Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Seong An | -- | 2902 | 2023-09-15 02:47:27 | | | |
| 2 | Peter Tang | Meta information modification | 2902 | 2023-09-15 03:02:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Giau, V.V.; Wu, S.Y.; Jamerlan, A.; An, S.S.A.; Kim, S.; Hulme, J. Neuroinflammatory Implications of Gut Microbiota in Alzheimer’s Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/49206 (accessed on 06 February 2026).
Giau VV, Wu SY, Jamerlan A, An SSA, Kim S, Hulme J. Neuroinflammatory Implications of Gut Microbiota in Alzheimer’s Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/49206. Accessed February 06, 2026.
Giau, Vo Van, Si Ying Wu, Angelo Jamerlan, Seong Soo A. An, Sangyun Kim, John Hulme. "Neuroinflammatory Implications of Gut Microbiota in Alzheimer’s Disease" Encyclopedia, https://encyclopedia.pub/entry/49206 (accessed February 06, 2026).
Giau, V.V., Wu, S.Y., Jamerlan, A., An, S.S.A., Kim, S., & Hulme, J. (2023, September 15). Neuroinflammatory Implications of Gut Microbiota in Alzheimer’s Disease. In Encyclopedia. https://encyclopedia.pub/entry/49206
Giau, Vo Van, et al. "Neuroinflammatory Implications of Gut Microbiota in Alzheimer’s Disease." Encyclopedia. Web. 15 September, 2023.
Copy Citation
The bidirectional communication between the central nervous system (CNS) and the gut microbiota plays a pivotal role in human health. Increasing numbers of studies suggest that the gut microbiota can influence the brain and behavior of patients. Various metabolites secreted by the gut microbiota can affect the cognitive ability of patients diagnosed with neurodegenerative diseases. Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by a progressive loss of memory, language, and cognitive ability.
gut microbiota
MGB axis
germ-free animal
probiotic
neurodegenerative diseases
Alzheimer’s disease
1. Introduction
Comprising trillions of symbiotic microorganisms, the gut microbiota is an essential element for the maintenance of the host’s health [1][2][3]. This microbial ecosystem consists mainly of bacteria, of which most are strict anaerobes, and also fungi and viruses. The four main phyla in adults consist of Bacteroidetes (~48%) and Firmicutes (~51%), which make up the highest proportion, as well as Proteobacteria and Actinobacteria, which are found in relatively low amounts (1%) [4]. Alterations in the composition of the gut microbiota, caused by dietary changes, antibiotic exposure, and infection, lead to the loss of homeostasis, which is implicated in the development of several diseases in humans, such as colorectal cancer, metabolic syndrome, obesity, allergies, inflammatory bowel disease (IBD), type 2 diabetes, heart failure, and neurodegenerative disorders [5][6][7][8]. Recent evidence points to a causative link between pathogens and changes in the intestinal microbiota composition, along with inflammatory changes in various tissues and organs including brain tissue [7]. Hence, gut microbes may alter levels of neurotransmitter-related metabolites, affecting gut-to-brain communication and/or altering brain function (Figure 1).
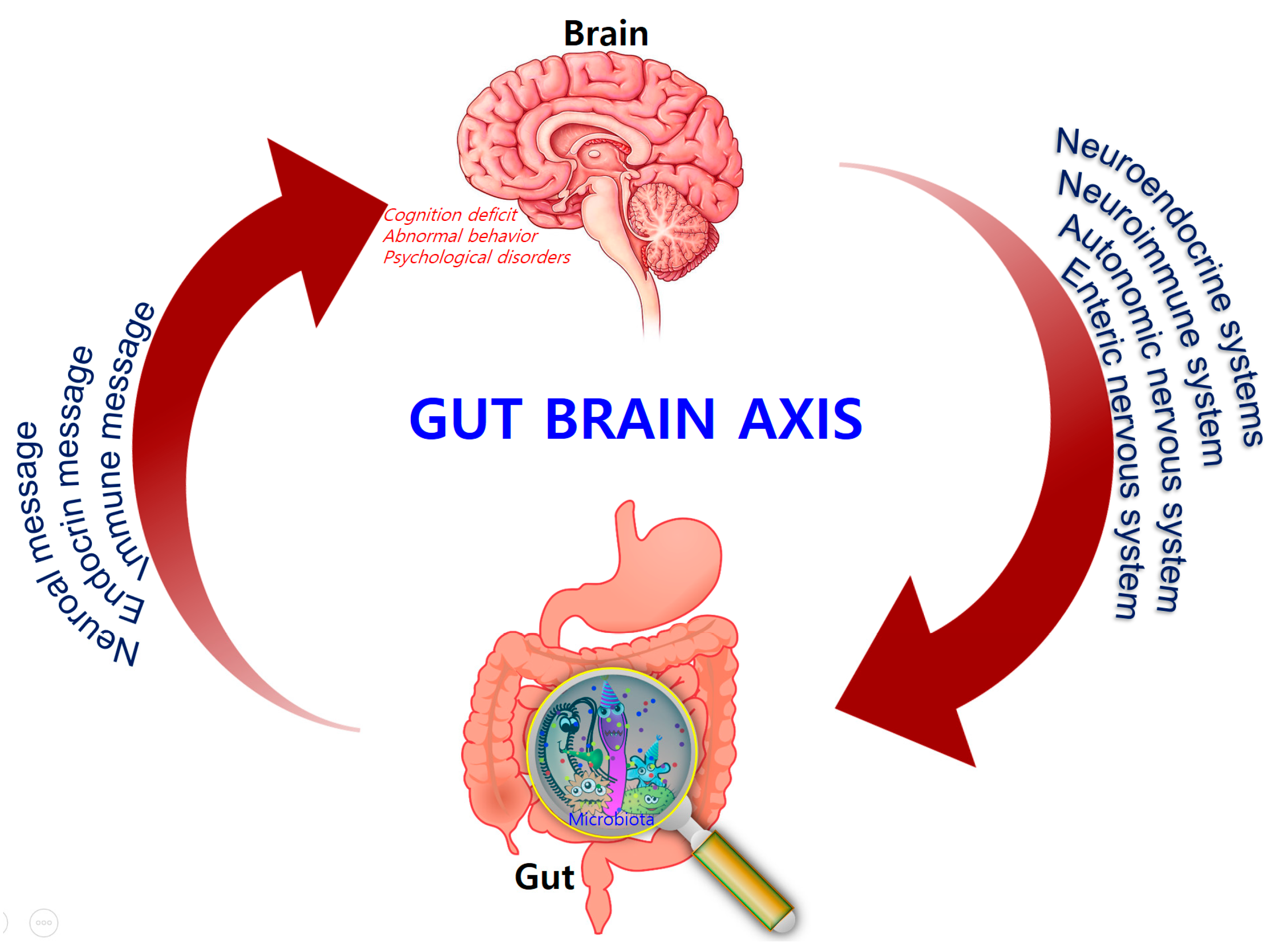
Figure 1. Bidirectional signaling between the gastrointestinal tract and the brain is regulated at the neural, hormonal, and immunological levels.
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by a progressive loss of memory, language, and cognitive ability. According to the classical “amyloid cascade” model, the disease results from the over production of amyloid-beta peptide (Aβ), following the disruption of homeostatic mechanisms which regulate the proteolytic cleavage of the amyloid precursor protein (APP). Amyloids associated with AD consist largely of perivascular amyloid enriched in the 42-amino-acid Aβ42 peptide. Aβ42 is thought to initiate the neuroinflammatory process characteristic of AD pathology [8]. Plaque formation is also a natural response to infection by trapping invading microorganisms, further contributing to the collateral damage of healthy tissue that results from neuroinflammation. However, recent work suggests that the cascade model does not fully explain AD pathogenesis [9] and that alterations in the gut microbiome may play a significant role in the progression of the disease [10].
Over the last decade, several publications demonstrated the regulatory influence of the gut microbiota on the innate and adaptive immune response, as well as the importance of the interactions between the endogenous microbiota and the host’s central nervous system (CNS) [11][12]. Given the unsuccessful AD treatments employed so far, gut modification or recondition strategies started attracting the attention of the scientific community regarding the pathogenesis of CNS diseases, with emphasis on Alzheimer’s and Parkinson’s diseases [13].
2. The Intestinal Microbiota and Homeostasis
Early colonization of certain enterotypes can have a long-lasting influence on the health status of the host [14][15][16]. Human microbial colonization begins at birth. Infants born vaginally are initially colonized with microbial colonies that have a maternal signature (enriched in Lactobacillus and Prevotella spp.), while those delivered by caesarean section harbor colonies that more closely resemble the skin microbiota (enriched in Staphylococcus and Propionibacterium spp.). The microbiota then diversifies over the first few weeks of life to form a complex, anaerobe-dominated microbial community [17]. At the same time, the hypothalamic–pituitary–adrenal (HPA) axis becomes activated, which has an impact on the enteric nervous system (ENS) that innervates the gastrointestinal tract (GIT). Finally, the human gut microbiota rapidly expands and reaches an adult-like stage by three years of age. Shifts from Bifidobacterium to Clostridia and Bacteriodetes occur as the host develops from an infant into an adult [18]. Reductions in the level of Faecalibacterium prauznitzii and its anti-inflammatory relatives occur as young adults mature [6]. The composition of the microbiota is altered throughout the lifespan and is dependent on dietary and environmental factors, disease state, and other factors. In addition, several strategies suggest that Porphyromonas gingivalis causes an inflammatory response in the liver through the increased expression of pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), as well as fat storage-inducing transmembrane protein 2 (Fitm2) and perilipin 2 (Plin2), associated with lipid droplet formation, which subsequently increases neuroinflammation and causes neurodegenerative changes and AD [12][19][20].
The microbiota has long been known to play a relevant role in the health of the host. It can help break down certain nutrients, which can then be metabolized by host cells, and some of these products are involved in neural function. As such, gut bacteria produce amino acids (i.e., gamma-amino butyric acid (GABA) and tryptophan) [21][22], and monoamines (i.e., serotonin, histamine, and dopamine), which play a significant role in the brain as neurotransmitters, or as neurotransmitter precursors [23][24]. These neuroactive products can target the CNS via the blood stream and can also influence neurons in the ENS.
In the homeostatic state, a healthy GIT has a normal and stable commensal intestinal microbiota. This provides the host with nutrition and energy through the production of vitamins [25][26][27], aids in the maintenance of intestinal epithelial barrier integrity, aids in resistance to pathogens, and plays a role in the metabolic and immune systems [28][29][30].
3. The Microbiota–Gut–Brain (MGB) Axis
In the 1880s, William James and Carl Lange first introduced the concept that bidirectional communication between the CNS and intestinal organs plays a role in emotional regulation [31][32]. Forty years later, the idea that the brain plays an important role in regulating GI function was developed by Walter Cannon [33]. A number of rodent studies also showed that the gut–brain axis is a focus of research in different fields, ranging from basic microbiology to translational applications. Consequently, the potential involvement of the gut microbiota in brain function emerged. This involvement pertains to the core microbiome, distinct enterotypes, and age-related shifts in composition, which are harmful to health [34]. The concept of the MGB axis is well established. The neuroendocrine and neuroimmune systems, in addition to the sympathetic and parasympathetic arms of the autonomic nervous system (ANS) and the ENS, are key pathways in gut–brain communication. Although the exact mechanisms mediating gut–brain interactions are not fully understood, they were suggested to involve endocrine, immune, and neural pathways (vagus nerve and enteric nervous system), leading to possible alteration in AD patients or aggravating inflammation.
The concept has now expanded and has become a quickly evolving area of research that led to convergence of research efforts in the fields of neuroscience, psychiatry, gastroenterology, and microbiology—disciplines that were previously considered to have distinct and separate research objectives and focuses.
The MGB axis is vital for maintaining gut homeostasis. Dysregulation of the MGB axis was implicated in various disease states, the most common being chronic functional GI disorders such as IBS, which can induce depression and can also result in decreased cognitive function [35][36]. The work done by Bravo and Tillisch et al. implicated microbiota–brain signaling in alterations of resting brain activity in key circuits involved in pain, emotion, and cognition [37]. Researchers in this area are increasingly giving recognition to the microbiota itself as being an active and highly influential contributing factor in this bidirectional communication network.
4. Disrupting Microbiota Effects on Brain and Behavior
The gut microbiota has become a focus of studies on the brain and behavior. Alterations in the gut microbiota can modulate the peripheral and central nervous systems, resulting in altered brain function, which gives further evidence for the existence of the MGB axis. Early studies in humans demonstrate that altering the microbiota with beneficial bacteria or probiotics can lead to changes in brain function, as well as subjective reports of mood. Experimental approaches in MGB-axis research include the use of germ-free animals, animals with pathogenic bacterial infections, and animals exposed to probiotic agents or antibiotics [38].
Germ-free animal studies are conducted in animals born and reared in sterile conditions, eliminating the opportunity for postnatal colonization of the GIT. Thus, research conducted in germ-free animals highlighted the important role the gut microbiota plays in the development of both physiologic and metabolic abnormalities [38]. When compared with conventional animals, germ-free animals exhibit abnormal gastrointestinal motility, increased expression of genes encoding transporters throughout the gut, and an altered response to inflammatory pain [39][40][41]. In addition, germ-free animals have an immature and dysregulated immune system, with abnormal immunoglobulin A (IgA) production [41][42][43][44][45] and decreased numbers of intestinal mast cells [46].
The absence of gut bacteria during development affects the HPA axis [47], which has a significant role in the stress response. Studies in germ-free animals clearly demonstrate a relationship between the gut microbiota and stress- and anxiety-related behaviors. Further studies should concentrate on the influence of time, sex, strain, and other species factors on this relationship.
Antibiotics are normally used to remove or prevent bacterial colonization in the human body, without targeting specific types of bacteria. As a result, broad-spectrum antibiotics can greatly affect the composition of the gut microbiota, reduce the bio-diversity of the fecal microbiota, and delay colonization for a long period after administration. A number of studies showed that different antibiotic treatments result in short- and/or long-term changes in the intestinal microbiota in both humans and animals.
5. Microbiota and Neurodegenerative Diseases
Amounting evidence suggests that gut microbiota plays an important role in the development of brain, and that there is a bidirectional relationship between the brain, gut, and the bacteria within the gut which is referred as the brain–gut–microbiome axis [48]. The microbiota can affect regulation of the MGB axis via immunological, neuroendocrine, and direct neural mechanisms. The gut microbiota is known to increase local and systemic inflammation due to lipopolysaccharide (LPS) from pathogenic bacteria and the synthesis of pro-inflammatory cytokines [49][50]. These microorganisms are able to produce neurotransmitters and neuromodulators, such as short-chain fatty acids (SCFAs), biogenic amines (e.g., histamine), and other amino-acid-derived metabolites such as serotonin [51] or GABA [52][53]. In addition, bacterial enzymes may also synthesize neurotoxic metabolites such as d-lactic acid and ammonia [54]. Signaling molecules secreted by the gut microbiota are transferred via the lymphatic and systemic circulation throughout the CNS where they then affect behavior and modulate brain plasticity and cognitive function [55]. This implicates the importance of the gut microbiota in the development and function of the CNS, and in the pathophysiology of chronic brain diseases [54]. Microbiome species and their secretory products are extremely powerful pro-inflammatory and innate-immune activators in the host [23][56][57][58][59][60][61][62][63][64].
The connection between the kind of gut microbiota and AD pathology was shown in a study that used transgenic mouse models. Harach et al. observed a significant shift in the diversity of gut microbiota of APP transgenic mice with that of non-transgenic wild-type mice through sequencing of 16S ribosomal RNA (rRNA) from their fecal samples [65]. Germ-free APP transgenic mice were generated, in which a significant decrease in cerebral Aβ pathology was observed when compared to control mice with intestinal microbiota. Interestingly, an increase in cerebral Aβ pathology was observed in germ-free APP transgenic mice when these were colonized with gut microbiota acquired from conventionally raised APP transgenic mice [66].
6. The Role of Inflammation in Alzheimer’s Disease
Inflammatory reactions could be both beneficial and detrimental to the brain, depending on strengths of their activation in various stages of neurodegeneration [67]. Regulation of immuno-inflammatory control is one of the relevant processes involved in the pathogenesis of neurodegenerative disorders. AD shares several common properties with other neurodegenerative disorders, such as accumulation of misfolded proteins (Aβ) and hyperphosphorylated tau, evidence for a prion-like spread of pathology with misfolded proteins and neuroinflammation [9]. The pro-inflammatory gut microbiota dysbiosis in AD patients could trigger inflammation-induced formation and aggregation of cerebral amyloid-β, proving to be an effective strategy for preventing or reducing the risk of AD [68]. Bacterial strains known to produce functional extracellular amyloid fibers are Escherichia coli, Salmonella enterica, Salmonella typhimurium, Bacillus subtilis, Mycobacterium tuberculosis, and Staphylococcus aureus [68]. For instance, E. coli endotoxin was shown to induce the formation of Aβ fibrils in vitro, implying their involvement in AD pathogenesis [68].
Activated astrocytes are supportive cells that provide trophic and metabolic maintenance for neurons and are also influential in neuroinflammation in AD. Changes in astrocyte morphology, gene expression, protein composition, and activity can be observed in AD, which in turn can compromise astrocyte function [69]. Several transgenic mouse models showed that activated astrocytes accumulate in the brain before any plaque or tangle pathology can be observed [70], suggesting that astrocyte activation may be involved in AD pathogenesis. Astrocytes outnumber microglia in the brain and have a greater influence on long-term neuroinflammation. They also secrete pro-inflammatory cytokines and chemokines to process and clear away accumulated Aβ. The additional deposition of Aβ then results in a positive feedback loop that furthers astrocyte activation resulting in the release of more pro-inflammatory factors [71]. Thus, it is essential to further explore the key inflammatory components in AD pathogenesis in order to develop better treatments for these disorders (Figure 2).
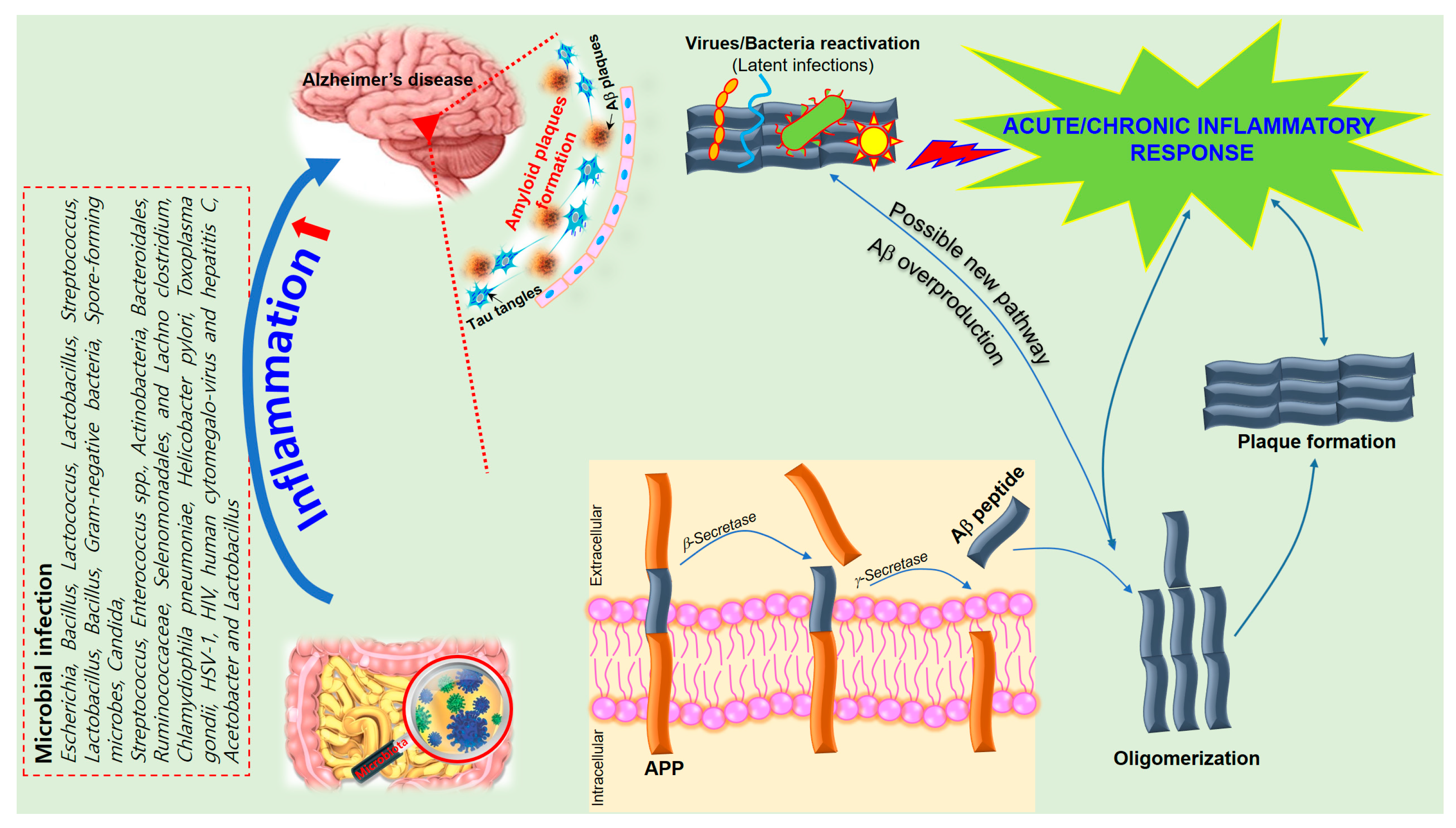
Figure 2. A schematic of the hypothetical chain of events via which brain infection may lead to pathological amyloid-β peptide (Aβ) plaque formation in the brain. The amyloid precursor protein (APP) is processed by secretases into different peptides, including Aβ. The gut microbiota plays a significant role in the development of Alzheimer’s disease (AD) since Aβ functions as an antimicrobial peptide via oligomerization and plaque formation, trapping invading microorganisms, including bacteria, fungi, viruses, and protist parasites. Aβ plaque formation in response to infection could result in a neuroinflammatory effect of microbiota on AD and neurodegeneration due to collateral damage in plaque-surrounding tissue.
7. Neuroinflammatory Effects of Microbiota on AD
In AD, the cerebellum is replete of microglia in areas of amyloid deposition, and cerebellar volume is reduced [72]. Molecular layer gliosis and atrophy in the vermis is also severe. Loss of Purkinje neurons occurs in the vermis, cerebellar hemispheres, and the inferior olivary nucleus [73][74]. Atrophy of the molecular layer by 24% and the granular layer by 22% correlates with a decrease in Purkinje cell numbers [75]. This might be related to BDNF, which is essential for the maintenance and survival of neurons, and which has pleiotropic effects on neuronal development, differentiation, synaptogenesis, and the synaptic plasticity that underlies neuronal circuit formation and cognition. Decreased amounts of BDNF were found in AD brains [76][77]. Interestingly, mice deficient in BDNF have altered development of GI-tract innervations [78][79]. BDNF expression was found to be reduced in the hippocampus and cortex of germ-free mice, and reduced expression of BDNF was found to be specifically associated with increased anxiety and progressive cognitive dysfunction [80][81].
Pro-inflammatory cytokines are already known to enhance APP expression, upregulate β-secretase messenger RNA (mRNA), and increase Aβ formation in the hippocampus. Similarly, bacterial amyloid is recognized as a pathogen-associated molecular pattern that can cause activation of Toll-like receptor-2 (TLR2), which was also reported to induce Notch1 upregulation and activation of microglia, and may enhance processes leading to the development of AD or Parkinson’s disease (PD) [81].
Chromogranin A (CHGA) is a neuroinflammatory factor which is frequently present in AD senile plaques associated with microglial activation [82]. Wu and Nakanishi found that CHGA activated both nuclear factor kappa B (NF-κB) and pro-caspase-1, while Aβ only activated pro-caspase-1. For activation of pro-caspase-1, both CHGA and Aβ require the enzymatic activity of cathepsin B (CatB). In the AD brain, highly activated microglia showed intense immunoreactivity for CatB and IL-1β, and they surround CHGA-positive plaques more frequently than Aβ-positive plaques. This suggests different pathways for CHGA- and Aβ-induced microglial production of IL-1β, which may help us gain a better understanding of the pathological significance of neuroinflammation in AD [83].
In addition, chronic gut inflammation may increase the breakdown of blood–brain barrier, LPS permeability, and the generation of pro-inflammatory cytokines. Elevated levels of LPS were found in the plasma of AD patients compared to healthy controls [56]. In an animal study, peripheral administration of LPS led to neuroinflammation with an increase in levels of IL-6. Moreover, overexpression of IL-1β resulted in a robust increase in tau phosphorylation in the triple transgenic mouse model of AD [81][84]. Furthermore, recent studies suggested that increased concentrations of circulating LPS, promoted by changes in intestinal permeability, may play a pivotal role in insulin resistance. Neuronal insulin resistance can increase the risk of developing AD, and insulin treatment may enhance memory function. Carvalho et al. found that antibiotic treatment greatly modifies the gut microbiota by reducing levels of Bacteroidetes and Firmicutes and circulating LPS levels. This modulation consequently improved glucose and insulin tolerance and activity in metabolically active tissues [85].
Furthermore, Villaran and co-workers reported that peripheral inflammation in the form of dextran sodium sulfate (DSS)-induced colitis can aggravate LPS-induced neuroinflammation and neurodegeneration as shown by increased mRNA transcripts of TNF-α, inducible nitric oxide synthase (iNOS), and IL-6 in the midbrain [4]. Interestingly, even rats with colitis alone, which received no midbrain injection of LPS, showed increased levels of TNF-α, iNOS, and IL-6 mRNA in the midbrain. Other studies also demonstrated that recurring systemic infections can increase the probability of developing multiple sclerosis (MS), AD or PD [86].
Hence, the microbiota is closely related to neurological dysfunction and plays a significant role in neuroinflammation through the secretion of pro-inflammatory cytokines. Changes in the homeostatic state of the microbiota leads to increased intestinal permeability, which may promote the translocation of bacteria and endotoxins across the epithelial barrier, inducing an immunological response associated with the production of pro-inflammatory cytokines. The activation of both enteric neurons and glial cells may result in various neurological disorders [86].
References
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155.
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340.
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65.
- Villaran, R.F.; Espinosa-Oliva, A.M.; Sarmiento, M.; De Pablos, R.M.; Arguelles, S.; Delgado-Cortes, M.J.; Sobrino, V.; Van Rooijen, N.; Venero, J.L.; Herrera, A.J.; et al. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: Potential risk factor in Parkinson’s disease. J. Neurochem. 2010, 114, 1687–1700.
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693.
- Morais, G.C.P.; Arruda, M.M.; Bonadia, J.C.A.; Pozzan, G. Cardiac amyloidosis: A challenging diagnosis. Autops. Case Rep. 2014, 4, 9–17.
- Sperry, B.W.; Tang, W.H.W. Amyloid heart disease: Genetics translated into disease-modifying therapy. Heart 2017, 103, 812–817.
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748.
- Bostanciklioğlu, M. Intestinal Bacterial Flora and Alzheimer’s Disease. Neurophysiology 2018, 50, 140–148.
- Morris, G.; Berk, M.; Maes, M.; Puri, B.K. Could Alzheimer’s Disease Originate in the Periphery and If So How So? Mol. Neurobiol. 2018.
- Lukiw, W.J.; Bazan, N.G. Survival signalling in Alzheimer’s disease. Biochem. Soc. Trans. 2006, 34 Pt 6, 1277–1282.
- Bagyinszky, E.; Giau, V.V.; Shim, K.; Suk, K.; An, S.S.A.; Kim, S. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J. Neurol. Sci. 2017, 376, 242–254.
- Cammarota, G.; Ianiro, G.; Bibbo, S.; Gasbarrini, A. Gut microbiota modulation: Probiotics, antibiotics or fecal microbiota transplantation? Intern. Emerg. Med. 2014, 9, 365–373.
- Schmidt, T.S.B.; Raes, J.; Bork, P. The Human Gut Microbiome: From Association to Modulation. Cell 2018, 172, 1198–1215.
- Cox, L.M.; Weiner, H.L. Microbiota Signaling Pathways that Influence Neurologic Disease. Neurotherapeutics 2018, 15, 135–145.
- O’Toole, P.W.; Jeffery, I.B. Microbiome-health interactions in older people. Cell. Mol. Life Sci. CMLS 2018, 75, 119–128.
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267.
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227.
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014, 4, 4828.
- Ding, Y.; Ren, J.; Yu, H.; Yu, W.; Zhou, Y. Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice. Immun. Ageing 2018, 15, 6.
- Briguglio, M.; Dell’Osso, B.; Panzica, G.; Malgaroli, A.; Banfi, G.; Zanaboni Dina, C.; Galentino, R.; Porta, M. Dietary Neurotransmitters: A Narrative Review on Current Knowledge. Nutrients 2018, 10, 591.
- Lyte, M.; Villageliú, D.N.; Crooker, B.A.; Brown, D.R. Symposium review: Microbial endocrinology—Why the integration of microbes, epithelial cells, and neurochemical signals in the digestive tract matters to ruminant health1. J. Dairy Sci. 2018, 101, 5619–5628.
- Thomas, C.M.; Hong, T.; van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE 2012, 7, e31951.
- Wall, R.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.F.; Dinan, T.G.; Stanton, C. Bacterial neuroactive compounds produced by psychobiotics. Adv. Exp. Med. Biol. 2014, 817, 221–239.
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome, and immune system: Envisioning the future. Nature 2011, 474, 327–336.
- Fukudo, S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J. Gastroenterol. 2007, 42 (Suppl. 17), 48–51.
- Nishino, R.; Mikami, K.; Takahashi, H.; Tomonaga, S.; Furuse, M.; Hiramoto, T.; Aiba, Y.; Koga, Y.; Sudo, N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. 2013, 25, 521–528.
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273.
- Holmes, E.; Kinross, J.; Gibson, G.R.; Burcelin, R.; Jia, W.; Pettersson, S.; Nicholson, J.K. Therapeutic modulation of microbiota-host metabolic interactions. Sci. Transl. Med. 2012, 4, 137rv6.
- Santilli, A.D.; Dawson, E.M.; Whitehead, K.J.; Whitehead, D.C. Nonmicrobicidal Small Molecule Inhibition of Polysaccharide Metabolism in Human Gut Microbes: A Potential Therapeutic Avenue. ACS Chem. Biol. 2018, 13, 1165–1172.
- Mulders, R.J.; de Git, K.C.G.; Schele, E.; Dickson, S.L.; Sanz, Y.; Adan, R.A.H. Microbiota in obesity: Interactions with enteroendocrine, immune and central nervous systems. Obes. Rev. 2018, 19, 435–451.
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behavior—Epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014, 13, 69–86.
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503.
- Kohler, C.A.; Maes, M.; Slyepchenko, A.; Berk, M.; Solmi, M.; Lanctot, K.L.; Carvalho, A.F. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 6152–6166.
- Distrutti, E.; Monaldi, L.; Ricci, P.; Fiorucci, S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J. Gastroenterol. 2016, 22, 2219–2241.
- Hestad, K.A.; Engedal, K.; Whist, J.E.; Farup, P.G. The Relationships among Tryptophan, Kynurenine, Indoleamine 2,3-Dioxygenase, Depression, and Neuropsychological Performance. Front. Psychol. 2017, 8, 1561.
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401.
- De Palma, G.; Collins, S.M.; Bercik, P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes 2014, 5, 419–429.
- Backhed, F. Programming of host metabolism by the gut microbiota. Ann. Nutr. Metab. 2011, 58 (Suppl. 2), 44–52.
- Luczynski, P.; McVey Neufeld, K.A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 2016, 19.
- Hansen, C.H.; Nielsen, D.S.; Kverka, M.; Zakostelska, Z.; Klimesova, K.; Hudcovic, T.; Tlaskalova-Hogenova, H.; Hansen, A.K. Patterns of early gut colonization shape future immune responses of the host. PLoS ONE 2012, 7, e34043.
- Olszak, T.; An, D.; Zeissig, S.; Vera, M.P.; Richter, J.; Franke, A.; Glickman, J.N.; Siebert, R.; Baron, R.M.; Kasper, D.L.; et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012, 336, 489–493.
- Pabst, O.; Cerovic, V.; Hornef, M. Secretory IgA in the Coordination of Establishment and Maintenance of the Microbiota. Trends Immunol. 2016, 37, 287–296.
- McCoy, K.D.; Ronchi, F.; Geuking, M.B. Host-microbiota interactions and adaptive immunity. Immunol. Rev. 2017, 279, 63–69.
- Macpherson, A.J.; Geuking, M.B.; McCoy, K.D. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. 2012, 33, 160–167.
- Girolamo, F.; Coppola, C.; Ribatti, D. Immunoregulatory effect of mast cells influenced by microbes in neurodegenerative diseases. Brain Behav. Immun. 2017, 65, 68–89.
- Lyte, M.; Li, W.; Opitz, N.; Gaykema, R.P.; Goehler, L.E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 2006, 89, 350–357.
- Panda, S.; El khader, I.; Casellas, F.; Lopez Vivancos, J.; Garcia Cors, M.; Santiago, A.; Cuenca, S.; Guarner, F.; Manichanh, C. Short-term effect of antibiotics on human gut microbiota. PLoS ONE 2014, 9, e95476.
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjolund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE 2010, 5, e9836.
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behave. Immunity 2017, 66, 9–17.
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572.
- Asti, A.; Gioglio, L. Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J. Alzheimer’s Dis. JAD 2014, 39, 169–179.
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742.
- Hanstock, T.L.; Mallet, P.E.; Clayton, E.H. Increased plasma d-lactic acid associated with impaired memory in rats. Physiol. Behav. 2010, 101, 653–659.
- De Haas, E.N.; van der Eijk, J.A.J. Where in the serotonergic system does it go wrong? Unravelling the route by which the serotonergic system affects feather pecking in chickens. Neurosci. Biobehav. Rev. 2018, 95, 170–188.
- Hernandez-Rapp, J.; Martin-Lannerée, S.; Hirsch, T.Z.; Launay, J.-M.; Mouillet-Richard, S. Hijacking PrP(c)-dependent signal transduction: When prions impair Aβ clearance. Front. Aging Neurosci. 2014, 6, 25.
- Daulatzai, M.A. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem. Res. 2014, 39, 624–644.
- Johnson, K.V.; Foster, K.R. Why does the microbiome affect behaviour? Nat. Rev. Microbiol. 2018, 16, 647–655.
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314.
- Tsavkelova, E.A.; Botvinko, I.V.; Kudrin, V.S.; Oleskin, A.V. Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl. Biochem. 2000, 372, 115–117.
- Shishov, V.A.; Kirovskaia, T.A.; Kudrin, V.S.; Oleskin, A.V. (Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12). Prikladnaia Biokhimiia i Mikrobiologiia 2009, 45, 550–554.
- Özogul, F. Effects of specific lactic acid bacteria species on biogenic amine production by foodborne pathogen. Int. J. Food Sci. Technol. 2011, 46, 478–484.
- Kawashima, K.; Misawa, H.; Moriwaki, Y.; Fujii, Y.X.; Fujii, T.; Horiuchi, Y.; Yamada, T.; Imanaka, T.; Kamekura, M. Ubiquitous expression of acetylcholine and its biological functions in life forms without nervous systems. Life Sci. 2007, 80, 2206–2209.
- Landete, J.M.; De las Rivas, B.; Marcobal, A.; Munoz, R. Updated molecular knowledge about histamine biosynthesis by bacteria. Crit. Rev. Food Sci. Nutr. 2008, 48, 697–714.
- Levi, M.; Keller, T.T.; van Gorp, E.; ten Cate, H. Infection and inflammation and the coagulation system. Cardiovasc. Res. 2003, 60, 26–39.
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802.
- Zhuang, Z.Q.; Shen, L.L.; Li, W.W.; Fu, X.; Zeng, F.; Gui, L.; Lu, Y.; Cai, M.; Zhu, C.; Tan, Y.L.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2018, 63, 1337–1346.
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634.
- Schwartz, K.; Boles, B.R. Microbial amyloids—Functions and interactions within the host. Curr. Opin. Microbiol. 2013, 16, 93–99.
- Schwab, C.; Klegeris, A.; McGeer, P.L. Inflammation in transgenic mouse models of neurodegenerative disorders. Biochim. Biophys. Acta 2010, 1802, 889–902.
- Lin, L.; Zheng, L.J.; Zhang, L.J. Neuroinflammation, Gut Microbiome, and Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 8243–8250.
- Andersen, K.; Andersen, B.B.; Pakkenberg, B. Stereological quantification of the cerebellum in patients with Alzheimer’s disease. Neurobiol. Aging 2012, 33, 197.e11–197.e20.
- Sjobeck, M.; Englund, E. Alzheimer’s disease and the cerebellum: A morphologic study on neuronal and glial changes. Dement. Geriatr. Cognit. Disord. 2001, 12, 211–218.
- Mavroudis, I.A.; Fotiou, D.F.; Adipepe, L.F.; Manani, M.G.; Njau, S.D.; Psaroulis, D.; Costa, V.G.; Baloyannis, S.J. Morphological changes of the human purkinje cells and deposition of neuritic plaques and neurofibrillary tangles on the cerebellar cortex of Alzheimer’s disease. Am. J. Alzheimer’s Dis. Dement. 2010, 25, 585–591.
- Wegiel, J.; Wisniewski, H.M.; Dziewiatkowski, J.; Badmajew, E.; Tarnawski, M.; Reisberg, B.; Mlodzik, B.; De Leon, M.J.; Miller, D.C. Cerebellar atrophy in Alzheimer’s disease-clinicopathological correlations. Brain Res. 1999, 818, 41–50.
- Reddy, Y.M.; Singh, D.; Nagarajan, D.; Pillarisetti, J.; Biria, M.; Boolani, H.; Emert, M.; Chikkam, V.; Ryschon, K.; Vacek, J.; et al. Atrial fibrillation ablation in patients with gastroesophageal reflux disease or irritable bowel syndrome-the heart to gut connection! J. Interv. Card. Electrophysiol. 2013, 37, 259–265.
- Churchill, L.; Taishi, P.; Wang, M.; Brandt, J.; Cearley, C.; Rehman, A.; Krueger, J.M. Brain distribution of cytokine mRNA induced by systemic administration of interleukin-1beta or tumor necrosis factor alpha. Brain Res. 2006, 1120, 64–73.
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136.
- Zhang, R.; Miller, R.G.; Gascon, R.; Champion, S.; Katz, J.; Lancero, M.; Narvaez, A.; Honrada, R.; Ruvalcaba, D.; McGrath, M.S. Circulating endotoxin and systemic immune activation in sporadic Amyotrophic Lateral Sclerosis (sALS). J. Neuroimmunol. 2009, 206, 121–124.
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.-C.; Ardura-Fabregat, A.; de Lima, K.A.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018, 557, 724–728.
- Ghosh, S.; Wu, M.D.; Shaftel, S.S.; Kyrkanides, S.; LaFerla, F.M.; Olschowka, J.A.; O’Banion, M.K. Sustained Interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J. Neurosci. 2013, 33, 5053–5064.
- Terada, K.; Yamada, J.; Hayashi, Y.; Wu, Z.; Uchiyama, Y.; Peters, C.; Nakanishi, H. Involvement of cathepsin B in the processing and secretion of interleukin-1beta in chromogranin A-stimulated microglia. Glia 2010, 58, 114–124.
- Wu, Z.; Sun, L.; Hashioka, S.; Yu, S.; Schwab, C.; Okada, R.; Hayashi, Y.; McGeer, P.L.; Nakanishi, H. Differential pathways for interleukin-1beta production activated by chromogranin A and amyloid beta in microglia. Neurobiol. Aging 2013, 34, 2715–2725.
- Moore, A.H.; Wu, M.; Shaftel, S.S.; Graham, K.A.; O’Banion, M.K. Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience 2009, 164, 1484–1495.
- Carvalho, B.M.; Guadagnini, D.; Tsukumo, D.M.L.; Schenka, A.A.; Latuf-Filho, P.; Vassallo, J.; Dias, J.C.; Kubota, L.T.; Carvalheira, J.B.C.; Saad, M.J.A. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia 2012, 55, 2823–2834.
- Tilvis, R.S.; Kahonen-Vare, M.H.; Jolkkonen, J.; Valvanne, J.; Pitkala, K.H.; Strandberg, T.E. Predictors of cognitive decline and mortality of aged people over a 10-year period. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, 268–274.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
15 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




