Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by claudia Sanjurjo Muñiz and Version 2 by Camila Xu.
Lubricants can be classified according to their physical state as solid, liquid, or semi-fluid (greases). The former is used when it is difficult to maintain contact with the fluid, while the latter is used in situations where liquid lubricants are not applicable. According to the UNE-EN 16807 standard, the term “bio” is considered synonymous with good for the environment. Its use in lubricants is linked to its environmental properties; therefore, it is expected that all compounds called bio-lubricants will degrade in the environment.
- biolubricant
- molecular structure
- tribological properties
1. Introduction
Water and food shortages are just some of the direct consequences of global warming caused by the increase in CO2 emissions in recent decades. According to Lindsey’s report [1], global sea levels have risen 24 cm since 1880, triggered by the start of the second industrial revolution. The highest peak emissions in history were recorded last year with 40.5 tons, behind pre-COVID-19 levels of 40.9 tons, with the use of fossil sources being the main emitter of greenhouse gases, leading with 90.47%, of which 25% came from energy losses [2].
Maintaining machinery properly, conserving energy, and finding potential substitutes for petroleum derivatives are necessary to combat accelerating climate change. One of the sciences that can contribute to this purpose is tribology, which is responsible for optimizing lubrication in the interaction between moving surfaces, and which has led to large amounts of energy and money being saved in industry. Jost [3] estimated that GBP 515 million could be saved by improving tribological conditions in the UK industry. In addition, recent research into natural lubricant sources is helping to move society toward more sustainable industries.
To achieve these goals, it is necessary to review the minimum requirements for lubricants, the advantages and disadvantages of natural sources, and the existing techniques for improving these materials to produce potential alternatives to petroleum-based lubricants. The basic functions of lubricants can be summarized as follows: (a) to reduce energy losses; (b) to protect surfaces from wear due to friction; (c) to protect against corrosion; (d) to reduce oxidation effects; (e) to cool down surfaces; (f) to decrease heat losses due to contact between moving surfaces; or (g) to increase tightness and prevent the leakage of contaminants and sediments [4][5][4,5], in addition to certain requirements depending on the application.
Lubricants can be classified according to their physical state as solid, liquid, or semi-fluid (greases). The former is used when it is difficult to maintain contact with the fluid, while the latter is used in situations where liquid lubricants are not applicable. They can be further classified according to their source material as mineral-, synthetic-, animal-, or vegetable-based lubricants (Figure 1). All base fluids directly refined from crude oil are called mineral bases, those refined from natural sources are called vegetable bases, and those synthesized (natural or mineral) are called synthetic bases [6].
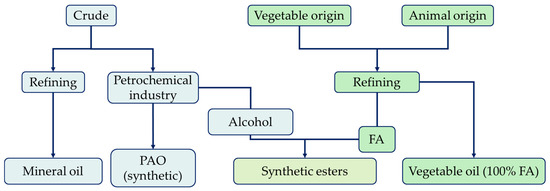
Figure 1.
Origin and classification of base fluids.
Environmental concerns about the accelerated development of global warming have led to the promotion of the use of new biodegradable and more environmentally sustainable materials. As part of this movement, new competitive lubricants are being sought from organic materials such as vegetable oils and animal fats to create what are known as biodegradable lubricants or biolubricants. As lubricants, they must fulfill the basic lubricating properties listed above. Thus, biolubricants show important attractive advantages such as high biodegradability, low toxicity, sustainability (eco-friendly), increased worker safety, increased machine life, reduced labor costs, and reduced energy consumption as well as other tribological and physicochemical properties such as increased lubricity, higher viscosity index, higher boiling point, or lower volatility [7][8][7,8]. On the contrary, they have certain disadvantages, including poor oxidative, thermal, and hydrolytic stability (leading to shorter shelf life); low corrosion inhibition properties; and poor to bad pour point (PP) [7][9][7,9].
According to Verified Market Research [10], the biolubricants market was valued at USD 2.82 billion in 2018 and is expected to reach USD 3.63 billion by 2026, growing at a Compound Annual Growth Rate (CAGR) of 3.2% from 2019 to 2026. Asia and the Americas are also expected to have the highest growth rate in this sector between 2019 and 2024 [11]. These data show the growing industry interest in replacing petroleum-based lubricants with more environmentally friendly ones, and therefore the need to find solutions to concerns about production costs and other more functional aspects, including poor oxidation stability.
2. Biolubricants
According to the UNE-EN 16807 standard [12], the term “bio” is considered synonymous with good for the environment. Its use in lubricants is linked to its environmental properties; therefore, it is expected that all compounds called bio-lubricants will degrade in the environment. According to this standard, bio-lubricants and bio-based lubricants must fulfill a minimum requirement:-
Biological carbon (C14) content greater than or equal to 25%.
-
Biodegradability of oils greater than or equal to 60% (50% for greases).
-
Ecotoxicity: not classified as “dangerous for the environment”.
Proper Classification for Biolubricants from Feedstock
An important characteristic of biodegradable lubricants is the raw material used to produce them. Therefore, these materials could be classified into first, second, third, and fourth generations based on the feedstock, as shown in Figure 2 [13][14][13,14]. The first generation would include all lubricants derived from edible crops like sunflower, rapeseed, soybean, palm, palm kernel, coconut, olive, or castor [15]. These oils are characterized by a low oil production yield and encourage the deforestation and destruction of ecosystems for their cultivation [16]. In addition, the use of arable land competes with food sources, which increases the cost of the final product and is counterproductive in the current context of global food shortages. The second generation comes from non-edible materials such as Jatropha, tobacco, or cotton seeds [17]. These materials are more widely available than the previous ones, but they do still require arable lands for their growth and compete with edible crops for land as in the first case. Third-generation biolubricants derived from microalgae are emerging to solve this problem. Also, biolubricants derived from macroalgae, bacteria, and fungi can also be included in this category [16][18][16,18].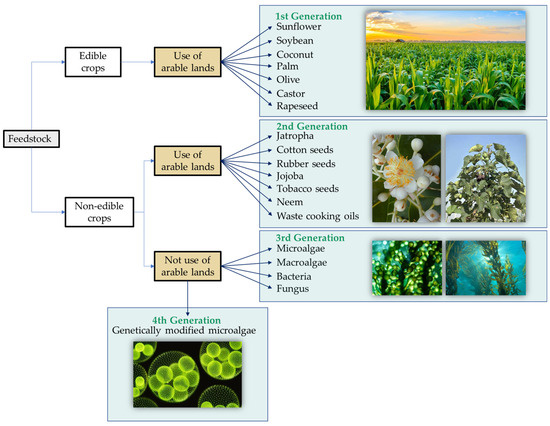
Figure 2.
Biolubricant classification according to feedstock.
3. Common Techniques for Biolubricant Production
Obtaining biomass from crops is the first step in the biolubricant production process. Depending on the type of crop, this can involve simple collection and purification processes, for example, using agricultural residues or waste cooking oil [25]. Raw materials such as macro- and microalgae require more elaborate harvesting processes, which can be physical, chemical, or biological (Figure 3). Centrifugation is the most widely used technique in the biomass-derived microalgae industry, usually complemented by drying systems such as lyophilization to efficiently remove moisture [13][22][13,22]. Figure 3 shows the bio-oil production chain including harvest, drying, pretreatment (if necessary), and extraction, and identifies the most used techniques at each stage.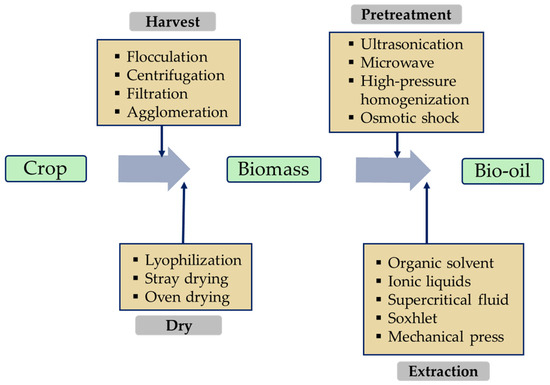
Figure 3.
Various techniques to produce bio-oil from plants.
3.1. Bio-Oils (Triglycerides) as Biolubricants
Vegetable oils are mainly composed of triglycerides: three fatty acids (FA) linked by a glycerol. Compared to the long-chain hydrocarbons of mineral oils, which have between 20 and 50 carbon atoms, FAs have shorter chains of between 4 and 26 carbon atoms [37]. They may also contain one or more double bonds and branches. Figure 4 shows the most common FA found in olive oil (mainly oleic acid).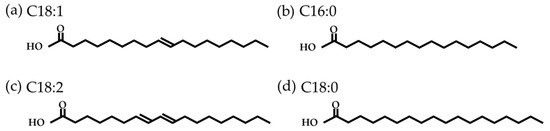
Figure 4.
Common fatty acids found in olive oil: (
a
) oleic acid; (
b
) palmitic acid; (
c
) linoleic acid; (
d
) and stearic acid.
3.2. Environmentally Friendly Modifications of Vegetable Oils
Despite the advantages offered by biolubricants based on bio-oils, it is inevitable highlight the need for improvement to provide functional storage properties, in addition to modifying psychochemical and tribological properties depending on the final applications. This led to the search for techniques capable of modifying the chemical structure of triglycerides, producing so-called modified esters, as shown in Figure 5.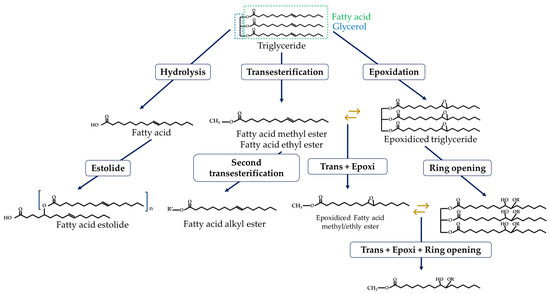
Figure 5.
Eco-friendly modifications of oil sources (esters)–palmitoleic acid example.
3.2.1. Hydrolysis
As explained above, bio-oils have poor hydrolytic stability and tend to hydrolyze easily in the presence of water or steam. This leads to the breakdown of their triglycerides and the formation of the corresponding free fatty acids (FFA). This is a spontaneous secondary reaction, promoted by the increase in temperature, which must be prevented [39][40][39,40].3.2.2. Transesterification
Transesterification reactions are the most widely used technique for ester modifications, especially in the biodiesel industry. As shown in Figure 6, it is based on the reaction between a triglyceride and an acyl acceptor (an alcohol) in the presence of a catalyst, and under temperature conditions, to produce fatty acid methyl ester (FAME) as the main product and glycerol as the secondary product. The catalyst function aims to assist the reaction and the deprotonation of the alcohol so that it can join the ester group. These can be acid, basic, or enzymatic catalysts, as well as homogeneous or heterogeneous (depending on the reaction phase) [4][13][41][4,13,41].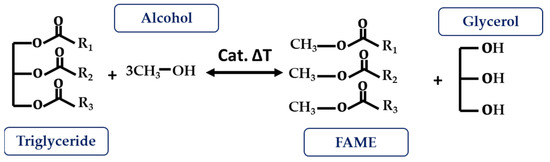
Figure 6.
Transesterification reaction.
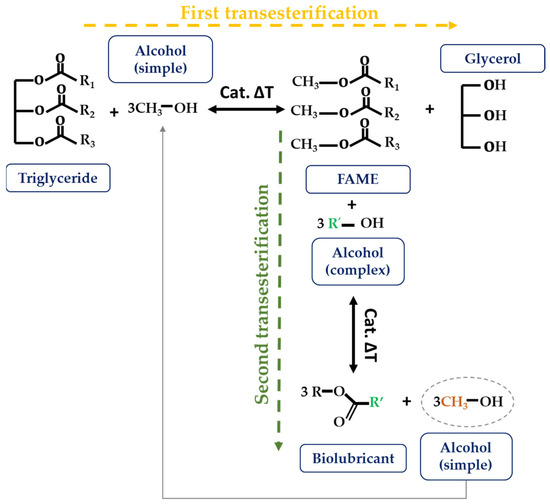
Figure 7.
Double transesterification reaction.
3.2.3. Epoxidation/Ring Opening
A significant problem with bio-oils is their poor thermal and oxidative stability due to the effect of double bonds on C-H bond strength. For this reason, the authors recommend raw materials that consist as much as possible of saturated fatty acids (SFAs) because they do not contain double bonds in their structure [20][56][20,56]. One way to neutralize these double bonds is through epoxidation reactions. These are based on the reaction of alkenes with peroxyacids to form a cyclic ether (epoxide) compound [57][58][57,58]. Epoxidized compounds have been shown to have superior frictional and anti-corrosive properties and better performance at low temperatures, providing a more economical, sustainable, and environmentally friendly alternative to mineral-based lubricants [58][59][60][58,59,60].3.2.4. Estolide Synthesis
Another eco-friendly modification of FFA is the formation of estolides. These are synthetic compounds derived from the linkage of the respective triglycerides or their FFA, which use their hydroxyl groups to form the estolide bonds [59][61][59,61]. The advantages of these structures include better oxidative stability, improved PP, and higher flash point (FP). However, this modification does not resolve the hydrolytic stability problems of the compound [62].Table 1.
Environmentally friendly modifications for the production of and improvement in biolubricants. Microalgae species in bold text.
| Feedstock | Technique | Molecular Structure | Ref. |
|---|---|---|---|
| High-oleic safflower oil | Double transesterification | FAAE | [52] |
| Cardoon oil | Double transesterification | FAAE | [50] |
| Cardoon oil | Double transesterification | FAAE | [53] |
| Rapeseed oil | Double transesterification | FAAE | [51] |
| Rapeseed and castor oils | Double transesterification | FAAE | [54] |
| Indian mustard seed oils | Double transesterification | FAAE | [63] |
| Rapeseed, seed and frying oils | Double transesterification | FAAE | [55] |
| Soybean oil | Double transesterification | FAAE | [64] |
| Jatropha oil | Double transesterification | FAAE | [65] |
| Coconut oil | Hydrogenation | [66] | |
| Waste cooking oil | Epoxidation + Transesterification | Epoxidized FAME | [60] |
| Karanja seed oil | Simple transesterification | FAME | [67] |
| Schlichera oleosa oil | Simple Transesterification | FAME | [68] |
| Refine bleached palm kernel oil | Simple Transesterification | FAME | [69] |
| Soybean oil | Epoxidation | Epoxidized triglycerides | [58] |
| Madhuca indica oil | Epoxidation | Epoxidized triglycerides | [70] |
| Michelia champaca oil | Epoxidation | Epoxidized triglycerides | [71] |
| Moringa olifera Lam oil | Epoxidation | Epoxidized triglycerides | [26] |
| Passiflora edulis oil | Epoxidation | Epoxidized triglycerides | |
| Crude Palm oil | Hydrolyzation + Esterification | Modified esters | [72] |
| Crude Jatropha oil | Esterification + Transesterification | FAME | [73] |
| Esterification + Ultrasound—assisted transesterification | |||
| Waste ayurvedic oil | Ultrasonic irradiation assisted Transesterification | FAME | [74] |
| Pequi oil | Hydrolyzation + Esterification | FA | [75] |
| Dunaliella salina | In situ transesterification | FAME | [44] |
| Chlorella vulgaris foamate | In situ transesterification | FAME | [45] |
| Chlorella pyrenoidosa | In situ transesterification | FAME | [46] |
| Chlorella vulgaris | In situ transesterification | FAME | [47] |
| Rubber seeds | In situ transesterification | FAME | [48] |
| Botryococcus braunii | In situ transesterification | FAME | [49] |
| Coccomyxa subellipsoidea | In situ transesterification |
