Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniele Lana | -- | 2238 | 2023-09-12 11:22:09 | | | |
| 2 | Catherine Yang | Meta information modification | 2238 | 2023-09-13 03:03:01 | | | | |
| 3 | Catherine Yang | -3 word(s) | 2235 | 2023-09-14 10:17:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lana, D.; Magni, G.; Landucci, E.; Wenk, G.L.; Pellegrini-Giampietro, D.E.; Giovannini, M.G. Microglia Phenomics in Alzheimer’s Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/49059 (accessed on 08 February 2026).
Lana D, Magni G, Landucci E, Wenk GL, Pellegrini-Giampietro DE, Giovannini MG. Microglia Phenomics in Alzheimer’s Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/49059. Accessed February 08, 2026.
Lana, Daniele, Giada Magni, Elisa Landucci, Gary L. Wenk, Domenico Edoardo Pellegrini-Giampietro, Maria Grazia Giovannini. "Microglia Phenomics in Alzheimer’s Disease" Encyclopedia, https://encyclopedia.pub/entry/49059 (accessed February 08, 2026).
Lana, D., Magni, G., Landucci, E., Wenk, G.L., Pellegrini-Giampietro, D.E., & Giovannini, M.G. (2023, September 12). Microglia Phenomics in Alzheimer’s Disease. In Encyclopedia. https://encyclopedia.pub/entry/49059
Lana, Daniele, et al. "Microglia Phenomics in Alzheimer’s Disease." Encyclopedia. Web. 12 September, 2023.
Copy Citation
Phenomics, the complexity of microglia phenotypes and their related functions compels the continuous study of microglia in disease animal models to find druggable targets for neurodegenerative disorders. Activation of microglia was long considered detrimental for neuron survival, but it has become apparent that the real scenario of microglia morphofunctional diversity is far more complex.
Alzheimer’s disease
Hippocampus CA1
Hippocampus CA3
α7AChNR
Aβ plaque
rod microglia
1. Introduction
For over a century, the brain was seen as a network of neurons, dendrites, axons, and synapses in a space embedded by other cells which, like glue, fill the empty space. Nevertheless, proper recruitment, activation, and intercommunication among neurons and glia is of fundamental importance for the functional organization of the brain. The research is now focused on understanding how microglia engage in morphological, ultrastructural, transcriptional, proteomic, and epigenetic switches that influence their functions, their responses, and their effects on the surrounding cells, suggesting that microglia states are modulated by local cues (for a comprehensive review, see [1]).
While for most of the last century “activation” of microglia was considered solely detrimental for neuron survival, it was later demonstrated that microglia are fundamental in early brain development (for ref., see [2]), for synaptogenesis, synaptic maintenance, maturation and synaptic pruning (for ref., see [3]), and in normal learning and memory in mice [4][5][6][7].
Microglia (5–10% of brain cells in number) are myeloid cells that invade the brain early during development [8][9] and coordinate the interactions between the immune system and cognitive functions [3][10][11][12][13][14][15][16][17][18]. Early studies in adult mice demonstrate that microglia acquire diverse morphologies in different brain areas, from radially orientated arborized cells in the grey matter to longitudinally branched elongated cells in the white matter and compact amoeboid cells around the circumventricular organs [19]. For many years, ramified microglia (Figure 1) were considered quiescent or ‘resting’, while they are now considered “homeostatic” [1].
In healthy conditions, microglia have small soma and fine, highly mobile ramified branches, which dynamically reorganize their shape and length, continuously elongating and withdrawing to patrol a defined, non-overlapping territory of the brain parenchyma [20] to detect and eliminate damaged neurons and maintain a healthy environment [21][22][23][24]. Recent studies have described hyper-ramified microglia (Figure 1D) in the medial prefrontal cortex of rats in response to chronic stress [25][26].
The ramified branches of microglia work as chemotactic sensors, moving their extensions towards injured cells for phagocytosis [27], and the impairment of their mobility can be deleterious in many conditions. Decreased migration of microglia hampers their phagocytic efficacy, increasing the degeneration of neurons and accumulation of toxic debris [28], and weakening microglia neuroprotective effects. Activation of microglia is a quick process that leads to morphological, phenotypic, and functional changes that stimulate the migration of microglia to the damaged brain area. Reactive microglia, after having fulfilled their phagocytotic function, regress rapidly to the homeostatic form [24]. Furthermore, during apoptotic clearance, spherical phagocytic pouches (ball) are formed at the tip of microglia terminal branches (chain), the so-called ball-and-chain structures (Figure 1C), which can phagocytose apoptotic debris [29] or a small quantity of other substances [29], with no modification of ramified morphology, in contrast to the phagocytosis performed by amoeboid microglia in pathological conditions (Figure 1E) [12]. In mouse organotypic hippocampal slices, ramified microglia exert neuroprotective effects during NMDA-induced excitotoxicity [30].
Microglia are plastic cells, and an oversimplified, already surpassed view, recognized two functional phenomic extremes acquired in response to cytokines, chemokines, and other soluble factors, namely the classical M1 pro-inflammatory and the M2 anti-inflammatory phenotypes [12][27][31][32][33][34][35]. M1 cells were classified as reactive cells that release pro-inflammatory cytokines, such as TNFα, IL-1, IL-6, and IL-18 [36], and have harmful properties. M2 were classified as non-reactive cells that secrete anti-inflammatory cytokines, such as IL-4, IL-10, IL-13, and TNF-ß, and have beneficial, neuroprotective properties, [36].
Nevertheless, the classification of microglia in these two all-or-none states [37] is too simplistic and does not correspond to the variety of different microglia phenotypes recently discovered [38][39]. Between these two extreme functional states, a plethora of phenotypically diverse intermediates with different functional states is now recognized. Indeed, a recent hypothesis based on accumulating data postulates that microglia, as neurons, exist physiologically as heterogeneous, mixed populations, which differ in their transcriptomic and morphofunctional characteristics. Consequently, microglia may exist in n possible phenomic states, diverse in health and disease conditions, and which depend not only on the type and intensity of insult and on the progression of the disease, but also on the brain structure where microglia are located [37][40][41][42][43][44]. The vast array of receptors expressed by microglia constantly surveying their surroundings constitutes a ‘sensome’, allowing detection and response to different stimuli that derive from sensory and behavioral experiences [1][45][46].

Figure 1. Diversity of microglia morphologies in the hippocampus. (A) Ramified, homeostatic microglia. IBA1: green (modified from [47]). Bar: 5 µm. (B) Jellyfish microglia in the ischemic CA1. IBA1: green, major histocompatibility complex type II (MHC II): red; merge: yellow–orange (modified from [48]). Bar: 2.5 µm. (C) Ball-and-chain-like structures at the end of microglia branches that can phagocytose small amounts of material. IBA1: green (modified from [49]). Bar: 5 µm. (D) Train of rod microglia in response to ischemia. IBA1: green; nuclei: blue (modified from [48]). Bar: 10 µm. (E) Phagoptotic microglia. IBA1: green; NeuN: red (modified from [50]). Bar: 5 µm. (F) Trogocytotic microglia. IBA1: green; NeuN: red (modified from [50]). Bar: 5 µm. (G) Amoeboid microglia with rounded morphology and phagocytosing pyknotic neurons after ischemia. IBA1: green; NeuN: red (modified from [48]). Bar: 10 µm. (H) Hyper-ramified microglia in CA1 of TGCRND8 mice. CD68: red (modified from [49]). Bar: 5 µm.
Microglia are anything but static, and, since they are exceptionally responsive to alterations in the surrounding environment, the states of microglia vary as a continuum rather than an all-or-none phenomenon. It will be interesting to understand whether microglia located in different brain areas acquire identical phenomics [51][52], or whether they react differently to the same insult. For instance, in the CA1 and CA3 hippocampus, microglia reactivity states are different in aging and acute inflammation [47][53], or after an excitotoxic insult [30]. Indeed, Vinet and colleagues [30] demonstrated that ramified microglia exert neuroprotective roles in pathologic processes, and that this function is region-specific, at least in the hippocampal areas. Region-specific differences in lysosome content and membrane properties of microglia [38][54], as well as the expression of genes related to the phagocytic capacity of microglia, have recently been demonstrated [55]. Furthermore, using scRNA-seq, it has been demonstrated that microglia populations display higher transcriptomic diversity in the developing, aged, and diseased brain [56][57] than in the adult brain. Many different receptors with a variety of functions are expressed by microglia. Their expression, and the outcome of their activation, depend not only on the pathological conditions, but also on the functional state of the cell. Depending on the nature of the ligand and on the receptor, downstream intracellular pathways translate their activation with detrimental or beneficial effects (for references, see [58]). The dysfunction of microglia has been described in many CNS disorders, such as AD [59], frontotemporal dementia [60][61], and PD [62].
2. Microglia Phenomics in Alzheimer’s Disease
Data from animal models of AD show that microglia are recruited at the site of Aβ deposition, and regulate Aβ levels in the brain [49], contributing to Aβ clearance and removal of cytotoxic debris from the brain [27][63][64][65][66]. Plaque-associated microglia inhibit additional fibrillization of Aβ and plaque growth [67], thus, protecting neighboring neurons [68]. However, the phagocytic activity and clearance capacity of microglia inversely correlate with Aβ plaque deposition and aging [69].
Microglia responses in AD are influenced by APOE and TREM2 [70]. TREM2 regulates microglia energetic and biosynthetic metabolism [71], maintaining the high activity microglia need to dispose of excess Aβ. However, the intense TREM2-dependent activation of microglia may in turn cause a harmful chronic inflammatory response of TREM2 [70]. Sustained activation of microglia can also cause phagoptosis of healthy neurons [64][72][73][74] intensifying neurodegeneration [75][76], and can cause phagocytosis of synapses in response to soluble Aβ [77], while microglia depletion prevents loss of neurons and dendritic spines [78], further suggesting a pathogenic role for microglia hyperactivation in AD. Variants of TREM2 impair microglia activation, phagocytic properties, inflammatory responses, energy metabolism, and plaque compaction, affecting the progression of AD [71][79][80]. Toll-like receptor 4 (TLR4) in microglia plays an important role in neuroinflammation [81], but studies on TLR4- and on TREM2-deficient mice give conflicting results on AD pathology [82]. In TREM2 deficient mice, loss of microglia clustering around Aβ plaques increases AD risk, supporting the idea that microglia are protective [68]. Nevertheless, it has also been shown that microglia associated with Aβ plaques have a neurodegenerative phenomic, regulated by the TREM2-APOE pathway, which suppresses the phagocytosis of apoptotic neurons [70]. TLR4, stimulated by both fibrillar and oligomeric forms of Aβ [83], seems to be protective [84]. Further, stimulation of Toll-like receptor 2 (TLR2) by fibrillar Aβ activates microglia into a more pro-inflammatory profile, with detrimental effects on AD pathology [83].
The age-related impairment of chemotactic sensors may weaken the neuroprotective activity of microglia which phagocytose Aβ fibrils less efficiently in old than in young mice [85]. On the contrary, amplified, exaggerated, or chronic microglia activation can lead to robust pathological changes and neurobehavioral complications, such as in chronic inflammatory diseases [39][86].
In both AD transgenic mice and human AD brains, a unique subtype of protective microglia, named disease-associated microglia (DAM), has recently been found [39]. DAM contribute to disease mitigation by expressing many factors that enhance microglia phagocytic activity [39], which do not represent the primary cause of the disease, per se, but do affect AD time-course and progression rate. The functions of the genes expressed by DAM are first TREM2-independent and later TREM2-dependent [39], and the transition to fully-activated DAM does not occur in the absence of TREM2 receptors. Increased expression of TREM2 during this stage is a defensive factor linked to Aβ clearance [39][87], indicating that TREM2 is necessary to support phagocytosis at a late stage of the disease. In more advanced stages of the disease, TREM2-expressing microglia, interacting with accumulating neurofibrillary tangles, cause extensive inflammation and neurodegeneration [88][89], but the absence of TREM2 in microglia at this stage of AD, but not at earlier stages, exacerbates AD symptomatology [90][91]. Complement C3, responsible for excessive release of pro-inflammatory mediators and induction of reactive astrocytes [37], is one of the most highly upregulated genes involved in this microglia reaction [92]. Distinct microglia subpopulations, located in different brain areas, seem to have different roles at different times in disease progression [39].
Dark microglia, immunonegative for the homeostatic microglial marker P2RY12 and weakly positive for CX3CR1 and IBA1, were identified from their condensed cytoplasm and nucleoplasm, and for their dilated endoplasmic reticulum and altered mitochondria, which make them distinguishable from typical microglia. Dark microglia interact with blood vessels, axon terminals, and dystrophic dendritic spines, and are highly immunoreactive for CD11b and TREM2 [93]. Dark microglia are found near fibrillar Aβ, near Aβ plaques and dystrophic neurites in the CA1 stratum lacunosum moleculare of the ventral hippocampus of the transgenic strain APP/PS1 [94][95]. Nevertheless, the exact role of dark microglia in the pathogenesis of AD remains unclear [93].
In the hippocampus, microglia have a high “immune-vigilant” phenotype that can be responsible for their higher activation in response to Aβ plaque formation, giving rise to a harmful chronic inflammatory response [79]. Furthermore, hippocampal microglia display lower expression of many proteins, including CXCR3 [81], one receptor involved in neuron–microglia communication, and in microglia recruitment, neuronal reorganization [82], and microglia activation during demyelination [83], as described above. Therefore, decreased levels of the CXCR3 receptor and other proteins in AD-vulnerable brain regions may impair microglia response and recruitment.
Microglia express α7nAChR [96] that possibly drive the “cholinergic anti-inflammatory pathway” that regulates systemic inflammatory responses [97]. In CA1 and CA3 of TGCRND8 mice, a transgenic model of AD, microglia surrounding Aβ plaques (plaque activated microglia, PAM) have a different phenotype and show differential expression of α7nAChR (Figure 2). In TGCRND8 mice, microglia in CA1 are bigger, more reactive, and express higher levels of α7nAChR than in WT mice and in CA3 of Tg mice (Figure 2). In CA3, microglia show a ramified state and express α7nAChR (Figure 2B), while in CA1 microglia have a round cell body with shorter branching, and the expression of α7nAChR is more intense (Figure 2C). This differential response of microglia in CA1 and CA3 around plaques confirms one more time the differential spatial states of the cells. Hart and colleagues [98] showed a further regional difference between microglia located in the white matter versus microglia located in the grey matter [80]. Region-specific variations in gene expression (both increases and decreases) may be implicated in the progression or in the resolution of neurodegenerative diseases [99].
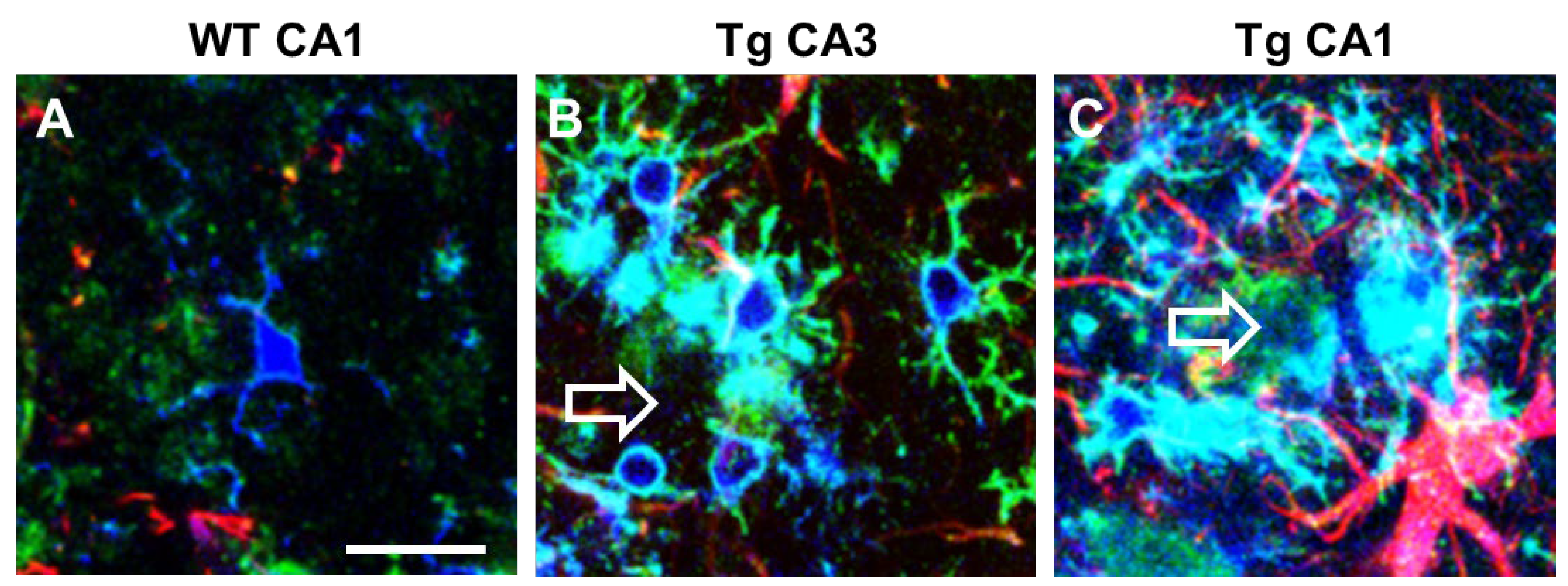
Figure 2. Intense upregulation of α7AChNR in reactive plaque-associated microglia in CA1 and CA3 of TgCRND8 mice. Comparison with WT mice. Anti IBA1 antibody (blue), anti α7AChNR antibody (green), anti GFAP antibody (red). Empty arrows indicate Aβ plaques. (A) A microglia cell with faint α7AChNR immunofluorescence in CA1 hippocampus of WT mouse. (B) Ramified plaque-associated microglia with intense α7AChNR immunofluorescence in CA3 of a TgCRND8 mouse. (C) Amoeboid plaque-associated microglia with very intense α7AChNR immunofluorescence in CA1 of a TgCRND8 mouse. Astrocytes are also visible in red. Scale bar: 20 µm (from [100]).
In the hippocampus of AD patients, the subregional pattern of atrophy is different from other groups of neurodegenerative conditions [101][102], and understanding the molecular and cellular mechanisms that lead to such a subregional vulnerability could unveil therapeutic strategies to alleviate the progression of memory decline. Genome-wide association studies (GWAS) identified AD onset risk loci that are associated with genes involved in microglia physiology and responses, such as CR1 (complement receptor type 1), SPI1 (transcription factor PU.1), TREM2 (triggering receptor expressed on myeloid cells 2), and CD33 [103].
Boosting microglia defensive capabilities with cell-specific therapies may offer new avenues for preventing or reversing neurodegeneration. Further work is needed to demonstrate and dissect these features.
References
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483.
- Salter, M.W.; Beggs, S. Sublime microglia: Expanding roles for the guardians of the CNS. Cell 2014, 158, 15–24.
- Schafer, D.P.; Stevens, B. Phagocytic glial cells: Sculpting synaptic circuits in the developing nervous system. Curr. Opin. Neurobiol. 2013, 23, 1034–1040.
- Derecki, N.C.; Cardani, A.N.; Yang, C.H.; Quinnies, K.M.; Crihfield, A.; Lynch, K.R.; Kipnis, J. Regulation of learning and memory by meningeal immunity: A key role for IL-4. J. Exp. Med. 2010, 207, 1067–1080.
- Ziv, Y.; Ron, N.; Butovsky, O.; Landa, G.; Sudai, E.; Greenberg, N.; Cohen, H.; Kipnis, J.; Schwartz, M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006, 9, 268–275.
- Pfrieger, F.W. Roles of glial cells in synapse development. Cell Mol. Life Sci. 2009, 66, 2037–2047.
- Heneka, M.T.; Rodríguez, J.J.; Verkhratsky, A. Neuroglia in neurodegeneration. Brain Res. Rev. 2010, 63, 189–211.
- Frost, J.L.; Schafer, D.P. Microglia: Architects of the Developing Nervous System. Trends Cell Biol. 2016, 26, 587–597.
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161.
- Boche, D.; Perry, V.H.; Nicoll, J.A.R. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18.
- Fetler, L.; Amigorena, S. Brain under surveillance: The microglia patrol. Science 2005, 309, 392–393.
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New Roles for the Synaptic Stripper. Neuron 2013, 77, 10–18.
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458.
- Wake, H.; Moorhouse, A.J.; Miyamoto, A.; Nabekura, J. Microglia: Actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 2013, 36, 209–217.
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R.; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609.
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705.
- Fitzner, D.; Schnaars, M.; Van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.K.; Simons, M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011, 124, 447–458.
- Safaiyan, S.; Kannaiyan, N.; Snaidero, N.; Brioschi, S.; Biber, K.; Yona, S.; Edinger, A.L.; Jung, S.; Rossner, M.J.; Simons, M. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 2016, 19, 995–998.
- Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990, 39, 151–170.
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Neuroscience: Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318.
- Koenigsknecht-Talboo, J.; Landreth, G.E. Microglial phagocytosis induced by fibrillar β-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 2005, 25, 8240–8249.
- Neumann, J.; Gunzer, M.; Gutzeit, H.O.; Ullrich, O.; Reymann, K.G.; Dinkel, K. Microglia provide neuroprotection after ischemia. FASEB J. 2006, 20, 714–716.
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758.
- Morsch, M.; Radford, R.; Lee, A.; Don, E.K.; Badrock, A.P.; Hall, T.E.; Cole, N.J.; Chung, R. In vivo characterization of microglial engulfment of dying neurons in the zebrafish spinal cord. Front. Cell. Neurosci. 2015, 9, 321.
- Hinwood, M.; Morandini, J.; Day, T.A.; Walker, F.R. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb. Cortex 2012, 22, 1442–1454.
- Hinwood, M.; Tynan, R.J.; Charnley, J.L.; Beynon, S.B.; Day, T.A.; Walker, F.R. Chronic stress induced remodeling of the prefrontal cortex: Structural re-organization of microglia and the inhibitory effect of minocycline. Cereb. Cortex 2013, 23, 1784–1797.
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394.
- Tian, L.; Ma, L.; Kaarela, T.; Li, Z. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. J. Neuroinflamm. 2012, 9, 155.
- Sierra, A.; Encinas, J.M.; Deudero, J.J.P.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495.
- Vinet, J.; van Weering, H.R.J.; Heinrich, A.; Kälin, R.E.; Wegner, A.; Brouwer, N.; Heppner, F.L.; van Rooijen, N.; Boddeke, H.W.G.M.; Biber, K. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J. Neuroinflamm. 2012, 9, 27.
- Ransohoff, R.M.; Perry, V.H. Microglial Physiology: Unique Stimuli, Specialized Responses. Annu. Rev. Immunol. 2009, 27, 119–145.
- Franco, R.; Fernández-Suárez, D. Alternatively activated microglia and macrophages in the central nervous system. Prog. Neurobiol. 2015, 131, 65–86.
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—New prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64.
- Jiang, X.; Pu, H.; Hu, X.; Wei, Z.; Hong, D.; Zhang, W.; Gao, Y.; Chen, J.; Shi, Y. A Post-stroke Therapeutic Regimen with Omega-3 Polyunsaturated Fatty Acids that Promotes White Matter Integrity and Beneficial Microglial Responses after Cerebral Ischemia. Transl. Stroke Res. 2016, 7, 548–561.
- Xiong, X.Y.; Liu, L.; Yang, Q.W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44.
- Allen, N.J.; Barres, B.A. Neuroscience: Glia—More than just brain glue. Nature 2009, 457, 675–677.
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967.
- De Biase, L.M.; Schuebel, K.E.; Fusfeld, Z.H.; Jair, K.; Hawes, I.A.; Cimbro, R.; Zhang, H.Y.; Liu, Q.R.; Shen, H.; Xi, Z.X.; et al. Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 2017, 95, 341–356.e6.
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17.
- Zhang, Y.; Barres, B.A. Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010, 20, 588–594.
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952.
- Ben Haim, L.; Rowitch, D.H. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 2016, 18, 31–41.
- Khakh, B.S.; Deneen, B. The Emerging Nature of Astrocyte Diversity. Annu. Rev. Neurosci. 2019, 42, 187–207.
- Pestana, F.; Edwards-Faret, G.; Belgard, T.G.; Martirosyan, A.; Holt, M.G. No longer underappreciated: The emerging concept of astrocyte heterogeneity in neuroscience. Brain Sci. 2020, 10, 168.
- Hughes, J.L.; Jones, P.S.; Beech, J.S.; Wang, D.; Menon, D.K.; Aigbirhio, F.I.; Fryer, T.D.; Baron, J.C. A microPET study of the regional distribution of -PK11195 binding following temporary focal cerebral ischemia in the rat. Correlation with post mortem mapping of microglia activation. Neuroimage 2012, 59, 2007–2016.
- De Felice, E.; Gonçalves de Andrade, E.; Golia, M.T.; González Ibáñez, F.; Khakpour, M.; Di Castro, M.A.; Garofalo, S.; Di Pietro, E.; Benatti, C.; Brunello, N.; et al. Microglial diversity along the hippocampal longitudinal axis impacts synaptic plasticity in adult male mice under homeostatic conditions. J. Neuroinflamm. 2022, 19, 292.
- Cerbai, F.; Lana, D.; Nosi, D.; Petkova-Kirova, P.; Zecchi, S.; Brothers, H.M.; Wenk, G.L.; Giovannini, M.G. The Neuron-Astrocyte-Microglia Triad in Normal Brain Ageing and in a Model of Neuroinflammation in the Rat Hippocampus. PLoS ONE 2012, 7, e45250.
- Lana, D.; Gerace, E.; Magni, G.; Cialdai, F.; Monici, M.; Mannaioni, G.; Giovannini, M.G. Hypoxia/Ischemia-Induced Rod Microglia Phenotype in CA1 Hippocampal Slices. Int. J. Mol. Sci. 2022, 23, 1422.
- Ugolini, F.; Lana, D.; Nardiello, P.; Nosi, D.; Pantano, D.; Casamenti, F.; Giovannini, M.G. Different Patterns of Neurodegeneration and Glia Activation in CA1 and CA3 Hippocampal Regions of TgCRND8 Mice. Front. Aging Neurosci. 2018, 10, 372.
- Lana, D.; Melani, A.; Pugliese, A.M.; Cipriani, S.; Nosi, D.; Pedata, F.; Giovannini, M.G. The neuron-astrocyte-microglia triad in a rat model of chronic cerebral hypoperfusion: Protective effect of dipyridamole. Front. Aging Neurosci. 2014, 6, 322.
- Martín-López, E.; García-Marques, J.; Núñez-Llaves, R.; López-Mascaraque, L. Clonal Astrocytic Response to Cortical Injury. PLoS ONE 2013, 8, e74039.
- Bribian, A.; Pérez-Cerdá, F.; Matute, C.; López-Mascaraque, L. Clonal glial response in a multiple sclerosis mouse model. Front. Cell. Neurosci. 2018, 12, 375.
- Lana, D.; Iovino, L.; Nosi, D.; Wenk, G.L.; Giovannini, M.G. The neuron-astrocyte-microglia triad involvement in neuroinflammaging mechanisms in the CA3 hippocampus of memory-impaired aged rats. Exp. Gerontol. 2016, 83, 71–88.
- De Biase, L.M.; Bonci, A. Region-Specific Phenotypes of Microglia: The Role of Local Regulatory Cues. Neuroscientist 2019, 25, 314–333.
- Ayata, P.; Badimon, A.; Strasburger, H.J.; Duff, M.K.; Montgomery, S.E.; Loh, Y.H.E.; Ebert, A.; Pimenova, A.A.; Ramirez, B.R.; Chan, A.T.; et al. Epigenetic regulation of brain region-specific microglia clearance activity. Nat. Neurosci. 2018, 21, 1049–1060.
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271.e6.
- Singh, N.; Benoit, M.R.; Zhou, J.; Das, B.; Davila-Velderrain, J.; Kellis, M.; Tsai, L.H.; Hu, X.; Yan, R. BACE-1 inhibition facilitates the transition from homeostatic microglia to DAM-1. Sci. Adv. 2022, 8, eabo1286.
- Gomes-Leal, W. Microglial physiopathology: How to explain the dual role of microglia after acute neural disorders? Brain Behav. 2012, 2, 345–356.
- Henneman, W.J.P.; Sluimer, J.D.; Barnes, J.; Van Der Flier, W.M.; Sluimer, I.C.; Fox, N.C.; Scheltens, P.; Vrenken, H.; Barkhof, F. Hippocampal atrophy rates in Alzheimer disease: Added value over whole brain volume measures. Neurology 2009, 72, 999–1007.
- Cagnin, A.; Rossor, M.; Sampson, E.L.; MacKinnon, T.; Banati, R.B. In vivo detection of microglial activation in frontotemporal dementia. Ann. Neurol. 2004, 56, 894–897.
- Rosso, S.M.; Landweer, E.J.; Houterman, M.; Donker Kaat, L.; Van Duijn, C.M.; Van Swieten, J.C. Medical and environmental risk factors for sporadic frontotemporal dementia: A retrospective case-control study. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1574–1576.
- Perry, V.H. Innate inflammation in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009373.
- Fu, R.; Shen, Q.; Xu, P.; Luo, J.J.; Tang, Y. Phagocytosis of microglia in the central nervous system diseases. Mol. Neurobiol. 2014, 49, 1422–1434.
- Vilalta, A.; Brown, G.C. Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J. 2018, 285, 3566–3575.
- ElAli, A.; Rivest, S. Microglia in Alzheimer’s disease: A multifaceted relationship. Brain. Behav. Immun. 2016, 55, 138–150.
- Daria, A.; Colombo, A.; Llovera, G.; Hampel, H.; Willem, M.; Liesz, A.; Haass, C.; Tahirovic, S. Young microglia restore amyloid plaque clearance of aged microglia. EMBO J. 2017, 36, 583–603.
- Bolmont, T.; Haiss, F.; Eicke, D.; Radde, R.; Mathis, C.A.; Klunk, W.E.; Kohsaka, S.; Jucker, M.; Calhoun, M.E. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J. Neurosci. 2008, 28, 4283–4292.
- Zhou, Y.; Ulland, T.K.; Colonna, M. TREM2-dependent effects on microglia in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 202.
- Blume, T.; Focke, C.; Peters, F.; Deussing, M.; Albert, N.L.; Lindner, S.; Gildehaus, F.J.; Von Ungern-Sternberg, B.; Ozmen, L.; Baumann, K.; et al. Microglial response to increasing amyloid load saturates with aging: A longitudinal dual tracer in vivo µpET-study 11 Medical and Health Sciences 1109 Neurosciences. J. Neuroinflamm. 2018, 15, 307.
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.e9.
- Ulland, T.K.; Song, W.M.; Huang, S.C.C.; Ulrich, J.D.; Sergushichev, A.; Beatty, W.L.; Loboda, A.A.; Zhou, Y.; Cairns, N.J.; Kambal, A.; et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 2017, 170, 649–663.e13.
- Brown, G.C.; Neher, J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014, 15, 209–216.
- Neher, J.J.; Neniskyte, U.; Zhao, J.-W.; Bal-Price, A.; Tolkovsky, A.M.; Brown, G.C. Inhibition of Microglial Phagocytosis Is Sufficient To Prevent Inflammatory Neuronal Death. J. Immunol. 2011, 186, 4973–4983.
- Neher, J.J.; Neniskyte, U.; Brown, G.C. Primary phagocytosis of neurons by inflamed microglia: Potential roles in neurodegeneration. Front. Pharmacol. 2012, 3, 27.
- Deleidi, M.; Jäggle, M.; Rubino, G. Immune ageing, dysmetabolism and inflammation in neurological diseases. Front. Neurosci. 2015, 9, 172.
- Giunta, B.; Fernandez, F.; Nikolic, W.V.; Obregon, D.; Rrapo, E.; Town, T.; Tan, J. Inflammaging as a prodrome to Alzheimer’s disease. J. Neuroinflamm. 2008, 5, 51.
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716.
- Spangenberg, E.E.; Lee, R.J.; Najafi, A.R.; Rice, R.A.; Elmore, M.R.P.; Blurton-Jones, M.; West, B.L.; Green, K.N. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β pathology. Brain 2016, 139, 1265–1281.
- Yin, Z.; Raj, D.; Saiepour, N.; Van Dam, D.; Brouwer, N.; Holtman, I.R.; Eggen, B.J.L.; Möller, T.; Tamm, J.A.; Abdourahman, A.; et al. Immune hyperreactivity of Aβ plaque-associated microglia in Alzheimer’s disease. Neurobiol. Aging 2017, 55, 115–122.
- Zhong, L.; Chen, X.F.; Wang, T.; Wang, Z.; Liao, C.; Wang, Z.; Huang, R.; Wang, D.; Li, X.; Wu, L.; et al. Soluble TREM2 induces inflammatory responses and enhances microglial survival. J. Exp. Med. 2017, 214, 597–607.
- Zhang, X.; Dong, H.; Zhang, S.; Lu, S.; Sun, J.; Qian, Y. Enhancement of LPS-induced microglial inflammation response via TLR4 under high glucose conditions. Cell. Physiol. Biochem. 2015, 35, 1571–1581.
- Jay, T.R.; Hirsch, A.M.; Broihier, M.L.; Miller, C.M.; Neilson, L.E.; Ransohoff, R.M.; Lamb, B.T.; Landreth, G.E. Disease progression-dependent effects of TREM2 deficiency in a mouse model of Alzheimer’s disease. J. Neurosci. 2017, 37, 637–647.
- Dansokho, C.; Heneka, M.T. Neuroinflammatory responses in Alzheimer’s disease. J. Neural Transm. 2018, 125, 771–779.
- Michaud, J.P.; Hallé, M.; Lampron, A.; Thériault, P.; Préfontaine, P.; Filali, M.; Tribout-Jover, P.; Lanteigne, A.M.; Jodoin, R.; Cluff, C.; et al. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proc. Natl. Acad. Sci. USA 2013, 110, 1941–1946.
- von Bernhardi, R.; Eugenín-von Bernhardi, L.; Eugenín, J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015, 7, 124.
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934.
- Parhizkar, S.; Arzberger, T.; Brendel, M.; Kleinberger, G.; Deussing, M.; Focke, C.; Nuscher, B.; Xiong, M.; Ghasemigharagoz, A.; Katzmarski, N.; et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat. Neurosci. 2019, 22, 191–204.
- Bemiller, S.M.; McCray, T.J.; Allan, K.; Formica, S.V.; Xu, G.; Wilson, G.; Kokiko-Cochran, O.N.; Crish, S.D.; Lasagna-Reeves, C.A.; Ransohoff, R.M.; et al. TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy. Mol. Neurodegener. 2017, 12, 74.
- Leyns, C.E.G.; Ulrich, J.D.; Finn, M.B.; Stewart, F.R.; Koscal, L.J.; Serrano, J.R.; Robinson, G.O.; Anderson, E.; Colonna, M.; Holtzman, D.M. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc. Natl. Acad. Sci. USA 2017, 114, 11524–11529.
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, J.D.; Young, K.L.; Robinette, M.L.; Gilfillan, S.; Krishnan, G.M.; Sudhakar, S.; Zinselmeyer, B.H.; et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 2015, 160, 1061–1071.
- Wang, Y.; Ulland, T.K.; Ulrich, J.D.; Song, W.; Tzaferis, J.A.; Hole, J.T.; Yuan, P.; Mahan, T.E.; Shi, Y.; Gilfillan, S.; et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med. 2016, 213, 667–675.
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905.
- St-Pierre, M.K.; Carrier, M.; González Ibáñez, F.; Šimončičová, E.; Wallman, M.J.; Vallières, L.; Parent, M.; Tremblay, M.È. Ultrastructural characterization of dark microglia during aging in a mouse model of Alzheimer’s disease pathology and in human post-mortem brain samples. J. Neuroinflamm. 2022, 19, 235.
- Bisht, K.; Sharma, K.P.; Lecours, C.; Gabriela Sánchez, M.; El Hajj, H.; Milior, G.; Olmos-Alonso, A.; Gómez-Nicola, D.; Luheshi, G.; Vallières, L.; et al. Dark microglia: A new phenotype predominantly associated with pathological states. Glia 2016, 64, 826–839.
- El Hajj, H.; Savage, J.C.; Bisht, K.; Parent, M.; Vallières, L.; Rivest, S.; Tremblay, M.È. Ultrastructural evidence of microglial heterogeneity in Alzheimer’s disease amyloid pathology. J. Neuroinflamm. 2019, 16, 87.
- Shytle, R.D.; Mori, T.; Townsend, K.; Vendrame, M.; Sun, N.; Zeng, J.; Ehrhart, J.; Silver, A.A.; Sanberg, P.R.; Tan, J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J. Neurochem. 2004, 89, 337–343.
- Wang, E.J.; Sun, J.; Pettoello-Mantovani, M.; Anderson, C.M.; Osiecki, K.; Zhao, M.L.; Lopez, L.; Lee, S.C.; Berman, J.W.; Goldstein, H. Microglia from mice transgenic for a provirus encoding a monocyte-tropic HIV type 1 isolate produce infectious virus and display in vitro and in vivo upregulation of lipopolysaccharide-induced chemokine gene expression. AIDS Res. Hum. Retroviruses 2003, 19, 755–765.
- Hart, A.D.; Wyttenbach, A.; Hugh Perry, V.; Teeling, J.L. Age related changes in microglial phenotype vary between CNS regions: Grey versus white matter differences. Brain. Behav. Immun. 2012, 26, 754–765.
- Minoretti, P.; Gazzaruso, C.; Di Vito, C.; Emanuele, E.; Bianchi, M.; Coen, E.; Reino, M.; Geroldi, D. Effect of the functional toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer’s disease. Neurosci. Lett. 2006, 391, 147–149.
- Giovannini, M.G.; Lana, D.; Pepeu, G. More than the cholinergic system: The evolving role of glia in memory, aging, and neurodegeneration. In Proceedings of the 17th International Symposium on Cholinergic Mechanisms, Dubrovnik, Croatia, 8–12 May 2022. Abstract Book p. 48.
- Fixemer, S.; Ameli, C.; Hammer, G.; Salamanca, L.; Uriarte Huarte, O.; Schwartz, C.; Gérardy, J.J.; Mechawar, N.; Skupin, A.; Mittelbronn, M.; et al. Microglia phenotypes are associated with subregional patterns of concomitant tau, amyloid-β and α-synuclein pathologies in the hippocampus of patients with Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 2022, 10, 36.
- Adler, D.H.; Wisse, L.E.M.; Ittyerah, R.; Pluta, J.B.; Ding, S.L.; Xie, L.; Wang, J.; Kadivar, S.; Robinson, J.L.; Schuck, T.; et al. Characterizing the human hippocampus in aging and Alzheimer’s disease using a computational atlas derived from ex vivo MRI and histology. Proc. Natl. Acad. Sci. USA 2018, 115, 4252–4257.
- McQuade, A.; Blurton-Jones, M. Microglia in Alzheimer’s Disease: Exploring How Genetics and Phenotype Influence Risk. J. Mol. Biol. 2019, 431, 1805–1817.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
745
Revisions:
3 times
(View History)
Update Date:
14 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




