Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | claudia Sanjurjo Muñiz | -- | 2797 | 2023-09-15 14:44:43 | | | |
| 2 | Camila Xu | Meta information modification | 2797 | 2023-09-18 02:48:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sanjurjo, C.; Rodríguez, E.; Viesca, J.L.; Battez, A.H. Common Techniques for Biolubricant Production. Encyclopedia. Available online: https://encyclopedia.pub/entry/49244 (accessed on 07 March 2026).
Sanjurjo C, Rodríguez E, Viesca JL, Battez AH. Common Techniques for Biolubricant Production. Encyclopedia. Available at: https://encyclopedia.pub/entry/49244. Accessed March 07, 2026.
Sanjurjo, Claudia, Eduardo Rodríguez, José L. Viesca, A. Hernández Battez. "Common Techniques for Biolubricant Production" Encyclopedia, https://encyclopedia.pub/entry/49244 (accessed March 07, 2026).
Sanjurjo, C., Rodríguez, E., Viesca, J.L., & Battez, A.H. (2023, September 15). Common Techniques for Biolubricant Production. In Encyclopedia. https://encyclopedia.pub/entry/49244
Sanjurjo, Claudia, et al. "Common Techniques for Biolubricant Production." Encyclopedia. Web. 15 September, 2023.
Copy Citation
Lubricants can be classified according to their physical state as solid, liquid, or semi-fluid (greases). The former is used when it is difficult to maintain contact with the fluid, while the latter is used in situations where liquid lubricants are not applicable. According to the UNE-EN 16807 standard, the term “bio” is considered synonymous with good for the environment. Its use in lubricants is linked to its environmental properties; therefore, it is expected that all compounds called bio-lubricants will degrade in the environment.
biolubricant
molecular structure
tribological properties
1. Introduction
Water and food shortages are just some of the direct consequences of global warming caused by the increase in CO2 emissions in recent decades. According to Lindsey’s report [1], global sea levels have risen 24 cm since 1880, triggered by the start of the second industrial revolution. The highest peak emissions in history were recorded last year with 40.5 tons, behind pre-COVID-19 levels of 40.9 tons, with the use of fossil sources being the main emitter of greenhouse gases, leading with 90.47%, of which 25% came from energy losses [2].
Maintaining machinery properly, conserving energy, and finding potential substitutes for petroleum derivatives are necessary to combat accelerating climate change. One of the sciences that can contribute to this purpose is tribology, which is responsible for optimizing lubrication in the interaction between moving surfaces, and which has led to large amounts of energy and money being saved in industry. Jost [3] estimated that GBP 515 million could be saved by improving tribological conditions in the UK industry. In addition, recent research into natural lubricant sources is helping to move society toward more sustainable industries.
To achieve these goals, it is necessary to review the minimum requirements for lubricants, the advantages and disadvantages of natural sources, and the existing techniques for improving these materials to produce potential alternatives to petroleum-based lubricants. The basic functions of lubricants can be summarized as follows: (a) to reduce energy losses; (b) to protect surfaces from wear due to friction; (c) to protect against corrosion; (d) to reduce oxidation effects; (e) to cool down surfaces; (f) to decrease heat losses due to contact between moving surfaces; or (g) to increase tightness and prevent the leakage of contaminants and sediments [4][5], in addition to certain requirements depending on the application.
Lubricants can be classified according to their physical state as solid, liquid, or semi-fluid (greases). The former is used when it is difficult to maintain contact with the fluid, while the latter is used in situations where liquid lubricants are not applicable. They can be further classified according to their source material as mineral-, synthetic-, animal-, or vegetable-based lubricants (Figure 1). All base fluids directly refined from crude oil are called mineral bases, those refined from natural sources are called vegetable bases, and those synthesized (natural or mineral) are called synthetic bases [6].
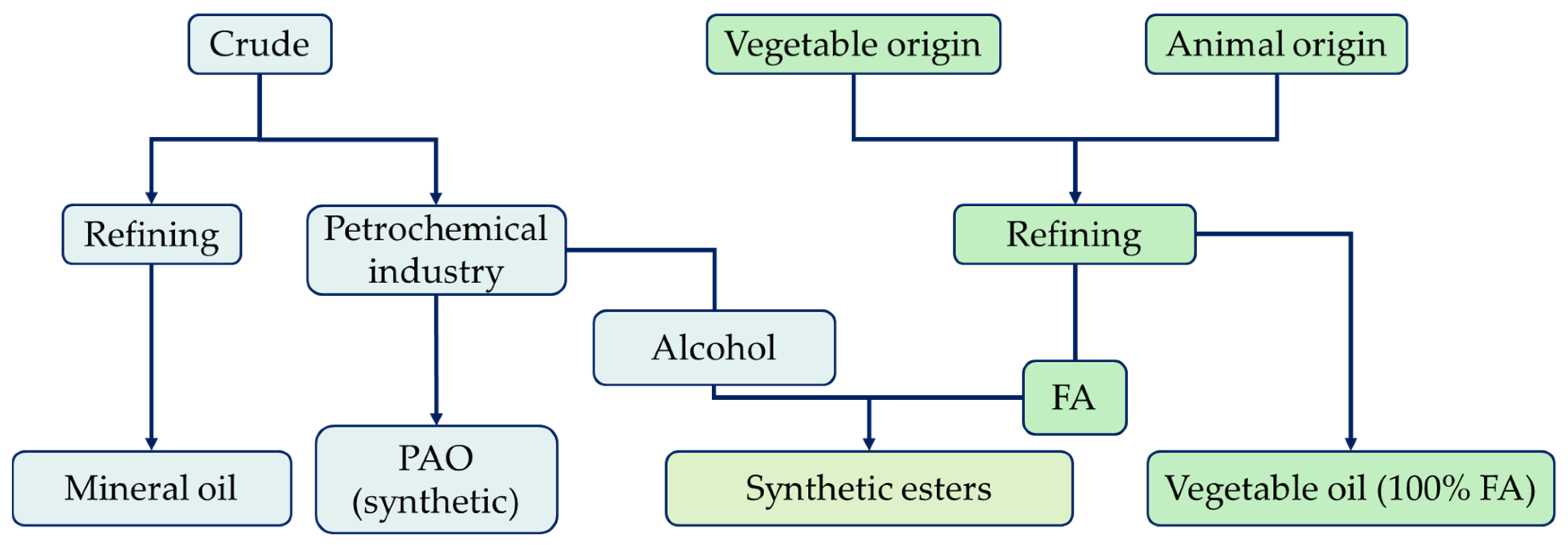
Figure 1. Origin and classification of base fluids.
Environmental concerns about the accelerated development of global warming have led to the promotion of the use of new biodegradable and more environmentally sustainable materials. As part of this movement, new competitive lubricants are being sought from organic materials such as vegetable oils and animal fats to create what are known as biodegradable lubricants or biolubricants. As lubricants, they must fulfill the basic lubricating properties listed above. Thus, biolubricants show important attractive advantages such as high biodegradability, low toxicity, sustainability (eco-friendly), increased worker safety, increased machine life, reduced labor costs, and reduced energy consumption as well as other tribological and physicochemical properties such as increased lubricity, higher viscosity index, higher boiling point, or lower volatility [7][8]. On the contrary, they have certain disadvantages, including poor oxidative, thermal, and hydrolytic stability (leading to shorter shelf life); low corrosion inhibition properties; and poor to bad pour point (PP) [7][9].
According to Verified Market Research [10], the biolubricants market was valued at USD 2.82 billion in 2018 and is expected to reach USD 3.63 billion by 2026, growing at a Compound Annual Growth Rate (CAGR) of 3.2% from 2019 to 2026. Asia and the Americas are also expected to have the highest growth rate in this sector between 2019 and 2024 [11]. These data show the growing industry interest in replacing petroleum-based lubricants with more environmentally friendly ones, and therefore the need to find solutions to concerns about production costs and other more functional aspects, including poor oxidation stability.
2. Biolubricants
According to the UNE-EN 16807 standard [12], the term “bio” is considered synonymous with good for the environment. Its use in lubricants is linked to its environmental properties; therefore, it is expected that all compounds called bio-lubricants will degrade in the environment. According to this standard, bio-lubricants and bio-based lubricants must fulfill a minimum requirement:
-
Biological carbon (C14) content greater than or equal to 25%.
-
Biodegradability of oils greater than or equal to 60% (50% for greases).
-
Ecotoxicity: not classified as “dangerous for the environment”.
Proper Classification for Biolubricants from Feedstock
An important characteristic of biodegradable lubricants is the raw material used to produce them. Therefore, these materials could be classified into first, second, third, and fourth generations based on the feedstock, as shown in Figure 2 [13][14]. The first generation would include all lubricants derived from edible crops like sunflower, rapeseed, soybean, palm, palm kernel, coconut, olive, or castor [15]. These oils are characterized by a low oil production yield and encourage the deforestation and destruction of ecosystems for their cultivation [16]. In addition, the use of arable land competes with food sources, which increases the cost of the final product and is counterproductive in the current context of global food shortages. The second generation comes from non-edible materials such as Jatropha, tobacco, or cotton seeds [17]. These materials are more widely available than the previous ones, but they do still require arable lands for their growth and compete with edible crops for land as in the first case. Third-generation biolubricants derived from microalgae are emerging to solve this problem. Also, biolubricants derived from macroalgae, bacteria, and fungi can also be included in this category [16][18].
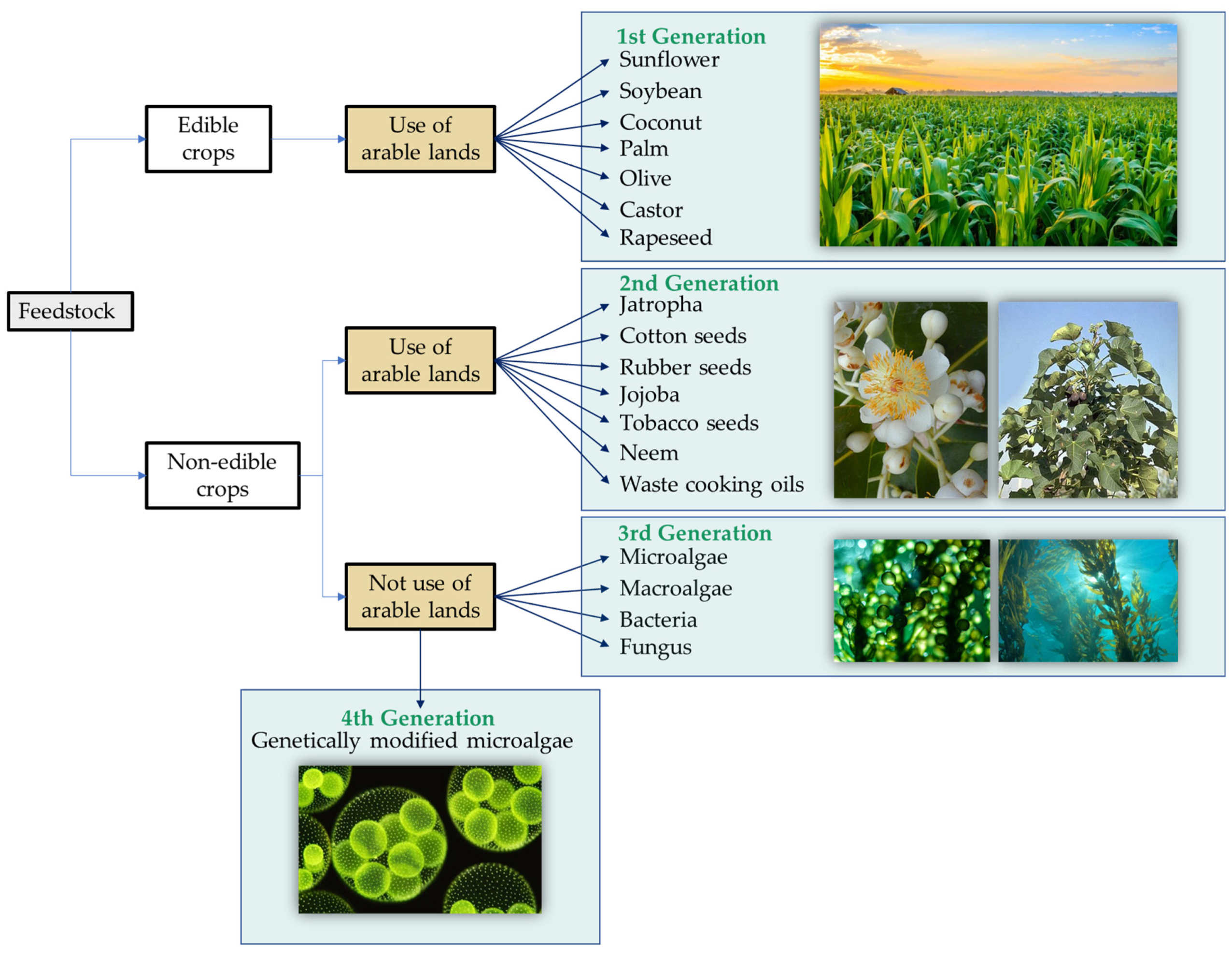
Figure 2. Biolubricant classification according to feedstock.
Microalgae are microorganisms capable of photosynthesis in freshwater, seawater, or wastewater and therefore do not require arable land for their cultivation. Among the requirements for the growth of these microorganisms, the presence of nutrients such as nitrogen, carbon, phosphorus, and potassium makes it possible to use them for wastewater treatment [19][20]. Compared to previous plant sources, they have additional economic and environmental advantages for their application as biolubricants: (a) high growth rate; (b) high biomass production; (c) high lipid content; (d) cultivable all-year-round; (e) higher CO2 reduction during photosynthesis; and (f) effective removal of phosphates and nitrates in wastewater during cultivation [21][22].
Finally, a fourth generation of biomass derived from genetically modified microalgae is being considered. The possibility of manipulating microalgae through mutagenesis or genetic transformation will open the door to the production of suitable bio-oils for biolubricant formulation without the need to improve them using chemical techniques such as epoxidation or the use of additives [18][23][24].
3. Common Techniques for Biolubricant Production
Obtaining biomass from crops is the first step in the biolubricant production process. Depending on the type of crop, this can involve simple collection and purification processes, for example, using agricultural residues or waste cooking oil [25]. Raw materials such as macro- and microalgae require more elaborate harvesting processes, which can be physical, chemical, or biological (Figure 3). Centrifugation is the most widely used technique in the biomass-derived microalgae industry, usually complemented by drying systems such as lyophilization to efficiently remove moisture [13][22]. Figure 3 shows the bio-oil production chain including harvest, drying, pretreatment (if necessary), and extraction, and identifies the most used techniques at each stage.
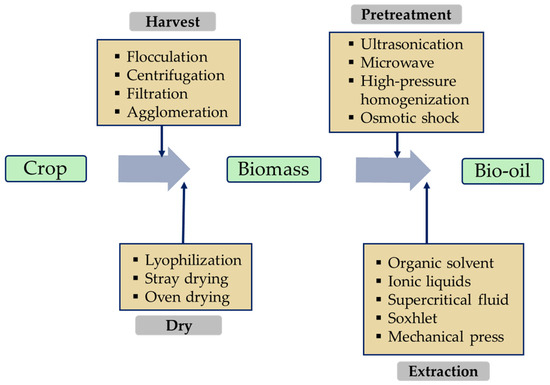
Figure 3. Various techniques to produce bio-oil from plants.
Once the biomass has been obtained, it is subjected to bio-oil production techniques. For materials with simple matrices, such as vegetable oils, more rudimentary methods are used, for example, mechanical or expeller pressing [26][27]. In the case of complex matrices like microalgae, pretreatment for cell disruption is required to facilitate bio-oil extraction. The use of high-pressure homogenization or ultra-high-pressure homogenization as a pretreatment resulted in high lipid extraction yields, reaching an increase of 30% in some cases [28][29][30]. The disadvantage of these techniques is the high operating cost due to the increased working pressures (supercritical conditions). As a solution, other procedures are being studied, including ultrasound, microwave, or osmotic shock [31][32][33].
In terms of extraction techniques, organic solvents are the most implemented, which can be alone or supported by complementary pretreatments [27]. If the biomass is semi-solid or solid, solvent extraction would be carried out using a Soxhlet device [34][35]. Ionic liquids with magnetic nanoparticles were also introduced as a potential alternative in 2021 by Egesa and Plucinski [36], where an extraction efficiency of 99% was achieved with a hexane-ionic liquid mixture.
3.1. Bio-Oils (Triglycerides) as Biolubricants
Vegetable oils are mainly composed of triglycerides: three fatty acids (FA) linked by a glycerol. Compared to the long-chain hydrocarbons of mineral oils, which have between 20 and 50 carbon atoms, FAs have shorter chains of between 4 and 26 carbon atoms [37]. They may also contain one or more double bonds and branches. Figure 4 shows the most common FA found in olive oil (mainly oleic acid).
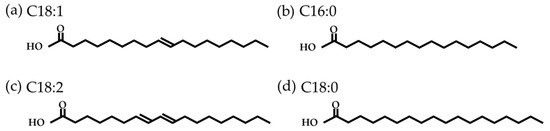
Figure 4. Common fatty acids found in olive oil: (a) oleic acid; (b) palmitic acid; (c) linoleic acid; (d) and stearic acid.
The use of these oils as lubricants has usually been studied as additives to improve a specific property, such as viscosity modifiers. Gallardo-Hernández [38] studied the use of Jatropha oil as an additive in a mineral oil (SAE40W oil) to evaluate its lubricating and thermal properties. The improvement in the lubricity in terms of friction and the deterioration in the anti-wear properties were observed, both related to the tribosystem created by the blend. A strong influence on the thermal properties was also observed at a content of less than 20%. Later, Contreras-Gallego [37] studied the variation in density and kinematic viscosity when the above bio-oil was added at 10% and 20% in four different mineral oils (SAE 5W-30, SAE 15W-40, SAE 25W-50, and SAE 40), as well as thermal conductivity and specific heat. An improvement in thermal properties associated with the increase in Jatropha oil was demonstrated. There was also a reduction in viscosity at higher additive contents, related to the substitution of long-chain hydrocarbons (mineral base oil) by shorter bio-oil ones. Recently, the feasibility of curcumin-extracted soybean waste cooking oil as a 10%, 20%, and 30% additive in N-150 mineral oil was verified by analyzing the tribological and physicochemical properties. In contrast to the previous case, a reduction in wear volume and coefficient of friction (COF) of up to 16% and 32%, respectively, was observed compared to N-150, due to a stronger tribofilm formed by the additive. The function of curcumin as a natural antioxidant to prevent the oxidation of soybean waste cooking oil was also confirmed. Finally, the increase in viscosity index with the molecular weight was confirmed and the decrease in PP from −12 °C (N-150) to −30 °C (10% bio-oil) was attributed to the increase in blend polarity [25].
3.2. Environmentally Friendly Modifications of Vegetable Oils
Despite the advantages offered by biolubricants based on bio-oils, it is inevitable highlight the need for improvement to provide functional storage properties, in addition to modifying psychochemical and tribological properties depending on the final applications. This led to the search for techniques capable of modifying the chemical structure of triglycerides, producing so-called modified esters, as shown in Figure 5.
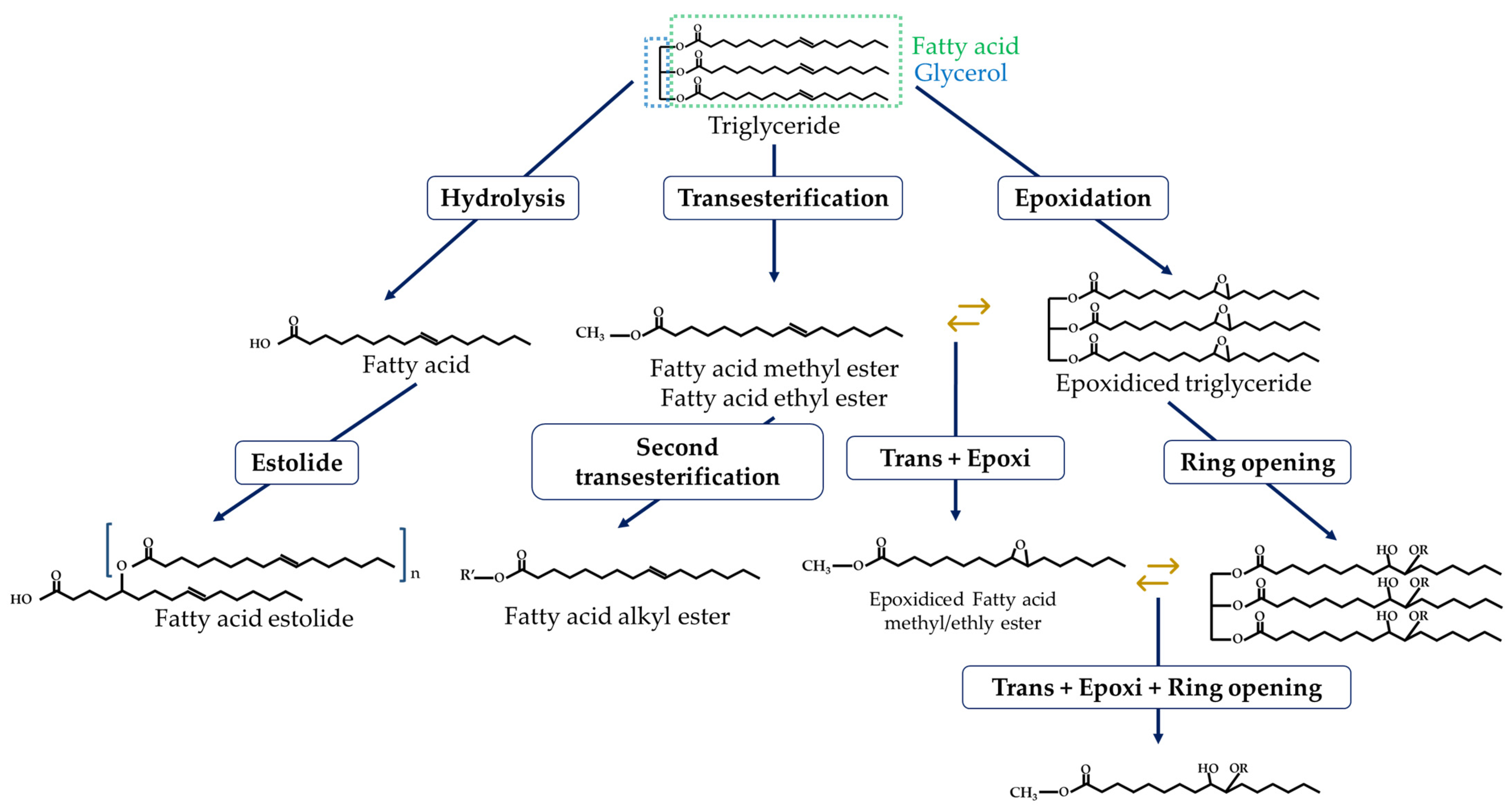
Figure 5. Eco-friendly modifications of oil sources (esters)–palmitoleic acid example.
3.2.1. Hydrolysis
As explained above, bio-oils have poor hydrolytic stability and tend to hydrolyze easily in the presence of water or steam. This leads to the breakdown of their triglycerides and the formation of the corresponding free fatty acids (FFA). This is a spontaneous secondary reaction, promoted by the increase in temperature, which must be prevented [39][40].
3.2.2. Transesterification
Transesterification reactions are the most widely used technique for ester modifications, especially in the biodiesel industry. As shown in Figure 6, it is based on the reaction between a triglyceride and an acyl acceptor (an alcohol) in the presence of a catalyst, and under temperature conditions, to produce fatty acid methyl ester (FAME) as the main product and glycerol as the secondary product. The catalyst function aims to assist the reaction and the deprotonation of the alcohol so that it can join the ester group. These can be acid, basic, or enzymatic catalysts, as well as homogeneous or heterogeneous (depending on the reaction phase) [4][13][41].
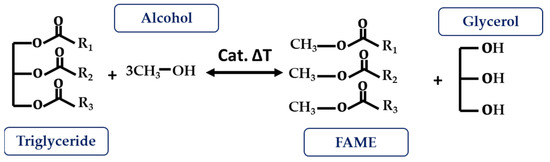
Figure 6. Transesterification reaction.
To avoid secondary reactions such as hydrolysis and thus soap formation at high FFA contents (>2–4 mg KOH/g), it is necessary to neutralize them through an esterification process using an acid catalyst, usually sulfuric acid [42]. It is also necessary to control the humidity of the bio-oil and remove any existing moisture, as this also promotes the saponification reaction.
In the last decade, a new modality called direct or in situ transesterification has attracted attention. It combines lipid extraction (bio-oil extraction) and the transesterification reaction into one process, using biomass as a reagent instead of bio-oil, which means energy and economic savings [43]. The water content is also no longer a critical parameter, as it has greater hydrolytic stability. However, it requires more severe temperature conditions and longer reaction times [13]. A notable application is the processing of microalgal biomass. A wide range of species of these organisms develop strong cell walls, which complicates the extraction process and thus requires an efficient prior cell disruption method. As mentioned above, the operating costs of lyophilization or high-pressure homogenization are high, even more so for complex matrices such as microalgae. The ability to skip the cell disruption and extraction steps is advantageous in terms of both operating cost and energy consumption. Table 1 shows the research that has used this technique for FAME (biodiesel) production in recent years, highlighting the presence of microalgae [44][45][46][47][48][49].
More recently, Encinar et al. [50][51][52][53][54] have worked on the modification of vegetable-oil-derived FA using double transesterification reactions. In the first step (Figure 7), the triglyceride is degraded to the corresponding FAME. The novelty of this process lies in the second step, in which another transesterification reaction is carried out on these FAMEs, this time using a complex alcohol as a reagent, to obtain the so-called fatty acid alkyl [54] esters (FAAEs). According to the researchers, the advantage of this refinery model is the diversity of the main products, starting with the use of bio-oil; the production of biodiesel (FAME) and biolubricants (FAAE); the economic and energy savings with the recovery of materials such as glycerol, as well as the recycling of methanol produced during the second transesterification, which can be reintroduced into the process as a reagent in the first transesterification [55]. So far, only vegetable feedstocks such as safflower, cardoon, rapeseed, soybean, or Jatropha oil have been subjected to this technique.
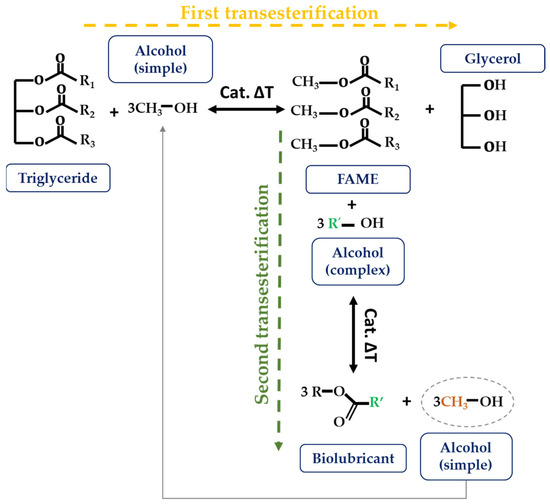
Figure 7. Double transesterification reaction.
3.2.3. Epoxidation/Ring Opening
A significant problem with bio-oils is their poor thermal and oxidative stability due to the effect of double bonds on C-H bond strength. For this reason, the authors recommend raw materials that consist as much as possible of saturated fatty acids (SFAs) because they do not contain double bonds in their structure [20][56]. One way to neutralize these double bonds is through epoxidation reactions. These are based on the reaction of alkenes with peroxyacids to form a cyclic ether (epoxide) compound [57][58]. Epoxidized compounds have been shown to have superior frictional and anti-corrosive properties and better performance at low temperatures, providing a more economical, sustainable, and environmentally friendly alternative to mineral-based lubricants [58][59][60].
3.2.4. Estolide Synthesis
Another eco-friendly modification of FFA is the formation of estolides. These are synthetic compounds derived from the linkage of the respective triglycerides or their FFA, which use their hydroxyl groups to form the estolide bonds [59][61]. The advantages of these structures include better oxidative stability, improved PP, and higher flash point (FP). However, this modification does not resolve the hydrolytic stability problems of the compound [62].
Table 1. Environmentally friendly modifications for the production of and improvement in biolubricants. Microalgae species in bold text.
| Feedstock | Technique | Molecular Structure | Ref. |
|---|---|---|---|
| High-oleic safflower oil | Double transesterification | FAAE | [52] |
| Cardoon oil | Double transesterification | FAAE | [50] |
| Cardoon oil | Double transesterification | FAAE | [53] |
| Rapeseed oil | Double transesterification | FAAE | [51] |
| Rapeseed and castor oils | Double transesterification | FAAE | [54] |
| Indian mustard seed oils | Double transesterification | FAAE | [63] |
| Rapeseed, seed and frying oils | Double transesterification | FAAE | [55] |
| Soybean oil | Double transesterification | FAAE | [64] |
| Jatropha oil | Double transesterification | FAAE | [65] |
| Coconut oil | Hydrogenation | [66] | |
| Waste cooking oil | Epoxidation + Transesterification | Epoxidized FAME | [60] |
| Karanja seed oil | Simple transesterification | FAME | [67] |
| Schlichera oleosa oil | Simple Transesterification | FAME | [68] |
| Refine bleached palm kernel oil | Simple Transesterification | FAME | [69] |
| Soybean oil | Epoxidation | Epoxidized triglycerides | [58] |
| Madhuca indica oil | Epoxidation | Epoxidized triglycerides | [70] |
| Michelia champaca oil | Epoxidation | Epoxidized triglycerides | [71] |
| Moringa olifera Lam oil | Epoxidation | Epoxidized triglycerides | [26] |
| Passiflora edulis oil | Epoxidation | Epoxidized triglycerides | |
| Crude Palm oil | Hydrolyzation + Esterification | Modified esters | [72] |
| Crude Jatropha oil | Esterification + Transesterification | FAME | [73] |
| Esterification + Ultrasound—assisted transesterification | |||
| Waste ayurvedic oil | Ultrasonic irradiation assisted Transesterification | FAME | [74] |
| Pequi oil | Hydrolyzation + Esterification | FA | [75] |
| Dunaliella salina | In situ transesterification | FAME | [44] |
| Chlorella vulgaris foamate | In situ transesterification | FAME | [45] |
| Chlorella pyrenoidosa | In situ transesterification | FAME | [46] |
| Chlorella vulgaris | In situ transesterification | FAME | [47] |
| Rubber seeds | In situ transesterification | FAME | [48] |
| Botryococcus braunii | In situ transesterification | FAME | [49] |
| Coccomyxa subellipsoidea | In situ transesterification |
References
- Climate Change: Global Sea Level | NOAA Climate.Gov. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-global-sea-level (accessed on 13 June 2023).
- Global Carbon Project (GCP). Available online: https://www.globalcarbonproject.org/ (accessed on 19 January 2023).
- Jost, H.P. Tribology—Origin and Future. Wear 1990, 136, 1–17.
- Panchal, T.M.; Patel, A.; Chauhan, D.D.; Thomas, M.; Patel, J.V. A Methodological Review on Bio-Lubricants from Vegetable Oil Based Resources. Renew. Sustain. Energy Rev. 2017, 70, 65–70.
- Biolubricantes Protegiendo El Medio Ambiente. Total Enery. 2012. Available online: https://docplayer.es/28005864-Biolubricantes-protegiendo-el-medio-ambiente.html (accessed on 19 January 2023).
- Torbacke, M.; Rudolphi, Å.K.; Kassfeldt, E. Lubricants: Introduction to Properties and Performance, 1st ed.; John Wiley and Sons: Hoboken, NJ, USA, 2014; ISBN 978-1-118-79974-1.
- Perera, M.; Yan, J.; Xu, L.; Yang, M.; Yan, Y. Bioprocess Development for Biolubricant Production Using Non-Edible Oils, Agro-Industrial Byproducts and Wastes. J. Clean. Prod. 2022, 357, 131956.
- Almasi, S.; Ghobadian, B.; Najafi, G.; Soufi, M.D. A Review on Bio-Lubricant Production from Non-Edible Oil-Bearing Biomass Resources in Iran: Recent Progress and Perspectives. J. Clean. Prod. 2021, 290, 125830.
- Sarma, R.N.; Vinu, R. Current Status and Future Prospects of Biolubricants: Properties and Applications. Lubricants 2022, 10, 70.
- Global Biolubricants Market Size by Base Oil Type, by Application, by End-User Industry, by Geographic Scope and Forecast. Available online: https://www.verifiedmarketresearch.com/product/bio-lubricants-market/ (accessed on 19 January 2023).
- Informe de Mercado de Biolubricantes | Tamaño, Participación, Crecimiento y Tendencias (2022–27). Available online: https://www.mordorintelligence.com/es/industry-reports/bio-lubricants-market (accessed on 15 March 2023).
- UNE-EN 16807:2017; Liquid Petroleum Products—Bio-Lubricants—Criteria and Requirements of Bio-Lubricants and Bio-Based Lubricants. UNE: Madrid, Spain, 2017.
- Eldiehy, K.S.H.; Bardhan, P.; Borah, D.; Gohain, M.; Ahmad Rather, M.; Deka, D.; Mandal, M.A. Comprehensive Review on Microalgal Biomass Production and Processing for Biodiesel Production. Fuel 2022, 324, 124773.
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A Review on the Chemistry, Production, and Technological Potential of Bio-Based Lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102.
- Brahma, S.; Nath, B.; Basumatary, B.; Das, B.; Saikia, P.; Patir, K.; Basumatary, S. Biodiesel Production from Mixed Oils: A Sustainable Approach towards Industrial Biofuel Production. Chem. Eng. J. Adv. 2022, 10, 100284.
- Abdul Hakim Shaah, M.; Hossain, M.S.; Salem Allafi, F.A.; Alsaedi, A.; Ismail, N.; Ab Kadir, M.O.; Ahmad, M.I. A Review on Non-Edible Oil as a Potential Feedstock for Biodiesel: Physicochemical Properties and Production Technologies. RSC Adv. 2021, 11, 25018–25037.
- Ulakpa, W.C.; Ulakpa, R.O.E.; Egwunyenga, M.C.; Egbosiuba, T.C. Transesterification of Non-Edible Oil and Effects of Process Parameters on Biodiesel Yield. Clean. Waste Syst. 2022, 3, 100047.
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.H.; Nguyen, T.H.P. Sustainability of the Four Generations of Biofuels—A Review. Int. J. Energy Res. 2020, 44, 9266–9282.
- Olia, M.S.J.; Azin, M.; Sepahi, A.A.; Moazami, N. Miniaturized Culture Method for the Statistical Study of Growth Rate and Carbohydrate Content of Picochlorum Sp. D8 Isolated from the Persian Gulf. Renew. Energy 2020, 149, 479–488.
- Farfan-Cabrera, L.I.; Franco-Morgado, M.; González-Sánchez, A.; Pérez-González, J.; Marín-Santibáñez, B.M. Microalgae Biomass as a New Potential Source of Sustainable Green Lubricants. Molecules 2022, 27, 1205.
- Sánchez-Bayo, A. Biorrefinería de Microalgas Para La Producción de Biocombustibles. Ph.D. Thesis, Universidad Rey Juan Carlos, Madrid, Spain, 2019.
- Peng, L.; Fu, D.; Chu, H.; Wang, Z.; Qi, H. Biofuel Production from Microalgae: A Review. Environ. Chem. Lett. 2020, 18, 285–297.
- Reeves, C.J.; Menezes, P.L.; Jen, T.C.; Lovell, M.R. The Influence of Fatty Acids on Tribological and Thermal Properties of Natural Oils as Sustainable Biolubricants. Tribol. Int. 2015, 90, 123–134.
- Vuttipongchaikij, S. Genetic Manipulation of Microalgae for Improvement of Biodiesel Production. Thai J. Genet. 2012, 5, 130–148.
- Singh, N.; Agarwal, P.; Porwal, S.K. Natural Antioxidant Extracted Waste Cooking Oil as Sustainable Biolubricant Formulation in Tribological and Rheological Applications. Waste Biomass Valorization 2022, 13, 3127–3137.
- Silva, M.S.; Foletto, E.L.; Alves, S.M.; de Castro Dantas, T.N.; Dantas Neto, A.A. New Hydraulic Biolubricants Based on Passion Fruit and Moringa Oils and Their Epoxy. Ind. Crops Prod. 2015, 69, 362–370.
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of Biofuels from Microalgae—A Review on Cultivation, Harvesting, Lipid Extraction, and Numerous Applications of Microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68.
- Imatoukene, N.; Koubaa, M.; Perdrix, E.; Benali, M.; Vorobiev, E. Combination of Cell Disruption Technologies for Lipid Recovery from Dry and Wet Biomass of Yarrowia lipolytica and Using Green Solvents. Process. Biochem. 2020, 90, 139–147.
- Onumaegbu, C.; Alaswad, A.; Rodriguez, C.; Olabi, A.G. Optimization of Pre-Treatment Process Parameters to Generate Biodiesel from Microalga. Energies 2018, 11, 806.
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Evaluating Microalgal Cell Disruption upon Ultra High Pressure Homogenization. Algal Res. 2019, 42, 101616.
- Onumaegbu, C.; Alaswad, A.; Rodriguez, C.; Olabi, A. Modelling and Optimization of Wet Microalgae Scenedesmus quadricauda Lipid Extraction Using Microwave Pre-Treatment Method and Response Surface Methodology. Renew. Energy 2019, 132, 1323–1331.
- Halim, R.; Papachristou, I.; Chen, G.Q.; Deng, H.; Frey, W.; Posten, C.; Silve, A. The Effect of Cell Disruption on the Extraction of Oil and Protein from Concentrated Microalgae Slurries. Bioresour. Technol. 2022, 346, 126597.
- Singh, S.; Meena, P.; Saharan, V.K.; Bhoi, R.; George, S. Enhanced Lipid Recovery from Chlorella Sp. Biomass by Green Approach: A Combination of Ultrasonication and Homogenization Pre-Treatment Techniques (Hybrid Method) Using Aqueous Deep Eutectic Solvents. Mater. Today Proc. 2022, 57, 179–186.
- Alrashidi, M.; Derawi, D.; Salimon, J.; Firdaus Yusoff, M. An Investigation of Physicochemical Properties of Nigella sativa L. Seed Oil from Al-Qassim by Different Extraction Methods. J.King Saud Univ. Sci. 2020, 32, 3337–3342.
- Hajinajaf, N.; Rabbani, Y.; Mehrabadi, A.; Tavakoli, O. Experimental and Modeling Assessment of Large-Scale Cultivation of Microalgae Nannochloropsis Sp. PTCC 6016 to Reach High Efficiency Lipid Extraction. Int. J. Environ. Sci. Technol. 2022, 19, 5511–5528.
- Egesa, D.; Plucinski, P. Efficient Extraction of Lipids from Magnetically Separated Microalgae Using Ionic Liquids and Their Transesterification to Biodiesel. Biomass Convers. Biorefin. 2022.
- Contreras-Gallegos, E.; Domínguez-Pacheco, F.A.; Hernández-Aguilar, C.; Bedoya, A.; Alvarado, S.; Marín, E.; Cruz-Orea, A. Study of Mineral-Based Oils with Jatropha curcas L. as Bio-Additive Through Thermal and Kinematic Viscosity Properties. Int. J. Thermophys. 2022, 43, 4.
- Gallardo-Hernández, E.A.; Lara-Hernández, G.; Nieto-Camacho, F.; Domínguez-Pacheco, A.; Cruz-Orea, A.; Hernández-Aguilar, C.; Contreras-Gallegos, E.; Torres, M.V.; Flores-Cuautle, J.J.A. Thermal and Tribological Properties of Jatropha Oil as Additive in Commercial Oil. Int. J. Thermophys. 2017, 38, 54.
- Ngaosuwan, K.; Lotero, E.; Suwannakarn, K.; Goodwin, J.G.; Praserthdam, P. Hydrolysis of Triglycerides Using Solid Acid Catalysts. Ind. Eng. Chem. Res. 2009, 48, 4757–4767.
- Ho, C.K.; McAuley, K.B.; Peppley, B.A. Biolubricants through Renewable Hydrocarbons: A Perspective for New Opportunities. Renew. Sustain. Energy Rev. 2019, 113, 109261.
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.M.A.; Soudagar, M.E.M.; Fattah, I.M.R. Current State and Perspectives on Transesterification of Triglycerides for Biodiesel Production. Catalysts 2021, 11, 1121.
- Azad, A.K.; Sharma, S.C.; Rasul, M.G. Clean Energy for Sustainable Development: Comparisons and Contrasts of New Approaches; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128054239.
- Langseter, A.M.; Dzurendova, S.; Shapaval, V.; Kohler, A.; Ekeberg, D.; Zimmermann, B. Evaluation and Optimisation of Direct Transesterification Methods for the Assessment of Lipid Accumulation in Oleaginous Filamentous Fungi. Microb. Cell Fact. 2021, 20, 59.
- Zorn, S.M.F.E.; da Silva, A.P.T.; Bredda, E.H.; Bento, H.B.S.; Pedro, G.A.; Carvalho, A.K.F.; Silva, M.B.; Da Rós, P.C.M. In Situ Transesterification of Microbial Biomass for Biolubricant Production Catalyzed by Heteropolyacid Supported on Niobium. Energies 2022, 15, 1591.
- Al-Humairi, S.T.; Lee, J.G.M.; Harvey, A.P. Direct and Rapid Production of Biodiesel from Algae Foamate Using a Homogeneous Base Catalyst as Part of an Intensified Process. Energy Convers. Manag. X 2022, 16, 100284.
- Sharma, A.K.; Ghodke, P.; Sharma, P.K.; Manna, S.; Pugazhendhi, A.; Matsakas, L.; Patel, A. Holistic Utilization of Chlorella pyrenoidosa Microalgae for Extraction of Renewable Fuels and Value-Added Biochar through in Situ Transesterification and Pyrolysis Reaction Process. Biomass Convers. Biorefin. 2022.
- Al-Humairi, S.T.; Lee, J.G.M.; Salihu, M.; Harvey, A.P. Biodiesel Production through Acid Catalyst In Situ Reactive Extraction of Chlorella Vulgaris Foamate. Energies 2022, 15, 4482.
- Tarigan, J.B.; Anggraini, R.; Sembiring, R.P.; Supeno, M.; Tarigan, K.; Ginting, J.; Karo-Karo, J.A.; Sitepu, E.K. Waste Rubber Seeds as a Renewable Energy Source: Direct Biodiesel Production Using a Controlled Crushing Device. RSC Adv. 2022, 12, 2094–2101.
- Chávez-Sandoval, B.E.; Hernández-Salgado, K.F.; Martínez-García, M.; Ávila-Paredes, H.J.; Díaz-álvarez, F.H.; García-Franco, F. Obtaining Biodiesel by Direct Transesterification of Botryococcus braunii and Coccomyxa subellipsoidea. J. Mex. Chem. Soc. 2021, 65, 318–330.
- Nogales-Delgado, S.; Encinar Martín, J.M. Cardoon Biolubricant through Double Transesterification: Assessment of Its Oxidative, Thermal and Storage Stability. Mater. Lett. 2021, 302, 130454.
- Encinar, J.M.; Nogales-Delgado, S.; Pinilla, A. Biolubricant Production through Double Transesterification: Reactor Design for the Implementation of a Biorefinery Based on Rapeseed. Processes 2021, 9, 1224.
- Encinar, J.M.; Nogales-Delgado, S.; Álvez-Medina, C.M. High Oleic Safflower Biolubricant through Double Transesterification with Methanol and Pentaerythritol: Production, Characterization, and Antioxidant Addition. Arab. J. Chem. 2022, 15, 103796.
- Nogales-Delgado, S.; Sánchez, N.; Encinar, J.M. Valorization of Cynara Cardunculus L. Oil as the Basis of a Biorefinery for Biodiesel and Biolubricant Production. Energies 2020, 13, 5085.
- Encinar, J.M.; Nogales-Delgado, S.; Sánchez, N.; González, J.F. Biolubricants from Rapeseed and Castor Oil Transesterification by Using Titanium Isopropoxide as a Catalyst: Production and Characterization. Catalysts 2020, 10, 366.
- Encinar, J.M.; Nogales, S.; González, J.F. Biodiesel and Biolubricant Production from Different Vegetable Oils through Transesterification. Eng. Rep. 2020, 2, e12190.
- Cecilia, J.A.; Plata, D.B.; Saboya, R.M.A.; de Luna, F.M.T.; Cavalcante, C.L.; Rodríguez-Castellón, E. An Overview of the Biolubricant Production Process: Challenges and Future Perspectives. Processes 2020, 8, 257.
- Do Valle, C.P.; Rodrigues, J.S.; Fechine, L.M.U.D.; Cunha, A.P.; Queiroz Malveira, J.; Luna, F.M.T.; Ricardo, N.M.P.S. Chemical Modification of Tilapia Oil for Biolubricant Applications. J. Clean. Prod. 2018, 191, 158–166.
- Cui, X.; Cao, P.; Guo, J.; Ming, P. Use and Performance of Soybean Oil Based Bio-Lubricant in Reducing Specific Cutting Energy during Biomimetic Machining. J. Manuf. Process. 2021, 62, 577–590.
- Hoong, S.S.; Arniza, M.Z.; Mariam, N.M.D.N.S.; Armylisas, A.H.N.; Yeong, S.K. Synthesis and Physicochemical Properties of Novel Lauric Acid Capped Estolide Esters and Amides Made from Oleic Acid and Their Evaluations for Biolubricant Basestock. Ind. Crops Prod. 2019, 140, 111653.
- Li, W.; Wang, X. Bio-Lubricants Derived from Waste Cooking Oil with Improved Oxidation Stability and Low-Temperature Properties. J. Oleo Sci. 2015, 64, 367–374.
- Cermak, S.C.; Isbell, T.A.; Bredsguard, J.W.; Thompson, T.D. Chapter 14—Estolides: Synthesis and Applications. In Fatty Acids Chemistry, Synthesis, and Applications; Ahmad, M.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 431–475.
- Salimon, J.; Nallathamby, N.; Salih, N.; Abdullah, B.M. Synthesis and Physical Properties of Estolide Ester Using Saturated Fatty Acid and Ricinoleic Acid. J. Autom. Methods Manag. Chem. 2011, 2011, 263624.
- Chen, J.; Bian, X.; Rapp, G.; Lang, J.; Montoya, A.; Trethowan, R.; Bouyssiere, B.; Portha, J.F.; Jaubert, J.N.; Pratt, P.; et al. From Ethyl Biodiesel to Biolubricants: Options for an Indian Mustard Integrated Biorefinery toward a Green and Circular Economy. Ind. Crops Prod. 2019, 137, 597–614.
- Shrivastava, S.; Prajapati, P.; Virendra; Srivastava, P.; Lodhi, A.P.S.; Kumar, D.; Sharma, V.; Srivastava, S.K.; Agarwal, D.D. Chemical Transesterification of Soybean Oil as a Feedstock for Stable Biodiesel and Biolubricant Production by Using Zn Al Hydrotalcites as a Catalyst and Perform Tribological Assessment. Ind. Crops Prod. 2023, 192, 116002.
- Edla, S.; Krishna, A.; Karthik, G.V.S.; Arif, M.M.; Rani, S. Potential Use of Transesterified Vegetable Oil Blends as Base Stocks for Metalworking Fluids and Cutting Forces Prediction Using Machine Learning Tool. Biomass Convers. Biorefin. 2021, 13, 10665–10676.
- Gasni, D.; Mulyadi, I.H.; Affi, J.; Miswar, A.Y. Investigation of Wear Mechanism in Ball Bearings Lubricated by a Bio-Lubricant. Int. J. Technol. 2017, 8, 1248–1257.
- Amriya Tasneem, H.R.; Ravikumar, K.P.; Ramakrishna, H.V. Performance and Wear Debris Characteristics of Karanja Biodiesel and Biolubricant as a Substitute in a Compression Ignition Engine. Fuel 2022, 319, 123870.
- Singh, Y.; Negi, P.; Yadav, A.; Tripathi, R. Development of Bio Based Lubricant from Schlichera Oleosa with Effect of Load during Tribological Analysis. Mater. Today Proc. 2021, 46, 10527–10529.
- Mohd Salleh, Z.A.; Syahrullail, S.; Norzita, N.; Nurun Najwa, R. Friction Study on Chemically Modified RBD PK Oil as a Potential Renewable Resource. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 127.
- Singh, Y.; Rahim, E.A.; Singh, N.K.; Sharma, A.; Singla, A.; Palamanit, A. Friction and Wear Characteristics of Chemically Modified Mahua (Madhuca indica) Oil Based Lubricant with SiO2 Nanoparticles as Additives. Wear 2022, 508–509, 204463.
- Singh, Y.; Abd Rahim, E. Michelia Champaca: Sustainable Novel Non-Edible Oil as Nano Based Bio-Lubricant with Tribological Investigation. Fuel 2020, 282, 118830.
- Nor, N.M.; Salih, N.; Salimon, J. Optimization and Lubrication Properties of Malaysian Crude Palm Oil Fatty Acids Based Neopentyl Glycol Diester Green Biolubricant. Renew. Energy 2022, 200, 942–956.
- Arce Saavedra, T.; Bueno-Borges, L.B.; Sangaletti-Gerhard, N.; de Alencar, S.M.; Regitano-d’Arce, M.A.B. Optimized Conventional and Ultrasound-Assisted Ethyl Transesterification of Jatropha (Jatropha curcas) and Palm (Elaeis guineensis) Oil Mixtures. Chem. Eng. Commun. 2022, 209, 1482–1495.
- Balakumar, R.; Sriram, G.; Arumugam, S. Effect of Engine Oil Dilution by Waste Ayurvedic Oil Biodiesel on Tribological Behavior of Liner-Ring Tribo Pair Material. Mater. Sci. Eng. 2020, 954, 012042.
- Ribeiro Filho, P.R.C.F.; da Silva, S.S.O.; do Nascimento, M.R.; de Aguiar Soares, S.; de Luna, F.M.T.; Cavalcante, C.L. Tribological Properties of Bio-Based Lubricant Basestock Obtained from Pequi Oil (Caryocar brasiliensis). J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 51.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Revisions:
2 times
(View History)
Update Date:
18 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





