadotropin (HCG) secretion [8] (Figure 1). These changes in trophoblast function could explain why both HSV-1 and HSV-2 have been associated with spontaneous pregnancy loss [9] and IUGR pregnancies [10][11] (Figure 1).
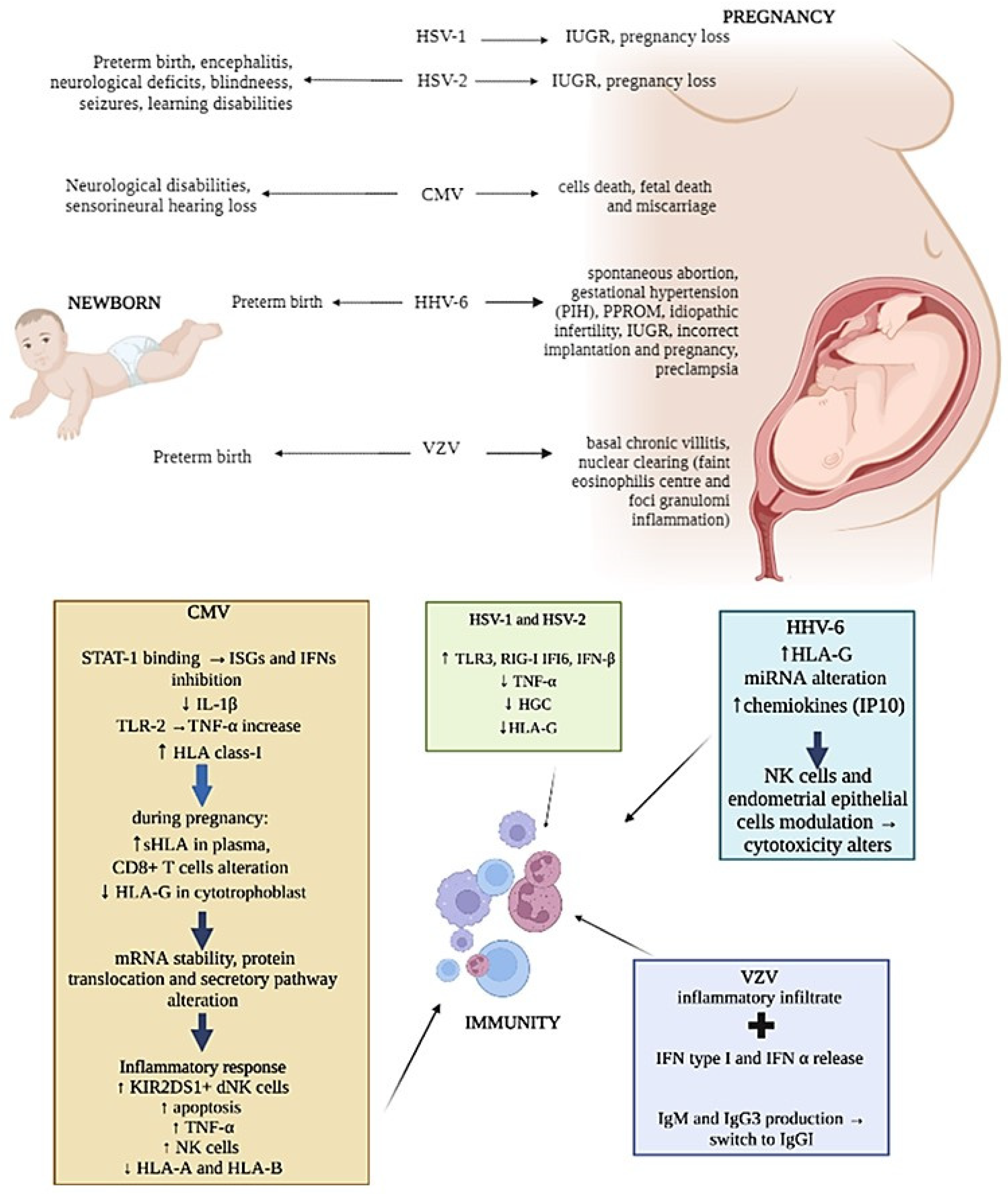
Figure 1. Representation of herpesviruses infection’s main effects on newborn, pregnancy and mother immunity.
Moreover, HSV is capable of increasing the expression of TLR3, RIG-I, IFI6 and IFN-β proteins as well as decreasing TNFα production in terms of human placental explant cultures [12] (Figure 1), affecting maternal innate immune system antiviral responses.
2. CMV
Among herpesviruses, CMV is one of the most vertically transmitted and it represents the most common cause of congenital infection in high-income countries, causing neurological disability and sensorineural hearing loss in newborns [13] (Figure 1). The intrauterine infection caused by CMV occurs in 0.3% to 2.3% of births [14]. CMV intrauterine transmission is more common after primary infection (30–40%) than after non-primary infection (1%) [15][16]. Nevertheless, it was estimated that non-primary maternal infections are responsible for the majority of congenital CMV infections [17].
CMV, such as many other viruses, employs multiple mechanisms to exploit the vulnerability of the placenta and impair the innate host response in order to spread the infection, including the expression of several viral proteins such as IE1 [18], IE86 [19], UL44, IE2 and UL94 [18][20][21].
The effect of CMV on the placenta has been confirmed by proving that ultraviolet-inactivated human CMV leads to syncytiotrophoblast apoptosis via TLR2, also increasing TNFα production [22][23]. Interestingly, in human villous explants, CMV did not induce the expression of RIG-I and MDA5 proteins and cytokine production [24], suggesting that RLRs could play a central role in the inhibition of vertical transmission of the virus as well as in the selectivity of vertical transmission of viruses across the placenta.
CMV can modulate the expression of HLA molecules by encoding specific viral proteins, mainly decreasing their expression on the cell surface to prevent immune cells recognition. In addition, CMV can selectively up-regulate specific HLA class-I molecules [25] (Figure 1). As aforementioned, HLA-G is physiologically expressed at the maternal–fetal interface on trophoblasts and is one of the major molecules targeted by viruses during pregnancy [26]. The concentrations of sHLA-G normally increases in the plasma of pregnant women during the first trimester of pregnancy [27], but during CMV infection, a reduction in HLA-G in cytotrophoblasts was observed [28] together with its up-regulation in peripheral blood cells [29] (Figure 1). This effect is due to the interaction of specific CMV proteins with the HLA-G promoter, which affects mRNA stability and protein translation and secretion [30][31][32] (Figure 1).
Several studies have reported the failure of dNK to control CMV infection. Since NK cells activity is regulated by the expression of activation/inhibitory killer imuunoglubulin-like receptors (KIRs), such as KIR2DL4, KIR2DS1/5, etc. as activating, and KIR2DL1/2/3, etc. as inhibiting, the expression of these receptors and of their ligands on target cells can be exploited by viruses as immune-escape mechanisms. Crespo et al. [33] demonstrate that CMV infection of human HLA-C2 + decidual stromal cells drives the cytotoxic activation of dNK and placental NK (pNK) cells in vitro by engaging KIR2DS2, and that KIR2DS1 or KIR2DS5-negative pregnant women have a lower ability to control placental CMV infection, developing complications. Van der Ploeg et al. [34] reported the molecular basis for the increased degranulation response of KIR2DS1 + dNK to CMV infection (Figure 1). Yan et al. [35] show that a KIR2DL4/HLA-G combination induces high NK cytotoxicity, which might be beneficial uterine CMV infection. Other studies described the presence of adaptive NK cell expansion found during different viral infections, concluding that in CMV-infected individuals, adaptive NK cells may be established probably as the result of opportunistic viral reactivation [36].
Moreover, the interaction between CMV and dNK cells can be the cause fetal death or miscarriage due to NK cell cytotoxic activity [37] (Figure 1). However, the CMV infection of EVT did not diminish the ability of EVT to increase FOXP3+ and PD1HI T-regs [38], suggesting that its infection does not alter the capacity of EVT to promote immune tolerance. This finding confirms the observation that dNK fails to degranulate in response to CMV-infected EVT, thus also maintaining immune tolerance in the presence of infection [39].
Moreover, the failure of dNK to respond to CMV-infected EVT during in vitro co-culture [39] may leave decidual CD8+ T cells as the predominant effector cell to clear pathogen-infected EVT.
Seropositive women during late pregnancy demonstrated an accumulation of highly differentiated CMV-specific T cells [40]. In fact, CMV seropositivity was shown to dramatically alter the maternal CD8+ T-cell repertoire during pregnancy [40], and T-cell responses to CMV rely heavily on HLA-C-restricted signals [41] (Figure 1). CMV CD8+ T cells were also found increased particularly in decidual tissue and were found able to produce IFNγ and restricted to recognizing viral peptides presented by HLA-A or HLA-B molecules, limiting the spread of infection to trophoblasts and/or the fetus [42].
3. HHV-6
HHV-6 is widely spread during pregnancy as well. HHV-6 DNA has been detected in blood and tissue samples from women with several types of gestational problems, including spontaneous abortions, gestational hypertension and preterm birth, in association with the detection of high anti-HHV-6 IgM and IgG titers [43] (Figure 1). HHV-6 DNA was also found in the amniotic fluid of women with gestational complications [44], as pregnancy induced hypertension (PIH) and the premature preterm rupture of membranes (PPROM) (Figure 1).
To date, despite different congenital herpetic infections having been associated with late IUGR, no direct implication of HHV-6 infection has been reported. In particular, HLA-G expression and HHV-6 infection have been evaluated in placentas from late-onset IUGR newborns compared to placentas from uncomplicated pregnancies [45], since HHV-6 is known to exploit the modulation of HLA-G as an immune-escape mechanism.
HLA-G increased and HHV-6 presence were found to correlate in IUGR placenta samples [45]. These preliminary results underline a direct relationship between HHV-6 infection and HLA-G deregulation that might affect vessel remodeling and prevent the correct pregnancy outcome in the IUGR condition (Figure 1).
However, HHV6 is comprised of two species, HHV-6A and HHV-6B [46][47]. While most of the population is infected by HHV-6B by 2 years of age, HHV-6A infection usually occurs later [48][49][50]. In particular, HHV-6A clinical manifestations are still unclear, but the presence of HHV-6A in endometrial epithelial cells of a subgroup of idiopathic infertile women [51] supported the role of HHV-6A [52] (Figure 1).
Moreover, it has been observed that HHV-6A infection induces a profound remodulation of miRNA expression in human cells of different origin [53], including human endometrial cells, in which HHV-6A modulates at least 16 miRNAs with potentially critical roles during embryo implantation [54]. These virus-induced alterations in the miRNA expression of endometrial cells might affect trophoblast cell behavior (Figure 1), supporting the hypothesis that HHV-6A might be associated with interference in correct implantation and pregnancy outcome [55].
The abilities of NK and endometrial cells have been described to be changed by HHV-6A infection. In fact, phenotypical and functional modifications of both endometrial NK (eNK) and epithelial cells have been reported in HHV-6A-positive infertile women samples, suggesting an imprint due to HHV-6A infection on both eNK cell immune-phenotype and receptors repertoire (Figure 1). In particular, during HHV-6A infection, eNK cells seem to acquire a cytotoxic profile as an attempt to limit the infection, which involves the NKG2D receptor [52]. The persistence of activated eNK and of subclinical HHV-6A infection could alter endometrial environment and disadvantage embryo implantation and placentation, and it could potentially have serious adverse side effects, such as pre-eclampsia, fetal growth restriction and stillbirth, as demonstrated by the increase in chemokines, mainly IP10 and FasL, in uterine flushing samples from HHV-6A-positive infertile women (Figure 1).
In addition, Rizzo et al. observed a lower percentage of KIR2DL4-positive eNK cells in primary infertile women in correlation to the diminished expression of soluble HLA-G [56]. This evidence supports the potential role of HHV-6 in female diseases, as a consequence of HLA-G modulation, that can in turn induce anergy to eNK cells via the inhibitory KIR2DL4 receptor [56].
4. VZV
VZV is the etiological agent for chicken pox at time of primary infection, and it is usually associated to mild clinical course, but in pregnant women, it may occasionally lead to serious maternal and fetal diseases. Maternal VZV can infect the baby by different routes: (a) transplacental viremia, (b) ascending infection during birth or (c) respiratory droplet/direct contact with infectious lesions after birth.
Even if the primary mechanism of VZV transfer across the placenta remains unclear, it is postulated that infected T cells might be present in the decidua basalis [57], where both CD4+ and CD8+ T cells are reprogrammed by the virus, becoming more capable of crossing into the intervillous space [58].
However, reports vary on the histological features of VZV placental infection, suggesting that VZV could be transmitted to the fetus via the placenta without apparent viral replication within the placenta [59] (Figure 1).
Interestingly, nearly 20% of infants with intrauterine-acquired VZV primary infection develop neonatal or infantile zoster, usually with uncomplicated course [60]. The disease is thought to represent reactivation of the virus after primary infection in utero, and the short viral latency may be explained by the immature cell-mediated immune response in young children.
Moreover, recurrent chickenpox has been documented in pregnant women [61], underlining again the key role of the immune system.
In addition to the tests of general antibody reactivity, tests of antibody avidity [62] and IgG isotype [63] can be used to assess the nature of VZV antibody responses. The avidity of antibodies seems to increase thereafter and during shingles, while there is a switch from IgM and IgG3 to IgG1 after primary disease [64] (Figure 1). Therefore, the clinical manifestation of these pregnant women can be due to the high virus load and low immune responses [65], whether the effect of pregnancy and associated hormones on VZV replication is not known.
VZV infection causes a very early release of IFN type I, which is particularly abundant at the lesion level [66] (Figure 1). NK cells can also be found early after VZV infection [67], suggesting their central role in controlling viral spread. In fact, while both cytotoxic NK and primed CD8+ T cells were nearly absent during the early phase of life-threatening primary VZV infection [68], their responses to VZV seem to be protective and associated to mild symptoms [69]. As an example, the detection of T cells within three days after the appearance of the varicella rash, with rapid host response to primary VZV infection, has been known to be associated with milder rash and a more rapid clearance of viremia in healthy subjects [67].
References
- Enright, A.M.; Prober, C.G. Neonatal herpes infection: Diagnosis, treatment and prevention. Semin. Neonatol. 2002, 7, 283–291.
- Kriebs, J.M. Understanding herpes simplex virus: Transmission, diagnosis, and considerations in pregnancy management. J. Midwifery Womens Health 2008, 53, 202–208.
- Dinc, B.; Bozdayi, G.; Biri, A.; Kalkanci, A.; Dogan, B.; Bozkurt, N.; Rota, S. Molecular detection of cytomegalovirus, herpes simplex virus 2, human papillomavirus 16-18 in Turkish pregnants. Braz. J. Infect. Dis. 2010, 14, 569–574.
- Brown, Z.A.; Wald, A.; Morrow, R.A.; Selke, S.; Zeh, J.; Corey, L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 2003, 289, 203–209.
- Brown, Z.A.; Gardella, C.; Wald, A.; Morrow, R.; Corey, L. Genital Herpes Complicating Pregnancy. Obstet. Gynecol. 2006, 107, 426.
- Brown, Z.A.; Benedetti, J.; Selke, S.; Ashley, R.; Watts, D.H.; Corey, L. Asymptomatic maternal shedding of herpes simplex virus at the onset of labor: Relationship to preterm labor. Obstet. Gynecol. 1996, 87, 483–488.
- Schust, D.J.; Hill, A.B.; Ploegh, H.L. Herpes simplex virus blocks intracellular transport of HLA-G in placentally derived human cells. J. Immunol. 1996, 157, 3375–3380.
- Norskov-Lauritsen, N.; Aboagye-Mathisen, G.; Juhl, C.B.; Petersen, P.M.; Zachar, V.; Ebbesen, P. Herpes simplex virus infection of cultured human term trophoblast. J. Med. Virol. 1992, 36, 162–166.
- Robb, J.A.; Benirschke, K.; Barmeyer, R. Intrauterine latent herpes simplex virus infection: I. Spontaneous abortion. Hum. Pathol. 1986, 17, 1196–1209.
- Arechavaleta-Velasco, F.; Koi, H.; Strauss, J.F., 3rd; Parry, S. Viral infection of the trophoblast: Time to take a serious look at its role in abnormal implantation and placentation? J. Reprod. Immunol. 2002, 55, 113–121.
- Brown, Z.A.; Vontver, L.A.; Benedetti, J.; Critchlow, C.W.; Sells, C.J.; Berry, S.; Corey, L. Effects on infants of a first episode of genital herpes during pregnancy. N. Engl. J. Med. 1987, 317, 1246–1251.
- Jabłońska, A.; Studzińska, M.; Suski, P.; Kalinka, J.; Paradowska, E. Enhanced expression of IFI16 and RIG-I in human third-trimester placentas following HSV-1 infection. Clin. Exp. Immunol. 2018, 193, 255–263.
- Leruez-Ville, M.; Foulon, I.; Pass, R.; Ville, Y. Cytomegalovirus infection during pregnancy: State of the science. Am. J. Obstet. Gynecol. 2020, 223, 330–349.
- Lycke, E.; Norrby, S.R. HerpesVirus Infections: State of the Art. Scand. J. Infect. Dis. 2015, 23, 1–118.
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276.
- Fowler, K.B.; Stagno, S.; Pass, R.F.; Britt, W.J.; Boll, T.J.; Alford, C.A. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 1992, 326, 663–667.
- de Vries, J.J.; van Zwet, E.W.; Dekker, F.W.; Kroes, A.C.; Verkerk, P.H.; Vossen, A.C. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: A population-based prediction model. Rev. Med. Virol. 2013, 23, 241–249.
- Amsler, L.; Verweij, M.; DeFilippis, V.R. The tiers and dimensions of evasion of the type I interferon response by human cytomegalovirus. J. Mol. Biol. 2013, 425, 4857–4871.
- Botto, S.; Abraham, J.; Mizuno, N.; Pryke, K.; Gall, B.; Landais, I.; Streblow, D.N.; Fruh, K.J.; DeFilippis, V.R. Human Cytomegalovirus Immediate Early 86-kDa Protein Blocks Transcription and Induces Degradation of the Immature Interleukin-1beta Protein during Virion-Mediated Activation of the AIM2 Inflammasome. mBio 2019, 10, e02510-18.
- Zou, H.M.; Huang, Z.F.; Yang, Y.; Luo, W.W.; Wang, S.Y.; Luo, M.H.; Fu, Y.Z.; Wang, Y.Y. Human Cytomegalovirus Protein UL94 Targets MITA to Evade the Antiviral Immune Response. J. Virol. 2020, 94, e00022-20.
- Fu, Y.Z.; Su, S.; Zou, H.M.; Guo, Y.; Wang, S.Y.; Li, S.; Luo, M.H.; Wang, Y.Y. Human Cytomegalovirus DNA Polymerase Subunit UL44 Antagonizes Antiviral Immune Responses by Suppressing IRF3- and NF-kappaB-Mediated Transcription. J. Virol. 2019, 93, e00181-19.
- Chan, G.; Guilbert, L.J. Ultraviolet-inactivated human cytomegalovirus induces placental syncytiotrophoblast apoptosis in a Toll-like receptor-2 and tumour necrosis factor-alpha dependent manner. J. Pathol. 2006, 210, 111–120.
- Chaudhuri, S.; Lowen, B.; Chan, G.; Davey, A.; Riddell, M.; Guilbert, L.J. Human cytomegalovirus interacts with toll-like receptor 2 and CD14 on syncytiotrophoblasts to stimulate expression of TNFalpha mRNA and apoptosis. Placenta 2009, 30, 994–1001.
- Jablonska, A.; Swierzko, A.S.; Studzinska, M.; Suski, P.; Kalinka, J.; Lesnikowski, Z.J.; Cedzynski, M.; Paradowska, E. Insight into the expression of RIG-I-like receptors in human third trimester placentas following ex vivo cytomegalovirus or vesicular stomatitis virus infection. Mol. Immunol. 2020, 126, 143–152.
- Lin, A.; Xu, H.; Yan, W. Modulation of HLA expression in human cytomegalovirus immune evasion. Cell. Mol. Immunol. 2007, 4, 91–98.
- Rizzo, R.; Vercammen, M.; van de Velde, H.; Horn, P.A.; Rebmann, V. The importance of HLA-G expression in embryos, trophoblast cells, and embryonic stem cells. Cell. Mol. Life Sci. 2011, 68, 341–352.
- Rizzo, R.; Andersen, A.S.; Lassen, M.R.; Sorensen, H.C.; Bergholt, T.; Larsen, M.H.; Melchiorri, L.; Stignani, M.; Baricordi, O.R.; Hviid, T.V. Soluble human leukocyte antigen-G isoforms in maternal plasma in early and late pregnancy. Am. J. Reprod. Immunol. 2009, 62, 320–338.
- Jun, Y.; Kim, E.; Jin, M.; Sung, H.C.; Han, H.; Geraghty, D.E.; Ahn, K. Human cytomegalovirus gene products US3 and US6 down-regulate trophoblast class I MHC molecules. J. Immunol. 2000, 164, 805–811.
- Onno, M.; Pangault, C.; Le Friec, G.; Guilloux, V.; Andre, P.; Fauchet, R. Modulation of HLA-G antigens expression by human cytomegalovirus: Specific induction in activated macrophages harboring human cytomegalovirus infection. J. Immunol. 2000, 164, 6426–6434.
- Park, B.; Spooner, E.; Houser, B.L.; Strominger, J.L.; Ploegh, H.L. The HCMV membrane glycoprotein US10 selectively targets HLA-G for degradation. J. Exp. Med. 2010, 207, 2033–2041.
- Barel, M.T.; Ressing, M.; Pizzato, N.; van Leeuwen, D.; Le Bouteiller, P.; Lenfant, F.; Wiertz, E.J. Human cytomegalovirus-encoded US2 differentially affects surface expression of MHC class I locus products and targets membrane-bound, but not soluble HLA-G1 for degradation. J. Immunol. 2003, 171, 6757–6765.
- Schust, D.J.; Tortorella, D.; Seebach, J.; Phan, C.; Ploegh, H.L. Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11. J. Exp. Med. 1998, 188, 497–503.
- Crespo, A.C.; van der Zwan, A.; Ramalho-Santos, J.; Strominger, J.L.; Tilburgs, T. Cytotoxic potential of decidual NK cells and CD8+ T cells awakened by infections. J. Reprod. Immunol. 2017, 119, 85–90.
- van der Ploeg, K.; Chang, C.; Ivarsson, M.A.; Moffett, A.; Wills, M.R.; Trowsdale, J. Modulation of Human Leukocyte Antigen-C by Human Cytomegalovirus Stimulates KIR2DS1 Recognition by Natural Killer Cells. Front. Immunol. 2017, 8, 298.
- Yan, W.H.; Lin, A.; Chen, B.G.; Zhou, M.Y.; Dai, M.Z.; Chen, X.J.; Gan, L.H.; Zhu, M.; Shi, W.W.; Li, B.L. Possible roles of KIR2DL4 expression on uNK cells in human pregnancy. Am. J. Reprod. Immunol. 2007, 57, 233–242.
- Beziat, V.; Hilton, H.G.; Norman, P.J.; Traherne, J.A. Deciphering the killer-cell immunoglobulin-like receptor system at super-resolution for natural killer and T-cell biology. Immunology 2017, 150, 248–264.
- Tanaka, K.; Yamada, H.; Minami, M.; Kataoka, S.; Numazaki, K.; Minakami, H.; Tsutsumi, H. Screening for vaginal shedding of cytomegalovirus in healthy pregnant women using real-time PCR: Correlation of CMV in the vagina and adverse outcome of pregnancy. J. Med. Virol. 2006, 78, 757–759.
- Salvany-Celades, M.; van der Zwan, A.; Benner, M.; Setrajcic-Dragos, V.; Bougleux Gomes, H.A.; Iyer, V.; Norwitz, E.R.; Strominger, J.L.; Tilburgs, T. Three Types of Functional Regulatory T Cells Control T Cell Responses at the Human Maternal-Fetal Interface. Cell. Rep. 2019, 27, 2537–2547 e2535.
- Crespo, A.C.; Strominger, J.L.; Tilburgs, T. Expression of KIR2DS1 by decidual natural killer cells increases their ability to control placental HCMV infection. Proc. Natl. Acad. Sci. USA 2016, 113, 15072–15077.
- Lissauer, D.; Choudhary, M.; Pachnio, A.; Goodyear, O.; Moss, P.A.; Kilby, M.D. Cytomegalovirus sero positivity dramatically alters the maternal CD8+ T cell repertoire and leads to the accumulation of highly differentiated memory cells during human pregnancy. Hum. Reprod. 2011, 26, 3355–3365.
- Ameres, S.; Mautner, J.; Schlott, F.; Neuenhahn, M.; Busch, D.H.; Plachter, B.; Moosmann, A. Presentation of an immunodominant immediate-early CD8+ T cell epitope resists human cytomegalovirus immunoevasion. PLoS Pathog. 2013, 9, e1003383.
- van Egmond, A.; van der Keur, C.; Swings, G.M.; Scherjon, S.A.; Claas, F.H. The possible role of virus-specific CD8(+) memory T cells in decidual tissue. J. Reprod. Immunol. 2016, 113, 1–8.
- Liu, W.; Niu, Z.; Li, Q.; Pang, R.T.; Chiu, P.C.; Yeung, W.S. MicroRNA and Embryo Implantation. Am. J. Reprod. Immunol. 2016, 75, 263–271.
- Cai, M.; Kolluru, G.K.; Ahmed, A. Small Molecule, Big Prospects: MicroRNA in Pregnancy and Its Complications. J. Pregnancy 2017, 2017, 6972732.
- Bortolotti, D.; Gentili, V.; Santi, E.; Taliento, C.; Vitagliano, A.; Schiuma, G.; Beltrami, S.; Rizzo, S.; Lanza, G.; Rizzo, R.; et al. Late-onset intrauterine growth restriction and HHV-6 infection: A pilot study. J. Med. Virol. 2021, 93, 6317–6322.
- Ablashi, D.; Agut, H.; Alvarez-Lafuente, R.; Clark, D.A.; Dewhurst, S.; DiLuca, D.; Flamand, L.; Frenkel, N.; Gallo, R.; Gompels, U.A.; et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch. Virol. 2014, 159, 863–870.
- Eliassen, E.; Marci, R.; Di Luca, D.; Rizzo, R. The use of heparin in infertility and recurrent pregnancy loss: Are its antiviral properties at play? Med. Hypotheses 2017, 102, 41–47.
- De Bolle, L.; Naesens, L.; De Clercq, E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin. Microbiol. Rev. 2005, 18, 217–245.
- Di Luca, D.; Dolcetti, R.; Mirandola, P.; De Re, V.; Secchiero, P.; Carbone, A.; Boiocchi, M.; Cassai, E. Human herpesvirus 6: A survey of presence and variant distribution in normal peripheral lymphocytes and lymphoproliferative disorders. J. Infect. Dis. 1994, 170, 211–215.
- Kondo, K.; Kondo, T.; Okuno, T.; Takahashi, M.; Yamanishi, K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J. Gen. Virol. 1991, 72 Pt 6, 1401–1408.
- Marci, R.; Gentili, V.; Bortolotti, D.; Lo Monte, G.; Caselli, E.; Bolzani, S.; Rotola, A.; Di Luca, D.; Rizzo, R. Presence of HHV-6A in Endometrial Epithelial Cells from Women with Primary Unexplained Infertility. PLoS ONE 2016, 11, e0158304.
- Caselli, E.; Bortolotti, D.; Marci, R.; Rotola, A.; Gentili, V.; Soffritti, I.; D’Accolti, M.; Lo Monte, G.; Sicolo, M.; Barao, I.; et al. HHV-6A Infection of Endometrial Epithelial Cells Induces Increased Endometrial NK Cell-Mediated Cytotoxicity. Front. Microbiol. 2017, 8, 2525.
- Rizzo, R.; Soffritti, I.; D’Accolti, M.; Bortolotti, D.; Di Luca, D.; Caselli, E. HHV-6A/6B Infection of NK Cells Modulates the Expression of miRNAs and Transcription Factors Potentially Associated to Impaired NK Activity. Front. Microbiol. 2017, 8, 2143.
- Ma, H.L.; Gong, F.; Tang, Y.; Li, X.; Li, X.; Yang, X.; Lu, G. Inhibition of Endometrial Tiam1/Rac1 Signals Induced by miR-22 Up-Regulation Leads to the Failure of Embryo Implantation During the Implantation Window in Pregnant Mice. Biol. Reprod. 2015, 92, 152.
- Bortolotti, D.; Soffritti, I.; D’Accolti, M.; Gentili, V.; Di Luca, D.; Rizzo, R.; Caselli, E. HHV-6A Infection of Endometrial Epithelial Cells Affects miRNA Expression and Trophoblast Cell Attachment. Reprod. Sci. 2020, 27, 779–786.
- Rizzo, R.; Lo Monte, G.; Bortolotti, D.; Graziano, A.; Gentili, V.; Di Luca, D.; Marci, R. Impact of soluble HLA-G levels and endometrial NK cells in uterine flushing samples from primary and secondary unexplained infertile women. Int. J. Mol. Sci. 2015, 16, 5510–5516.
- Hoo, R.; Nakimuli, A.; Vento-Tormo, R. Innate Immune Mechanisms to Protect Against Infection at the Human Decidual-Placental Interface. Front. Immunol. 2020, 11, 2070.
- Sen, N.; Mukherjee, G.; Sen, A.; Bendall, S.C.; Sung, P.; Nolan, G.P.; Arvin, A.M. Single-cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus. Cell. Rep. 2014, 8, 633–645.
- Kawana, K.; Yoshikawa, H.; Sata, T. Post-partum detection of varicella-zoster virus DNA in the placenta. Int. J. Gynaecol. Obstet. 1996, 55, 165–166.
- Sauerbrei, A.; Wutzler, P. Herpes simplex and varicella-zoster virus infections during pregnancy: Current concepts of prevention, diagnosis and therapy. Part 2: Varicella-zoster virus infections. Med. Microbiol. Immunol. 2007, 196, 95–102.
- McGregor, J.A.; Mark, S.; Crawford, G.P.; Levin, M.J. Varicella zoster antibody testing in the care of pregnant women exposed to varicella. Am. J. Obstet. Gynecol. 1987, 157, 281–284.
- Kangro, H.O.; Manzoor, S.; Harper, D.R. Antibody avidity following varicella-zoster virus infections. J. Med. Virol. 1991, 33, 100–105.
- Asano, Y.; Hiroishi, Y.; Itakura, N.; Hirose, S.; Kajita, Y.; Nagai, T.; Yazaki, T.; Takahashi, M. Immunoglobulin Subclass Antibodies to Varicefla-Zoster Virus. Pediatrics 1987, 80, 933–936.
- Junker, A.K.; Tilley, P. Varicella-zoster virus antibody avidity and IgG-subclass patterns in children with recurrent chickenpox. J. Med. Virol. 1994, 43, 119–124.
- Asano, Y.; Itakura, N.; Kajita, Y.; Suga, S.; Yoshikawa, T.; Yazaki, T.; Ozaki, T.; Yamanishi, K.; Takahashi, M. Severity of viremia and clinical findings in children with varicella. J. Infect. Dis. 1990, 161, 1095–1098.
- Gerlini, G.; Mariotti, G.; Bianchi, B.; Pimpinelli, N. Massive recruitment of type I interferon producing plasmacytoid dendritic cells in varicella skin lesions. J. Investig. Dermatol. 2006, 126, 507–509.
- Arvin, A.M.; Koropchak, C.M.; Williams, B.R.; Grumet, F.C.; Foung, S.K. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J. Infect. Dis. 1986, 154, 422–429.
- Banovic, T.; Yanilla, M.; Simmons, R.; Robertson, I.; Schroder, W.A.; Raffelt, N.C.; Wilson, Y.A.; Hill, G.R.; Hogan, P.; Nourse, C.B. Disseminated varicella infection caused by varicella vaccine strain in a child with low invariant natural killer T cells and diminished CD1d expression. J. Infect. Dis. 2011, 204, 1893–1901.
- Duncan, C.J.; Hambleton, S. Varicella zoster virus immunity: A primer. J. Infect. 2015, 71 (Suppl. S1), S47–S53.
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
20 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




