Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alice Vilela | -- | 3145 | 2023-09-13 13:01:38 | | | |
| 2 | Lindsay Dong | + 5 word(s) | 3150 | 2023-09-15 02:49:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pinto, T.; Pinto, A.; Vilela, A. Techniques of Making Edible Coatings. Encyclopedia. Available online: https://encyclopedia.pub/entry/49110 (accessed on 07 February 2026).
Pinto T, Pinto A, Vilela A. Techniques of Making Edible Coatings. Encyclopedia. Available at: https://encyclopedia.pub/entry/49110. Accessed February 07, 2026.
Pinto, Teresa, Ana Pinto, Alice Vilela. "Techniques of Making Edible Coatings" Encyclopedia, https://encyclopedia.pub/entry/49110 (accessed February 07, 2026).
Pinto, T., Pinto, A., & Vilela, A. (2023, September 13). Techniques of Making Edible Coatings. In Encyclopedia. https://encyclopedia.pub/entry/49110
Pinto, Teresa, et al. "Techniques of Making Edible Coatings." Encyclopedia. Web. 13 September, 2023.
Copy Citation
Edible coatings are made from natural food-grade materials, such as hydrocolloids (polysaccharides, proteins), lipids, and emulsifiers, produced with different techniques, such as dipping (immersing), spraying, spreading, brushing, pressing them/thermoforming, or extrusion. The most used method for coating is immersing, where food is dipped in a liquid containing food matrices, forming a film around the food and protecting all the components present.
casting
extrusion
chitosan
cellulose
pectin
carrageenan
alginate
1. Introduction
Currently, one of the alternatives for prolonging food shelf-life is the creation of plastic packaging, which is already implemented in the market and has been used in consumers' daily lives for many years. The high usage of this alternative relies on its low cost and resistance, as well as being easy to mold [1][2]. Despite the aspects mentioned above, several studies [3][4][5] show that this solution is not a promising alternative, as it implies an overall increase in the use of plastic. In 2021, 390.700 million tonnes of plastic were produced worldwide, of which 57.200 million tonnes were made in Europe, with a gradual increase over the years [6]. Another disadvantage of this material is that it is not biodegradable, constituting a harmful environmental alternative. It is not beneficial for the lifestyle of the entire population and for all biodiversity. It is increasingly a more debatable and worrying issue. Because of this, several alternatives have been investigated to substitute this alternative for something more sustainable and not harmful to the environment.
The alternative to replacing plastic and consequently meeting the requirements of the extension of the shelf-life of food is the creation of edible coatings that are progressively a more cost-effective, sustainable, and environmentally effective solution in the use of primary packaging in the food industry. They can also be an alternative to replace commercial synthetic waxes made of oxidized polyethylene. These coatings protect food ingredients from oxidation and degradation caused by enzymes while also helping to preserve the flavors originating from each food, thus ensuring the viability of the active ingredients for a long time. Moreover, they may contain valuable additives, like antioxidants and phytonutrients [7][8][9]. In addition, depending on the coating, beneficial properties may be added, such as preventing chronic degenerative and cardiovascular diseases due to phenolic compounds and preventing reproductive, nervous system, inflammation, and immune system degeneration [10][11].
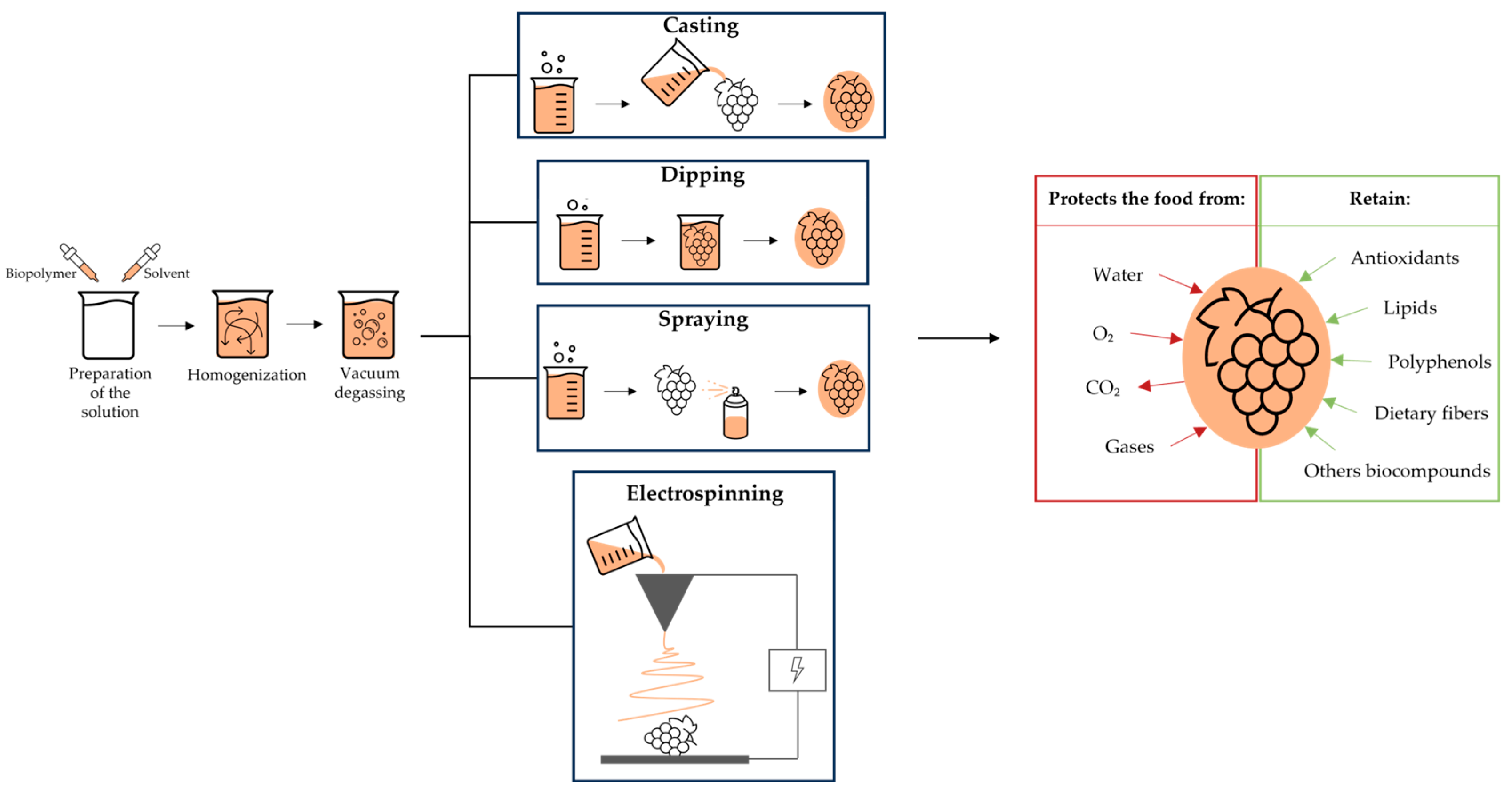
2. Techniques of Making Edible Coatings
2.1. Casting and Extrusion
Two techniques can be used to make edible films, namely casting and extrusion (Figure 1). Casting is a technique used in the laboratory that has the advantage of being accessible and straightforward. This technique is divided into three phases of preparation: solubilization, casting, and drying. Firstly, one dispersion/solution of a suitable solvent is prepared and then spread on the material's surface. Secondly, the prepared is dried since this technique uses the evaporation of the solvent to increase the solution viscosity, which will help to make an edible film with desirable properties [12]. The processing time, temperature, and drying conditions condition the effectiveness of creating a strong and promising film. As this technique is produced in three phases, the processing time is long and has an extended drying time, so it is not practicable for commercial film production [13][14]. This technique has been used in several studies with many different foods like okra leaves [15], cherry tomato [16], and mangoes [17] and plants like yerba mate [18].

Figure 1. Illustration of the application and protection of edible coatings in grapevine by-products.
Extrusion is a technique that can be applied in the industry, unlike the previous method. It begins with preparing the film-forming solution and introducing it in the feeding zone. The next step is kneading, where the mix is compressed, and the pressure, temperature, and density increase. The last stage is the heating stage, where the solution is extruded through a nozzle at a defined speed, and finally, the films are dried [19]. This technique is more sensitive since a high temperature and unsuitable pH may break the polypeptide chain of the protein, conditioning the formation of the edible film [20]. The advantage of this process is that it allows for a continuous operation with control of the temperature, size, form, and humidity, providing a more structured film and allowing for better dispersion of the active compounds [13].
2.2. Dipping (Immersion)
The dipping process has a wide application, even in the laboratory, for simplicity (Figure 1). First, the coating solution is prepared by dipping the food into the coat-forming solution for a specific time. Then, the coated food is left to dry at room temperature, and the excess solution is removed not to become a film with a large layer [21]. This method can be repeated with another solution for better coating [19]. This technique has the advantage that it is simple, short, and low-cost. The only downside of this process is that it uses a lot of film-making solutions, taking longer in preparation [20].
2.3. Electrospinning
Electrospinning is used chiefly in edible film packaging [22], Figure 1. This filmmaking process is inexpensive, easy to use, and versatile because it only uses an electric field to stretch a polymeric solution’s forming structures with a high specific surface so it is possible to coat the materials; that is, an electrostatic force is used to transform a polymeric solution into nanofibrous films. This process demonstrates a high surface–volume ratio and a small pore size distribution [12]. The only disadvantage is that this process presents a weak interaction between the electrolyte fibers, conferring destructive mechanical properties on the film [20].
2.4. Spraying
Spraying is a method where the coating solution is distributed on the surface of the food pieces from the drops that form in the equipment's nozzle, and it is the most used in the food industry (Figure 1). The advantage of this method is that it forms a coating with uniform thickness over the whole surface of the food, the quantity of the necessary solution is less, the possibility of contamination is lower, and it can be applied to food with large surfaces [19].
3. Edible Films and Coating Materials
3.1. Polysaccharides
Polysaccharides are used to make edible films to preserve food, especially fruits and vegetables. Using these polysaccharides to make coatings is very popular due to their low cost and availability. Polysaccharide coatings offer barrier properties, such as water and gaseous permeability, and create modified internal atmospheric conditions, resulting in significant preservative effects [15]. Antioxidants and antibacterial characteristics are related to polysaccharide-based edible covering [7].
Chitosan is a natural cationic polysaccharide created by deacetylating the chitin found in animal shells, such as crustaceans, insect cuticles, and yeast [23]. In recent years, chitosan has been applied in several foods, like sweet cherries [24], kiwifruit [25], mango [26], litchi [27], grape [28][29][30], apricot [31], banana [32], and papaya [33], among others. Prathibhani et al. [25] studied the effect of an edible chitosan coating on kiwifruit. They showed that this coating has an effective preservation technique, reducing respiration activity and delaying weight loss and firmness changes, thereby delaying ripening.
Cellulose is the most widespread polymer in nature and is produced by various sources, such as plants, animals, and bacteria [7]. Bacterial cellulose is the most widely used biopolymer in making edible films and coatings, with the disadvantage of this polysaccharide having no antimicrobial activity [34]. Strnad et al. [35] showed that the combination of cellulose and chitosan is beneficial to producing the perfect edible film since the cellulose improved the mechanical strength, and the chitosan improved the antibacterial and biocompatible properties of the composites. The cellulose and chitin junction also produces a film that is biocompatible, biodegradable, non-toxic, antimicrobial, presents antioxidant activity, has low gas permeability, and, compared with protein cellulose, is less immunogenic and non-hemolytic [36].
Pectin is the most used polysaccharide in food processing [37]. It can be found in many fruits and vegetables, such as banana peels, red pomelo peels, watermelon rinds, sugar beet pulp, sunflower heads, tomato pomace, and carrot pomace. However, most commercial pectin is obtained from citrus or apple pomace wastes [38][39]. Pectin polysaccharide structures contain β − 1, 4-linked galacturonic acid residues. Extraction of pectin allows for reusing the pomace that is not used in the fruit juice industry.
Regarding carrageenan, it is a natural polysaccharide extracted from red seaweed, such as Eucheuma cottonii, Mastocarpus stellatus, and Hypnea musciformis [20]. It is an absorbing polymer because it forms thermoreversible gels and viscous solutions. It becomes elastic and stable if Ca2+ is added, which is ideal for developing edible films [37].
Alginate is a polymer in brown algae's cell walls [40]. Alginate covering materials combine divalent cations, such as Mg2+, Ca2+, Al2+, Mn2+, and others. Due to their hydrophilic aspect, they are used to make edible solid coatings or films with low water resistance [41]. A disadvantage of this polymer is its poor mechanical properties; however, according to several studies, the alginate mixture with pectin and carrageenan improves the missing properties and forms a more protective and effective film [36].
Gums are naturally occurring polysaccharides with worldwide industrial uses and have been increasingly investigated for their desirable advantages in creating edible films [42]. Two categories divide gums: exudate gums and seed gums. Seed gums are the best known and used because they are easily accessible since they are extracted from plants, specifically from the epidermis of seeds, leaves, and bark. This category includes guar, locust bean, tara, tamarind, basil, and fenugreek gums. The exudate gum is used as a thickener, stabilizer, rheology modifier, soluble fiber, and fat replacer. Examples of exudate gum are gum ghatti, Persian, tragacanth, and shellac. Other gums are extensively used, like Arabic gum and xanthan gum [43]. Arabic gum is produced from the Acacia Senegal tree, and it has antioxidant and antifungal characteristics against fungi and antimicrobial properties advantageous for the creation of films. It has already been applied in the edible coatings industry in cereals [44] and fruit conservation [45].
Guar gum is obtained from the seed endosperm of Cyamopsis tetragonolobus. According to several studies, it has excellent film-forming capacities, is biocompatible, delays the loss of quality, and prolongs the shelf life of food. The study by Jiang et al. [46] demonstrates several procedures to improve this film: changes to the biopolymer, the addition of plasticizers, mixing with other polymers, the layer-by-layer (LBL) assembly technique, or the addition of plant extracts (Pes) and essential oils (Eos), thus showing improvements in the realization of edible coatings. In the study by Shubham et al. [47], the guar gum (1.5%) edible film created to preserve the quality of litchi was promising because it was capable of forming a protective barrier on the surface of litchi, maintaining fruit total soluble solids, pH, acidity, ascorbic acid, total sugar, reducing sugar, and non-reducing sugar over 12 days. Locust bean gum is a natural polymer obtained by crushing the endosperm of the seeds from a carob fruit pod [48]. To create an edible film, it is necessary to put the locust bean gum in hot water (80 °C) for solubilization [34].
Gum ghatti is an exudate gum from the tree Anogeissus Indifolia, which has good water oil emulsification stability and is a non-starch polysaccharide. The study by Eshghi et al. [49] that produced an edible coating to improve the quality of a specific type of table grape (Rishbaba) with a mix of chitosan and gum ghatti in different concentrations showed that the addition of gum ghatti to chitosan presented extra beneficial effects for weight loss and the titratable acidity, pH, and total soluble solids of the table grape. The mix of chitosan (1%) and gum ghatti (1%) showed the best concentration in forming an edible coating because it delayed losses in ascorbic acid, presented good membrane stability, and improved antioxidant enzyme activities of the Rishbaba grape during 60 days of storage at 0 ± 1 °C and 85% relative humidity. The film extended the shelf life and preserved the bioactive ingredients [49]. Tragacanth gum is an anionic biopolymer obtained from the dry sap of the species Astragalus that contains intumesce and water-soluble parts. It has a hydrophilic property that causes stabilizing and emulsifying properties [50][51].
Starch is a natural polymer composed of amylose (water-soluble) and amylopectin (water-insoluble) molecules that can form strong gels [7]. It is one of the most widely used polymers in the packaging and food industries. Several foods with starch can include films, such as cassava, corn, millet, wheat, rice, quinoa, sweet potatoes, peas, and tef [52]. Like so many others, the disadvantage of this polymer is that the mechanical quality is not good [53], and its hydrophilic nature is high, thus making the film permeable to water and sensitive to moisture, leading to reduced film permanence [54].
Agar is a polysaccharide extracted from specific red seaweed that possesses excellent film-forming ability and has the particularity of being insoluble in cold water but soluble in boiling water [52]. The application of this polymer is limited due to its shortcomings of strong hydrophilicity and weak mechanical properties, and it is relatively brittle, has low elasticity, and has poor thermal stability [55][56]. To overcome these limitations, the solution is in combination with other polymers, hydrophobic materials, plasticizers, nanoparticles, and antimicrobial agents [55].
Inulin is a fructan polysaccharide found in several plant species, like Liliaceae, Amaryllidaceae, Gramineae, and Compositae, that is soluble in hot water and slightly soluble in cold water. Despite its viscous properties, the viscosity decreases with an increase in temperature.
Konjac glucomannan is a water-soluble natural polysaccharide extracted from the tuber of Amorphophallus konjac that has been extensively used as a material of film that exhibits good film-forming ability. The low water resistance and low mechanical properties are disadvantages and limit the use of this polymer [57]. For this, the combination of Konjac glucomannan with other polymers is beneficial.
Pullulan is a protein developed by microbial fermentation and has a starch-like structure. The existing limitations of pullulan-based films are the hydrophilicity, brittleness, high cost, and lack of active functions [58]. Improving this characteristic can be performed with physical and chemical crosslinking, plasticizers, and combination with other polymers, such as pectin [59], chitosan [60][61][62], starch [63], sodium alginate [64], gelatin [65], konjac glucomannan [57], and others.
3.2. Proteins
Gelatin comes from animal sub-products, specifically pig skin, beef skin, pork, and cattle bones [54]. An edible film based on gelatin proves to be a cheap, biodegradable option and can absorb ultraviolet light due to the aromatic amino acids present in its structure. It has the disadvantage of having low thermal stability and weak mechanical and barrier properties, being more advantageous with the combination of other biopolymers [50].
In the study by Chen et al. [66], cherry tomatoes were coated with edible gelatin, chitosan, nisin, and cornstarch film. They showed that weight loss and total bacterial count were reduced, and higher firmness and better color were observed during the storage over 22 days.
Biodegradable polymers have experienced a notable upswing in popularity as of late, motivated by two main reasons: firstly, the rapid decrease in oil reserves worldwide and second, the urgency to reduce environmental pollution resulting from the use of non-biodegradable resources [67]. Zein, a prolamin polymer commercially available since 1938 [68], is a storage protein extracted from corn that is present mainly in corn residues [53][69]. It has the particularity of not dissolving in water, and the film must be prepared in a mixture of alcohol–water and acetic acid at alkaline pH and high concentrations of urea [70].
Whey protein is a valuable by-product of cheese manufacturing that presents a sound barrier against aromas, lipids, and oxygen and has good mechanical properties. Still, its hydrophilic nature limits its capacity to act as a barrier against water vapor [71]. This limitation can be improved with enzymatic modification, like adding transglutaminase and numerous hydrophobic compounds, waxes, fatty acids, or acetylated monoglycerides like vegetable oils [72]. This protein needs a plasticizing agent to minimize the brittleness and extensibility of the film structure [73]. Glycerol is an example that can be used to improve the characteristics of an edible film with whey protein. It is promising because it can create a film with good mechanical and excellent oxygen barrier properties [74].
3.3. Lipids
Different types of waxes can be used in the food industry. An example is carnauba wax, which is extracted from the leaves of the carnauba trees and has more stable characteristics; it has excellent water vapor barrier properties, which have been widely recognized, and exhibits a high melting point and hardness, which can be used to develop a durable coating [14].
Beeswax is produced naturally by honeybees in the bees’ hive. It is well-known as hydrophobic and is used in making candles, metal casting, cosmetic products, textiles, polishes, and food processing [75]. It presents high plasticity, is viscoelastic, and has a low melting point [14]. This wax is beneficial with other polymers to make an edible film with good characteristics. For example, adding different concentrations (0, 2.5, 5, and 10 wt%) of beeswax in the edible film of cassava starch improved the moisture barrier and reduced the water solubility properties. Nevertheless, it also reduced the tensile strength, elongation, and flexural strength while enhancing the tensile and flexural modulus until five wt% beeswax [75].
4. Using Essential Oils, Plasticizers, Extracts, and Crosslinker Agents in the Edible Film with Biopolymers
Some strategies have been applied to improve the efficiency of some edible films and coatings. Adding essential oils is an example that can help in the antibacterial effect [76][77] and retain the qualities and benefits of food. There are several studies with the incorporation of different essential oils, such as thyme [78], oregano [79], grape seed, sea buckthorn [21], lemon [80], ginger [81], rosemary [82], clove [83], cinnamon [84], and lemongrass [85], among others.
Aloe vera is a plasticizer that can decrease weight loss and inhibit the maturity stage, as shown in the titratable acidity, pH, and total soluble solids. It has also been studied in several foods, such as tomatoes [86] and apples [80].
On the other hand, vanillin is a phenolic aldehyde organic compound derived from the vanilla bean that has inhibitory effects against yeasts, molds, and bacteria, thus controlling the decay of the fruit. Vanillin was studied in the conservation of tomato fruit [87] but had few studies with utilization in edible films.
Crosslinker agents are another alternative to improve the quality of edible films and have been studied recently. There are two types of crosslinker agents: green and chemical. Chemical crosslinker agents like, for example, glutaraldehyde have high reactivity, crosslinking solid properties, and increased stability, but their toxicity limits their application in food packaging films [88][89]. To resolve this problem, green crosslinker agents have been studied, like, for example, tannic acid, which has excellent antibacterial and antioxidant properties that improve the biopolymers' performance in food packaging films [88][90].
Deep eutectic solvents (DES) were defined as one system composed of a mixture of at least two compounds, a hydrogen-bond acceptor (tetraalkylammonium, quarternary ammonium, or phosphonium salts-HBA) and a hydrogen-bond donor (acids, amines, or alcohols-HBD), which are capable of self-associating [91][92]. It is a safe chemical that recently became a hot topic for use in the manufacturing of biopolymer-based films due to it being inexpensive, presenting a shallow environmental impact, having low toxicity, high renewability, high biodegradability, remarkable tunability, low cost, and particularity of having outstanding solvation properties that make it ideally suited for the extraction of bioactive compounds from various natural matrices [88][93]. Only in the last six years was it commonly used as a substitute for expensive organic solvents; year by year, it attracted more attention [94]. The mixture of choline chloride–citric acid (ChCl-CA), choline chloride–urea (ChCl-Urea), and choline chloride–glycerol (ChCl-Gly) are the DESs most used for plasticizing edible film [95].
References
- Socaciu, M.; Câmpia, V.; Dabija, D.; Fogarasi, M.; Semeniuc, C.; Podar, A.; Vodnar, D. Assessing Consumers’ Preference and Loyalty towards Biopolymer Films for Food Active Packaging. Coatings 2022, 12, 1770.
- Ștefănescu, B.; Socaciu, C.; Vodnar, D. Recent Progress in Functional Edible Food Packaging Based on Gelatin and Chitosan. Coatings 2022, 12, 1815.
- Navarre, N.; Mogollón, J.; Tukker, A.; Barbarossa, V. Recycled plastic packaging from the Dutch food sector pollutes Asian Oceans. Resour. Conserv. Recycl. 2022, 185, 106508.
- Paolo, L.; Abbate, S.; Celani, E.; Battista, D.; Candeloro, G. Carbon Footprint of Single-Use Plastic Items and Their Substitution. Sustainability 2022, 14, 16563.
- Bargagli, R.; Rota, E. Microplastic Interactions and Possible Combined Biological Effects in Antarctic Marine Ecosystems. Animals 2023, 13, 162.
- Plastics Europe. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 27 June 2023).
- Khalid, M.; Niaz, B.; Saeed, F.; Afzaal, M.; Islam, F.; Hussain, M.; Mahwish; Khalid, H.; Siddeeg, A. Edible coatings for enhancing safety and quality attributes of fresh produce: A comprehensive review. Int. J. Food Prop. 2022, 25, 1817–1847.
- Tee, Z.; Zaidel, D.; Jusoh, Y.; Muhamad, I.; Hashim, Z. Encapsulation of Milk Protein with Inulin for Improved Digestibility. J. Hum. Centered Technol. 2022, 1, 26–32.
- Tauferova, A.; Pospiech, M.; Javurkova, Z.; Tremlova, B.; Dordevic, D.; Jancikova, S.; Tesikova, K.; Zdarsky, M.; Vitez, T.; Vitezova, M. Plant Byproducts as Part of Edible Coatings: A Case Study with Parsley, Grape and Blueberry Pomace. Polymers 2021, 13, 2578.
- Tsali, A.; Goula, A. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207.
- Zabot, G.; Rodrigues, F.; Ody, L.; Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194.
- Wang, P.; Li, Y.; Zhang, C.; Que, F.; Weiss, J.; Zhang, H. Characterization and antioxidant activity of trilayer gelatin/dextran-propyl gallate/gelatin films: Electrospinning versus solvent casting. LWT 2020, 128, 109536.
- Roy, S.; Zhang, W.; Biswas, D.; Ramakrishnan, R.; Rhim, J. Grapefruit Seed Extract-Added Functional Films and Coating for Active Packaging Applications: A Review. Molecules 2023, 28, 730.
- Cheng, Y.; Zhai, X.; Wu, Y.; Li, C.; Zhang, R.; Sun, C.; Wang, W.; Hou, H. Effects of natural wax types on the physicochemical properties of starch/gelatin edible films fabricated by extrusion blowing. Food Chem. 2023, 401, 134081.
- Olawuyi, I.; Lee, W. Development and Characterization of Biocomposite Films Based on Polysaccharides Derived from Okra Plant Waste for Food Packaging Application. Polymers 2022, 14, 4884.
- Sharma, S.; Perera, K.; Pradhan, D.; Duffy, B.; Jaiswal, A.; Jaiswal, S. Active Packaging Film Based on Poly Lactide-Poly(butylene adipate-co-terephthalate) Blends Incorporated with Tannic Acid and Gallic Acid for the Prolonged Shelf Life of Cherry Tomato. Coatings 2022, 12, 1902.
- Oldoni, F.; Bernardo, M.; Filho, J.; Aguiar, A.; Moreira, F.; Mattoso, L.; Colnago, L.; Ferreira, M. Valorization of mangoes with internal breakdown through the production of edible films by continuous solution casting. LWT 2021, 145, 111339.
- Agüero, A.; Perianes, E.; Muelas, S.; Lascano, D.; García-Soto, M.; Peltzer, M.; Balart, R.; Arrieta, M. Plasticized Mechanical Recycled PLA Films Reinforced with Microbial Cellulose Particles Obtained from Kombucha Fermented in Yerba Mate Waste. Polymers 2023, 15, 285.
- Sáez-Orviz, S.; Rendueles, M.; Días, M. Impact of adding prebiotics and probiotics on the characteristics of edible films and coatings—A review. Food Res. Int. 2023, 164, 112381.
- Cheng, C.; Chen, S.; Su, J.; Zhu, M.; Zhou, M.; Chen, T.; Han, Y. Recent advances in carrageenan-based films for food packaging applications. Front. Nutr. 2022, 9, 1004588.
- Popescu, P.; Palade, L.; Nicolae, I.; Popa, E.; Miteluț, A.; Drăghici, M.; Matei, F.; Popa, M. Chitosan-Based Edible Coatings Containing Essential Oils to Preserve the Shelf Life and Postharvest Quality Parameters of Organic Strawberries and Apples during Cold Storage. Foods 2022, 11, 3317.
- Tampau, A.; González-Martínez, C.; Chiralt, A. Polyvinyl alcohol-based materials encapsulating carvacrol obtained by solvent casting and electrospinning. React. Funtional Polym. 2020, 153, 104603.
- Herliana, H.; Yusuf, H.; Laviana, A.; Wandawa, G.; Cahyanto, A. Characterization and Analysis of Chitosan-Gelatin Composite-Based Biomaterial Effectivity as Local Hemostatic Agent: A Systematic Review. Polymers 2023, 15, 575.
- Tokatli, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Sci. Hortic. 2020, 259, 108656.
- Prathibhani, H.; Kumarihami, C.; Kwack, Y.; Kim, J.; Kim, J. Application of chitosan as edible coating to enhance storability and fruit quality of Kiwifruit: A Review. Sci. Hortic. 2022, 292, 110647.
- Parvin, N.; Rahman, A.; Roy, J.; Rashid, H.; Paul, N.; Mahamud, A.; Imran, S.; Sakil, A.; Uddin, F.; Molla, E.; et al. Chitosan Coating Improves Postharvest Shelf-Life of Mango (Mangifera indica L.). Horticulturae 2023, 9, 64.
- Nhung, D.; Thao, C.; Tu, N.; Phong, T. Effect of chitosan coating on quality of lychee during storage. Vietnam J. Chem. 2018, 56, 292–296.
- Melo, N.; Soares, B.; Diniz, K.; Leal, C.; Canto, D.; Flores, M.; Tavares-Filho, J.; Galembeck, A.; Stamford, T.; Stamford-Arnaud, T.; et al. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of postharvest table grapes. Postharvest Biol. Technol. 2018, 139, 56–66.
- Zhao, X.; Tian, R.; Zhou, J.; Liu, Y. Multifunctional chitosan/grape seed extract/silver nanoparticle composite for food packaging application. Int. J. Biol. Macromol. 2022, 207, 152–160.
- Vilela, A.; Cruz, I.; Oliveira, I.; Pinto, A.; Pinto, T. Sensory and Nutraceutical Properties of Infusions Prepared with Grape Pomace and Edible-Coated Dried–Minced Grapes. Foods 2022, 12, 443.
- Algarni, E.; Elnaggar, I.; El-wahed, A.; Taha, I.; AL-Jumayi, H.; Elhamamsy, S.; Mahmoud, S.; Fahmy, A. Effect of Chitosan Nanoparticles as Edible Coating on the Storability and Quality of Apricot Fruits. Polymers 2022, 14, 2227.
- Parijadi, A.; Yamamoto, K.; Ikram, M.; Dwivany, F.; Wikantika, K.; Putri, S.; Fukusaki, E. Metabolome Analysis of Banana (Musa acuminata) Treated With Chitosan Coating and Low Temperature Reveals Different Mechanisms Modulating Delayed Ripening. Front. Sustain. Food Syst. 2022, 6, 835978.
- Ali, A.; Muhammad, M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626.
- Tudu, M.; Samanta, A. Natural polysaccharides: Chemical properties and application in pharmaceutical formulations. Eur. Polym. J. 2023, 184, 111801.
- Strnad, S.; Zemljič, L. Cellulose–Chitosan Functional Biocomposites. Polymers 2023, 15, 425.
- Yu, Z.; Ji, Y.; Bourg, V.; Bilgen, M.; Meredith, J. Chitin- and cellulose-based sustainable barrier materials: A review. Emergent Mater. 2020, 3, 919–936.
- Stach, M.; Kolniak-Ostek, J. The Influence of the Use of Different Polysaccharide Coatings on the Stability of Phenolic Compounds and Antioxidant Capacity of Chokeberry Hydrogel Microcapsules Obtained by Indirect Extrusion. Foods 2023, 12, 515.
- Júnior, L.; Gonçalves, S.; Silva, R.; Martins, J.; Vicente, A.; Alves, R.; Vieira, R. Effect of green propolis extract on functional properties of active pectin-based films. Food Hydrocoll. 2022, 131, 107746.
- Roman-Benn, A.; Contador, C.; Li, M.; Lam, H.; Ah-Hen, K.; Ulloa, P.; Ravanal, M. Pectin: An overview of sources, extraction and applications in food products and health. Food Chem. Adv. 2023, 2, 100192.
- Sun, X.; Xue, Z.; Sun, M.; Zhang, Y.; Zhang, Y.; Fu, H.; Zhang, Y.; Wang, P. Characterization of a Novel Alginate Lyase with Two Alginate Lyase Domains from the Marine Bacterium Vibrio sp. C42. Mar. Drugs 2022, 20, 746.
- Borchard, W.; Kenning, A.; Kapp, A.; Mayer, C. Phase diagram of the system sodium alginate/water: A model for biofilms. Int. J. Biol. Macromol. 2005, 35, 247–256.
- Annisa, V.; Sulaiman, T.; Nugroho, A.K.; Nugroho, A.G.; Kutsyk, R. Characterization of Alginate with Natural Polymers Combination for Drug Encapsulation. J. Pharm. Sci. 2022, 31, 150–159.
- Nehra, A.; Biswas, D.; Siracusa, V.; Roy, S. Natural Gum-Based Functional Bioactive Films and Coatings: A Review. Int. J. Mol. Sci. 2023, 24, 485.
- Prasad, N.; Thombare, N.; Sharma, S.; Kumar, S. Gum arabic—A versatile natural gum: A review on production, processing, properties and applications. Ind. Crops Prod. 2022, 187, 115304.
- Salehi, F. Edible Coating of Fruits and Vegetables Using Natural Gums: A Review. Int. J. Fruit Sci. 2020, 20, S570–S589.
- Jiang, H.; Zhang, W.; Chen, L.; Cao, J.; Jiang, W. Recent advances in guar gum-based films or coatings: Diverse property enhancement strategies and applications in foods. Food Hydrocoll. 2023, 136, 108278.
- Shubham; Mishra, N.; Dongariyal, A.; Rai, R.; Arya, M. Effect of edible coating and packaging on postharvest life and quality of litchi (Litchi chinensis Sonn.) fruits during storage. J. Pharmacogn. Phytochem. 2020, 9, 517–526.
- Agarwal, D.; Kim, E.; Feng, L.; Wade, C.; Moggré, G.; Morgenstern, M.; Hedderley, D. Microstructure, rheological and water mobility behaviour of plant-based protein isolates (pea and quinoa) and locust bean gum mixtures. Food Res. Int. 2023, 164, 112311.
- Eshghi, S.; Karimi, R.; Shiri, A.; Karami, M.; Moradi, M. The novel edible coating based on chitosan and gum ghatti to improve the quality and safety of ‘Rishbaba’ table grape during cold storage. J. Food Meas. Charact. 2021, 15, 3683–3693.
- Amani, F.; Rezaei, A.; Akbari, H.; Dima, C.; Jafari, S. Active Packaging Films Made by Complex Coacervation of Tragacanth Gum and Gelatin Loaded with Curcumin; Characterization and Antioxidant Activity. Foods 2022, 11, 3168.
- Boamah, P.; Afoakwah, N.; Onumah, J.; Osei, E.; Mahunu, G. Physicochemical Properties, Biological Properties and Applications of Gum Tragacanth—A Review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100288.
- Tafa, K.; Satheesh, N.; Abera, W. Mechanical properties of tef starch based edible films: Development and process optimization. Heliyon 2023, 9, e13160.
- Perera, K.; Prendeville, J.; Jaiswal, A.; Jaiswal, S. Cold Plasma Technology in Food Packaging. Coatings 2022, 12, 1896.
- Zhang, Z.; Fang, C.; Zhang, W.; Lei, W.; Wang, D.; Zhou, X. Novel grasshopper protein/soy protein isolate/pullulan ternary blend with hesperidin derivative for antimicrobial edible film. Arab. J. Chem. 2023, 16, 104563.
- Mostafavi, F.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176.
- Zhao, J.; Liu, T.; Xia, K.; Liu, X.; Zhang, X. Preparation and application of edible agar-based composite films modified by cellulose nanocrystals. Food Packag. Shelf Life 2022, 34, 100936.
- Yan, Y.; Duan, S.; Zhang, H.; Liu, Y.; Li, C.; Hu, B.; Liu, A.; Wu, D.; He, J.; Wu, W. Preparation and characterization of Konjac glucomannan and pullulan composite films for strawberry preservation. Carbohydr. Polym. 2020, 243, 116446.
- Ghosh, T.; Priyadarshi, R.; Souza, C.; Angioletti, B.; Rhim, J. Advances in pullulan utilization for sustainable applications in food packaging and preservation: A mini-review. Trends Food Sci. Technol. 2022, 125, 43–53.
- Priyadarhi, R.; Riahi, Z.; Rhim, J. Antioxidant pectin/pullulan edible coating incorporated with Vitis vinifera grape seed extract for extending the shelf life of Peanuts. Postharvest Biol. Technol. 2022, 183, 111740.
- Roy, S.; Rhim, J. Effect of chitosan modified halloysite on the physical and functional properties of pullulan/chitosan biofilm integrated with rutin. Appl. Clay Sci. 2021, 211, 106205.
- Gan, L.; Jiang, G.; Yang, Y.; Zheng, B.; Zhang, S.; Li, X.; Tian, Y.; Peng, B. Development and characterization of levan/pullulan/chitosan edible films enriched with ε-polylysine for active food packaging. Food Chem. 2022, 388, 132989.
- Kumar, N.; Neeraj; Ojha, A.; Singh, R. Preparation and characterization of chitosan—Pullulan blended edible films enrich with pomegranate peel extract. React. Funtional Polym. 2019, 144, 104350.
- Kanmani, P.; Lim, S. Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 2013, 141, 1041–1049.
- Xiao, Q.; Tong, Q.; Lim, L. Pullulan-sodium alginate based edible films: Rheological properties of film forming solutions. Carbohydr. Polym. 2012, 87, 1689–1695.
- Liu, J.; Zhang, L.; Liu, C.; Zheng, X.; Tang, K. Tuning structure and properties of gelatin edible films through pullulan dialdehyde crosslinking. LWT 2021, 138, 110607.
- Chen, J.; Zhang, J.; Liu, D.; Zhang, C.; Yi, H.; Liu, D. Preparation, characterization, and application of edible antibacterial three-layer films based on gelatin–chitosan–corn starch–incorporated nisin. Food Packag. Shelf Life 2022, 34, 100980.
- Jones, A.; Sharma, S. Surface and degradation properties of thermoplastic blends from albumin and zein-based plastics. J. Appl. Polym. Sci. 2017, 134, e44646.
- Liu, Z.; Cao, X.; Ren, S.; Wang, J.; Zhang, H. Physicochemical characterization of a zein prepared using a novel aqueous extraction technology and tensile properties of the zein film. Ind. Crops Prod. 2019, 130, 57–62.
- Tadele, D.; Shorey, R.; Mekonnen, T. Fatty acid modified zein films: Effect of fatty acid chain length on the processability and thermomechanical properties of modified zein films. Ind. Crops Prod. 2023, 192, 116028.
- Parlak, M.; Uzuner, K.; Kirac, F.; Ozdemir, S.; Dundar, A.; Sahin, O.; Dagdelen, A.; Saricaoglu, F. Production and characterization of biodegradable bi-layer films from poly(lactic) acid and zein. Int. J. Biol. Macromol. 2023, 227, 1027–1037.
- Robalo, J.; Lopes, M.; Cardoso, O.; Silva, A.; Ramos, F. Efficacy of Whey Protein Film Incorporated with Portuguese Green Tea (Camellia sinensis L.). Extract for the Preservation of Latin-Style Fresh Cheese. Foods 2022, 11, 1158.
- Xin, Y.; Yang, C.; Zhang, J.; Xiong, L. Application of Whey Protein-Based Emulsion Coating Treatment in Fresh-Cut Apple Preservation. Foods 2023, 12, 1140.
- Tonyali, B.; Cikrikci, S.; Oztop, M. Physicochemical and microstructural characterization of gum tragacanth added whey protein based films. Food Res. Int. 2018, 105, 1–9.
- Ramos, Ó.; Reinas, I.; Silva, S.; Fernandes, J.; Cerqueira, M.; Pereira, R.; Vicente, A.; Poças, M.; Pintado, M.; Malcata, F. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122.
- Diyana, Z.; Jumaidin, R.; Selamat, M.; Suan, M. Thermoplastic starch/beeswax blend: Characterization on thermal mechanical and moisture absorption properties. Int. J. Biol. Macromol. 2021, 190, 224–232.
- György, É.; Laslo, É.; Salamon, B. Antimicrobial impacts of selected Lamiaceae plants on bacteria isolated from vegetables and their application in edible films. Food Biosci. 2023, 51, 102280.
- Prasetyaningrum, A.; Utomo, D.; Raemas, A.; Kusworo, T.; Jos, B.; Djaeni, M. Alginate/κ-Carrageenan-Based Edible Films Incorporated with Clove Essential Oil: Physico-Chemical Characterization and Antioxidant-Antimicrobial Activity. Polymers 2021, 13, 354.
- Liu, Z.; Lin, D.; Shen, R.; Zhang, R.; Liu, L.; Yang, X. Konjac glucomannan-based edible films loaded with thyme essential oil: Physical properties and antioxidant-antibacterial activities. Food Packag. Shelf Life 2021, 29, 100700.
- Matadamas-Ortiz, A.; Hernández- Hernández, E.; Castaño-Tostado, E.; Amaro-Reyes, A.; García-Almendárez, B.; Velasquez, G.; Regalado-González, C. Long-Term Refrigerated Storage of Beef Using an Active Edible Film Reinforced with Mesoporous Silica Nanoparticles Containing Oregano Essential Oil (Lippia graveolens Kunth). Mol. Sci. 2023, 24, 92.
- Farina, V.; Passafiume, R.; Tinebra, I.; Palazzolo, E.; Sortino, G. Use of Aloe Vera Gel-Based Edible Coating with Natural Anti-Browning and Antioxidant Additives to Improve Post-Harvest Quality of Fresh-Cut ‘Fuji’ Apple. Agronomy 2020, 10, 515.
- Al-Harrasi, A.; Bhtaia, S.; Al-Azrii, M.; Makeen, H.; Albratty, M.; Alhazmi, H.; Mohan, S.; Sharma, A.; Behl, T. Correction: Al-Harrasi et al. Development and Characterization of Chitosan and Porphyran Based Composite Edible Films Containing Ginger Essential Oil. Polymers 2022, 14, 2518.
- Tsironi, M.; Kosma, I.; Badeka, A. The Effect of Whey Protein Films with Ginger and Rosemary Essential Oils on Microbiological Quality and Physicochemical Properties of Minced Lamb Meat. Sustainability 2022, 14, 3434.
- Ansarian, E.; Aminzare, M.; Azar, H.; Mehrasbi, M.; Bimakr, M. Nanoemulsion-based basil seed gum edible film containing resveratrol and clove essential oil: In vitro antioxidant properties and its effect on oxidative stability and sensory characteristic of camel meat during refrigeration storage. Meat Sci. 2022, 185, 108716.
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236.
- Kawhena, T.; Opara, U.; Fawole, O. Effects of Gum Arabic Coatings Enriched with Lemongrass Essential Oil and Pomegranate Peel Extract on Quality Maintenance of Pomegranate Whole Fruit and Arils. Foods 2022, 11, 593.
- Jati, I.; Setijawaty, E.; Utomo, A.; Darmoatmodjo, L. The Application of Aloe vera Gel as Coating Agent to Maintain the Quality of Tomatoes during Storage. Coatings 2022, 12, 1480.
- Safari, Z.; Ding, P.; Nakasha, J.; Yusoff, S. Controlling Fusarium oxysporum Tomato Fruit Rot under Tropical Condition Using Both Chitosan and Vanillin. Coatings 2021, 11, 367.
- Zhang, W.; Roy, S.; Ezati, P.; Yang, D.; Rhim, J. Tannic acid: A green crosslinker for biopolymer-based food packaging films. Trends Food Sci. Technol. 2023, 136, 11–23.
- Garg, R.; Rana, H.; Singh, N.; Goswami, S. Guargum/nanocellulose based novel crosslinked antimicrobial film with enhanced barrier and mechanical properties for food packaging. J. Environ. Chem. Eng. 2023, 11, 109254.
- Venkatesan, R.; Sivaprakash, P.; Kim, I.; Eldesoky, G.; Kim, S. Tannic acid as a crosslinking agent in poly(butylene adipate-co-terephthalate) composite films enhanced with carbon nanoparticles: Processing, characterization, and antimicrobial activities for food packaging. J. Environ. Chem. Eng. 2023, 11, 110194.
- Cabezas, R.; Zurob, E.; Gomez, B.; Merlet, G.; Plaza, A.; Araya-Lopez, C.; Romero, J.; Olea, F.; Quijada-Maldonado, Q.; Pino-Soto, L.; et al. Challenges and Possibilities of Deep Eutectic Solvent-Based Membranes. Ind. Eng. Chem. Res. 2022, 61, 17397–17422.
- Meenu, M.; Bansal, V.; Rana, S.; Sharma, N.; Kumar, V.; Arora, V.; Garg, M. Deep eutectic solvents (DESs) and natural deep eutectic solvents (NADESs): Designer solvents for green extraction of anthocyanin. Sustain. Chem. Pharm. 2023, 34, 101168.
- Yu, J.; Liu, X.; Xu, S.; Shao, P.; Li, J.; Chen, Z.; Wang, X.; Lin, Y.; Renard, C. Advances in green solvents for production of polysaccharide-based packaging films: Insights of ionic liquids and deep eutectic solvents. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1030–1057.
- Lin, Z.; Lialu, X.; Jiao, B. Deep eutectic solvents-modified advanced functional materials for pollutant detection in food and the environment. TrAC Trends Anal. Chem. 2023, 159, 116923.
- Teixeira-Costa, B.; Andrade, C. Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging. Biomolecules 2021, 11, 1599.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.4K
Revisions:
2 times
(View History)
Update Date:
15 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




