Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Seong An and Version 2 by Peter Tang.
The bidirectional communication between the central nervous system (CNS) and the gut microbiota plays a pivotal role in human health. Increasing numbers of studies suggest that the gut microbiota can influence the brain and behavior of patients. Various metabolites secreted by the gut microbiota can affect the cognitive ability of patients diagnosed with neurodegenerative diseases. Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by a progressive loss of memory, language, and cognitive ability.
- gut microbiota
- MGB axis
- germ-free animal
- probiotic
- neurodegenerative diseases
- Alzheimer’s disease
1. Introduction
Comprising trillions of symbiotic microorganisms, the gut microbiota is an essential element for the maintenance of the host’s health [1][2][3][1,2,3]. This microbial ecosystem consists mainly of bacteria, of which most are strict anaerobes, and also fungi and viruses. The four main phyla in adults consist of Bacteroidetes (~48%) and Firmicutes (~51%), which make up the highest proportion, as well as Proteobacteria and Actinobacteria, which are found in relatively low amounts (1%) [4]. Alterations in the composition of the gut microbiota, caused by dietary changes, antibiotic exposure, and infection, lead to the loss of homeostasis, which is implicated in the development of several diseases in humans, such as colorectal cancer, metabolic syndrome, obesity, allergies, inflammatory bowel disease (IBD), type 2 diabetes, heart failure, and neurodegenerative disorders [5][6][7][8][5,6,7,8]. Recent evidence points to a causative link between pathogens and changes in the intestinal microbiota composition, along with inflammatory changes in various tissues and organs including brain tissue [7]. Hence, gut microbes may alter levels of neurotransmitter-related metabolites, affecting gut-to-brain communication and/or altering brain function (Figure 1).
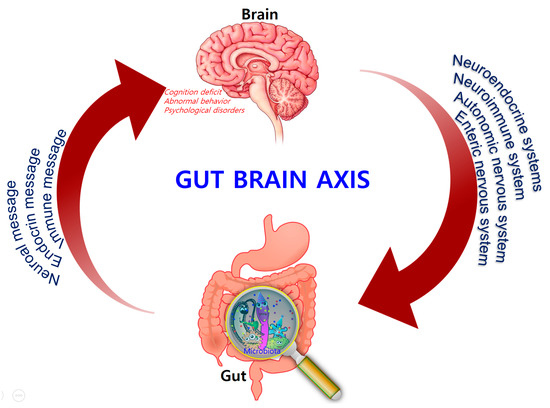
Figure 1.
Bidirectional signaling between the gastrointestinal tract and the brain is regulated at the neural, hormonal, and immunological levels.
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by a progressive loss of memory, language, and cognitive ability. According to the classical “amyloid cascade” model, the disease results from the over production of amyloid-beta peptide (Aβ), following the disruption of homeostatic mechanisms which regulate the proteolytic cleavage of the amyloid precursor protein (APP). Amyloids associated with AD consist largely of perivascular amyloid enriched in the 42-amino-acid Aβ42 peptide. Aβ42 is thought to initiate the neuroinflammatory process characteristic of AD pathology [8]. Plaque formation is also a natural response to infection by trapping invading microorganisms, further contributing to the collateral damage of healthy tissue that results from neuroinflammation. However, recent work suggests that the cascade model does not fully explain AD pathogenesis [9] and that alterations in the gut microbiome may play a significant role in the progression of the disease [10].
Over the last decade, several publications demonstrated the regulatory influence of the gut microbiota on the innate and adaptive immune response, as well as the importance of the interactions between the endogenous microbiota and the host’s central nervous system (CNS) [11][12][11,12]. Given the unsuccessful AD treatments employed so far, gut modification or recondition strategies started attracting the attention of the scientific community regarding the pathogenesis of CNS diseases, with emphasis on Alzheimer’s and Parkinson’s diseases [13].
2. The Intestinal Microbiota and Homeostasis
Early colonization of certain enterotypes can have a long-lasting influence on the health status of the host [14][15][16][14,15,16]. Human microbial colonization begins at birth. Infants born vaginally are initially colonized with microbial colonies that have a maternal signature (enriched in Lactobacillus and Prevotella spp.), while those delivered by caesarean section harbor colonies that more closely resemble the skin microbiota (enriched in Staphylococcus and Propionibacterium spp.). The microbiota then diversifies over the first few weeks of life to form a complex, anaerobe-dominated microbial community [17]. At the same time, the hypothalamic–pituitary–adrenal (HPA) axis becomes activated, which has an impact on the enteric nervous system (ENS) that innervates the gastrointestinal tract (GIT). Finally, the human gut microbiota rapidly expands and reaches an adult-like stage by three years of age. Shifts from Bifidobacterium to Clostridia and Bacteriodetes occur as the host develops from an infant into an adult [18]. Reductions in the level of Faecalibacterium prauznitzii and its anti-inflammatory relatives occur as young adults mature [6]. The composition of the microbiota is altered throughout the lifespan and is dependent on dietary and environmental factors, disease state, and other factors. In addition, several strategies suggest that Porphyromonas gingivalis causes an inflammatory response in the liver through the increased expression of pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), as well as fat storage-inducing transmembrane protein 2 (Fitm2) and perilipin 2 (Plin2), associated with lipid droplet formation, which subsequently increases neuroinflammation and causes neurodegenerative changes and AD [12][19][20][12,19,20]. The microbiota has long been known to play a relevant role in the health of the host. It can help break down certain nutrients, which can then be metabolized by host cells, and some of these products are involved in neural function. As such, gut bacteria produce amino acids (i.e., gamma-amino butyric acid (GABA) and tryptophan) [21][22][21,22], and monoamines (i.e., serotonin, histamine, and dopamine), which play a significant role in the brain as neurotransmitters, or as neurotransmitter precursors [23][24][23,24]. These neuroactive products can target the CNS via the blood stream and can also influence neurons in the ENS. In the homeostatic state, a healthy GIT has a normal and stable commensal intestinal microbiota. This provides the host with nutrition and energy through the production of vitamins [25][26][27][25,26,27], aids in the maintenance of intestinal epithelial barrier integrity, aids in resistance to pathogens, and plays a role in the metabolic and immune systems [28][29][30][28,29,30].3. The Microbiota–Gut–Brain (MGB) Axis
In the 1880s, William James and Carl Lange first introduced the concept that bidirectional communication between the CNS and intestinal organs plays a role in emotional regulation [31][32][38,39]. Forty years later, the idea that the brain plays an important role in regulating GI function was developed by Walter Cannon [33][40]. A number of rodent studies also showed that the gut–brain axis is a focus of research in different fields, ranging from basic microbiology to translational applications. Consequently, the potential involvement of the gut microbiota in brain function emerged. This involvement pertains to the core microbiome, distinct enterotypes, and age-related shifts in composition, which are harmful to health [34][41]. The concept of the MGB axis is well established. The neuroendocrine and neuroimmune systems, in addition to the sympathetic and parasympathetic arms of the autonomic nervous system (ANS) and the ENS, are key pathways in gut–brain communication. Although the exact mechanisms mediating gut–brain interactions are not fully understood, they were suggested to involve endocrine, immune, and neural pathways (vagus nerve and enteric nervous system), leading to possible alteration in AD patients or aggravating inflammation. The concept has now expanded and has become a quickly evolving area of research that led to convergence of research efforts in the fields of neuroscience, psychiatry, gastroenterology, and microbiology—disciplines that were previously considered to have distinct and separate research objectives and focuses. The MGB axis is vital for maintaining gut homeostasis. Dysregulation of the MGB axis was implicated in various disease states, the most common being chronic functional GI disorders such as IBS, which can induce depression and can also result in decreased cognitive function [35][36][46,47]. The work done by Bravo and Tillisch et al. implicated microbiota–brain signaling in alterations of resting brain activity in key circuits involved in pain, emotion, and cognition [37][48]. Researchers in this area are increasingly giving recognition to the microbiota itself as being an active and highly influential contributing factor in this bidirectional communication network.4. Disrupting Microbiota Effects on Brain and Behavior
The gut microbiota has become a focus of studies on the brain and behavior. Alterations in the gut microbiota can modulate the peripheral and central nervous systems, resulting in altered brain function, which gives further evidence for the existence of the MGB axis. Early studies in humans demonstrate that altering the microbiota with beneficial bacteria or probiotics can lead to changes in brain function, as well as subjective reports of mood. Experimental approaches in MGB-axis research include the use of germ-free animals, animals with pathogenic bacterial infections, and animals exposed to probiotic agents or antibiotics [38][49]. Germ-free animal studies are conducted in animals born and reared in sterile conditions, eliminating the opportunity for postnatal colonization of the GIT. Thus, research conducted in germ-free animals highlighted the important role the gut microbiota plays in the development of both physiologic and metabolic abnormalities [38][49]. When compared with conventional animals, germ-free animals exhibit abnormal gastrointestinal motility, increased expression of genes encoding transporters throughout the gut, and an altered response to inflammatory pain [39][40][41][50,51,52]. In addition, germ-free animals have an immature and dysregulated immune system, with abnormal immunoglobulin A (IgA) production [41][42][43][44][45][52,53,54,55,56] and decreased numbers of intestinal mast cells [46][57]. The absence of gut bacteria during development affects the HPA axis [47][58], which has a significant role in the stress response. Studies in germ-free animals clearly demonstrate a relationship between the gut microbiota and stress- and anxiety-related behaviors. Further studies should concentrate on the influence of time, sex, strain, and other species factors on this relationship. Antibiotics are normally used to remove or prevent bacterial colonization in the human body, without targeting specific types of bacteria. As a result, broad-spectrum antibiotics can greatly affect the composition of the gut microbiota, reduce the bio-diversity of the fecal microbiota, and delay colonization for a long period after administration. A number of studies showed that different antibiotic treatments result in short- and/or long-term changes in the intestinal microbiota in both humans and animals.5. Microbiota and Neurodegenerative Diseases
Amounting evidence suggests that gut microbiota plays an important role in the development of brain, and that there is a bidirectional relationship between the brain, gut, and the bacteria within the gut which is referred as the brain–gut–microbiome axis [48][74]. The microbiota can affect regulation of the MGB axis via immunological, neuroendocrine, and direct neural mechanisms. The gut microbiota is known to increase local and systemic inflammation due to lipopolysaccharide (LPS) from pathogenic bacteria and the synthesis of pro-inflammatory cytokines [49][50][75,76]. These microorganisms are able to produce neurotransmitters and neuromodulators, such as short-chain fatty acids (SCFAs), biogenic amines (e.g., histamine), and other amino-acid-derived metabolites such as serotonin [51][77] or GABA [52][53][78,79]. In addition, bacterial enzymes may also synthesize neurotoxic metabolites such as d-lactic acid and ammonia [54][80]. Signaling molecules secreted by the gut microbiota are transferred via the lymphatic and systemic circulation throughout the CNS where they then affect behavior and modulate brain plasticity and cognitive function [55][81]. This implicates the importance of the gut microbiota in the development and function of the CNS, and in the pathophysiology of chronic brain diseases [54][80]. Microbiome species and their secretory products are extremely powerful pro-inflammatory and innate-immune activators in the host [23][56][57][58][59][60][61][62][63][64][23,82,83,84,85,86,87,88,89,90]. The connection between the kind of gut microbiota and AD pathology was shown in a study that used transgenic mouse models. Harach et al. observed a significant shift in the diversity of gut microbiota of APP transgenic mice with that of non-transgenic wild-type mice through sequencing of 16S ribosomal RNA (rRNA) from their fecal samples [65][97]. Germ-free APP transgenic mice were generated, in which a significant decrease in cerebral Aβ pathology was observed when compared to control mice with intestinal microbiota. Interestingly, an increase in cerebral Aβ pathology was observed in germ-free APP transgenic mice when these were colonized with gut microbiota acquired from conventionally raised APP transgenic mice [66][98].6. The Role of Inflammation in Alzheimer’s Disease
Inflammatory reactions could be both beneficial and detrimental to the brain, depending on strengths of their activation in various stages of neurodegeneration [67][112]. Regulation of immuno-inflammatory control is one of the relevant processes involved in the pathogenesis of neurodegenerative disorders. AD shares several common properties with other neurodegenerative disorders, such as accumulation of misfolded proteins (Aβ) and hyperphosphorylated tau, evidence for a prion-like spread of pathology with misfolded proteins and neuroinflammation [9]. The pro-inflammatory gut microbiota dysbiosis in AD patients could trigger inflammation-induced formation and aggregation of cerebral amyloid-β, proving to be an effective strategy for preventing or reducing the risk of AD [68][115]. Bacterial strains known to produce functional extracellular amyloid fibers are Escherichia coli, Salmonella enterica, Salmonella typhimurium, Bacillus subtilis, Mycobacterium tuberculosis, and Staphylococcus aureus [68][115]. For instance, E. coli endotoxin was shown to induce the formation of Aβ fibrils in vitro, implying their involvement in AD pathogenesis [68][115]. Activated astrocytes are supportive cells that provide trophic and metabolic maintenance for neurons and are also influential in neuroinflammation in AD. Changes in astrocyte morphology, gene expression, protein composition, and activity can be observed in AD, which in turn can compromise astrocyte function [69][126]. Several transgenic mouse models showed that activated astrocytes accumulate in the brain before any plaque or tangle pathology can be observed [70][119], suggesting that astrocyte activation may be involved in AD pathogenesis. Astrocytes outnumber microglia in the brain and have a greater influence on long-term neuroinflammation. They also secrete pro-inflammatory cytokines and chemokines to process and clear away accumulated Aβ. The additional deposition of Aβ then results in a positive feedback loop that furthers astrocyte activation resulting in the release of more pro-inflammatory factors [71][118]. Thus, it is essential to further explore the key inflammatory components in AD pathogenesis in order to develop better treatments for these disorders (Figure 2).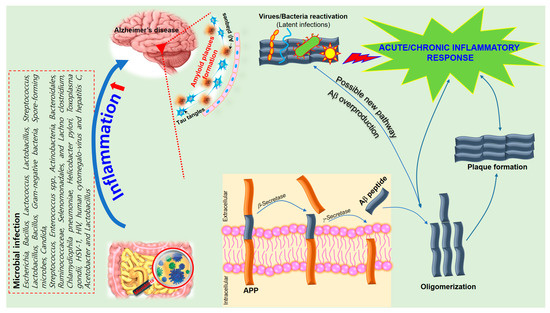
Figure 2. A schematic of the hypothetical chain of events via which brain infection may lead to pathological amyloid-β peptide (Aβ) plaque formation in the brain. The amyloid precursor protein (APP) is processed by secretases into different peptides, including Aβ. The gut microbiota plays a significant role in the development of Alzheimer’s disease (AD) since Aβ functions as an antimicrobial peptide via oligomerization and plaque formation, trapping invading microorganisms, including bacteria, fungi, viruses, and protist parasites. Aβ plaque formation in response to infection could result in a neuroinflammatory effect of microbiota on AD and neurodegeneration due to collateral damage in plaque-surrounding tissue.
