
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | John Morser | -- | 6190 | 2023-09-12 02:44:49 | | | |
| 2 | Jessie Wu | -2 word(s) | 6188 | 2023-09-12 04:23:26 | | |
Video Upload Options
Osteopontin (OPN) is a multi-functional protein that is involved in various cellular processes such as cell adhesion, migration, and signaling. There is a single conserved thrombin cleavage site in OPN that, when cleaved, yields two fragments with different properties from full-length OPN. In cancer, OPN has tumor-promoting activity and plays a role in tumor growth and metastasis. High levels of OPN expression in cancer cells and tumor tissue are found in various types of cancer, including breast, lung, prostate, ovarian, colorectal, and pancreatic cancer, and are associated with poor prognosis and decreased survival rates. OPN promotes tumor progression and invasion by stimulating cell proliferation and angiogenesis and also facilitates the metastasis of cancer cells to other parts of the body by promoting cell adhesion and migration. Furthermore, OPN contributes to immune evasion by inhibiting the activity of immune cells.
1. Introduction
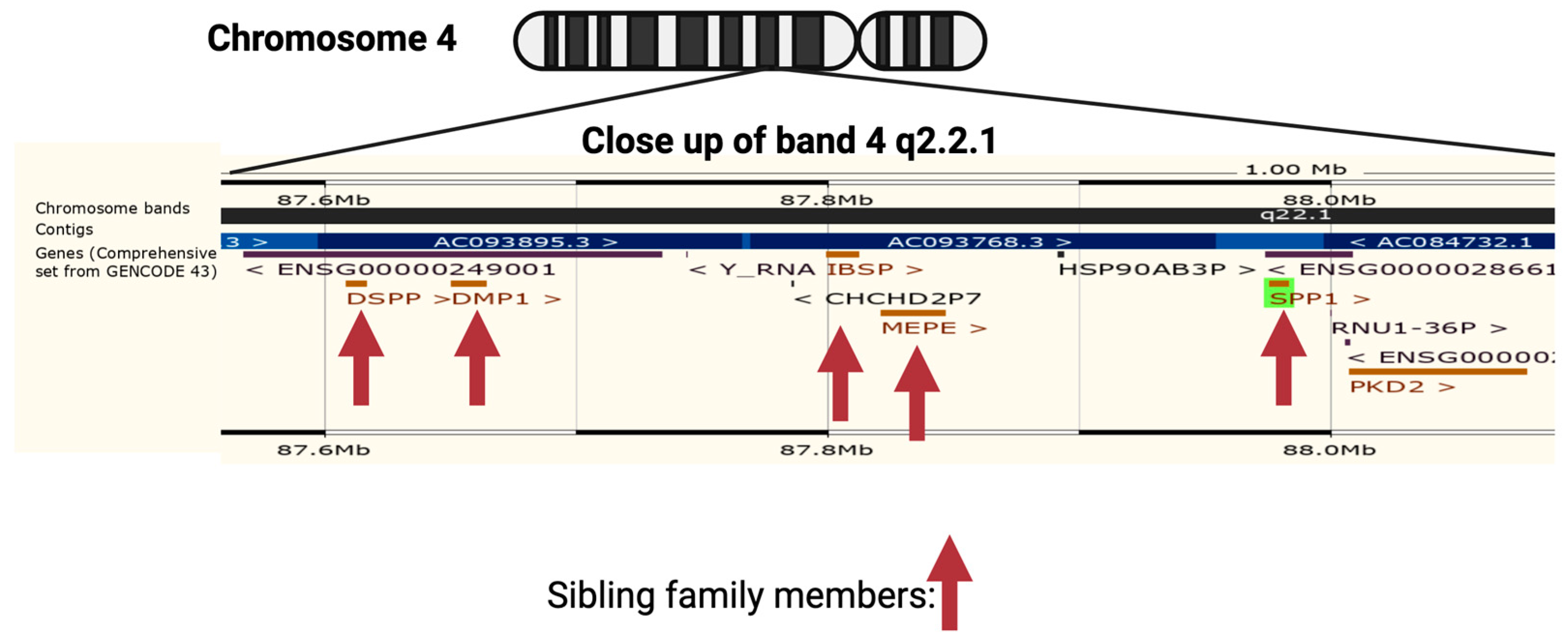
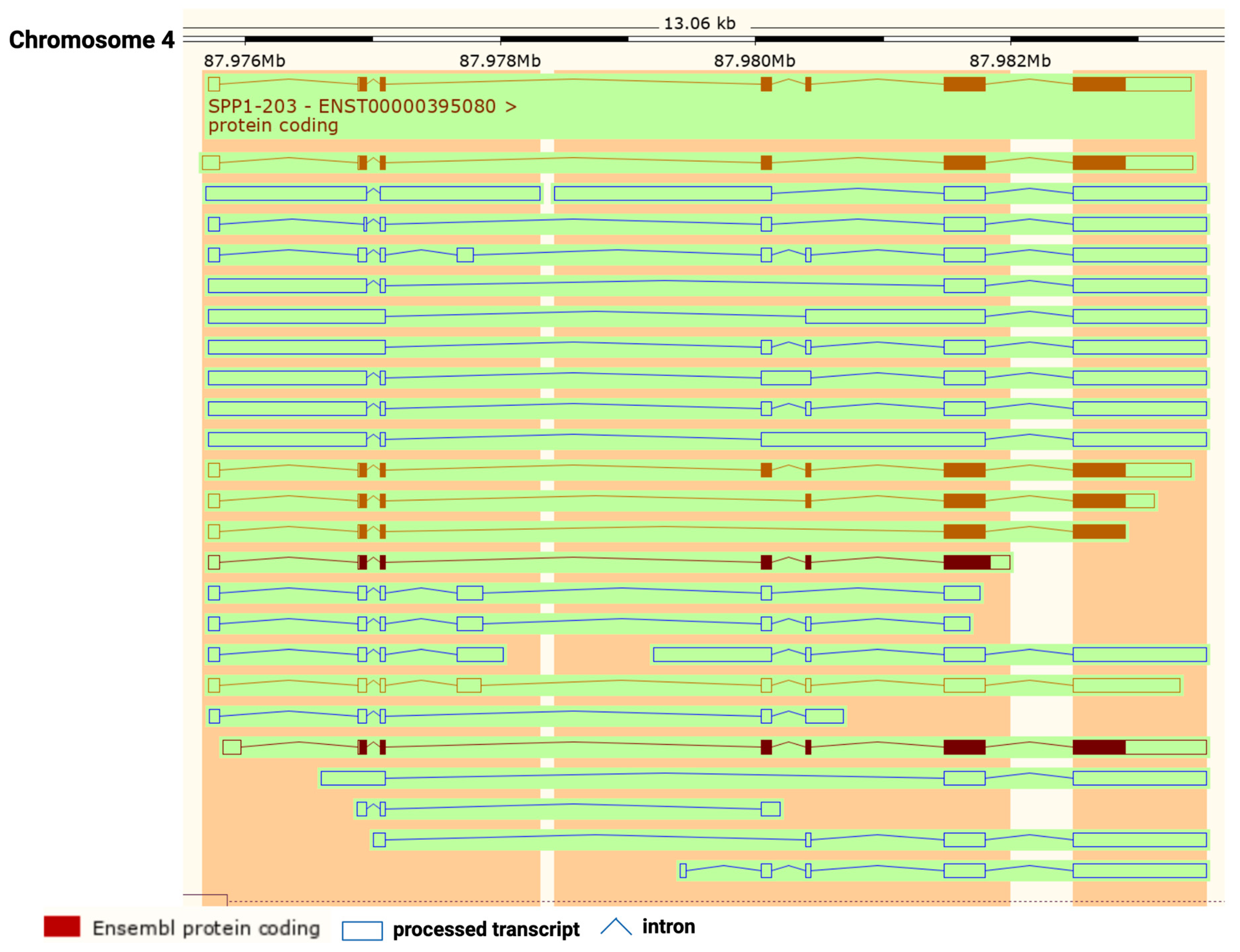
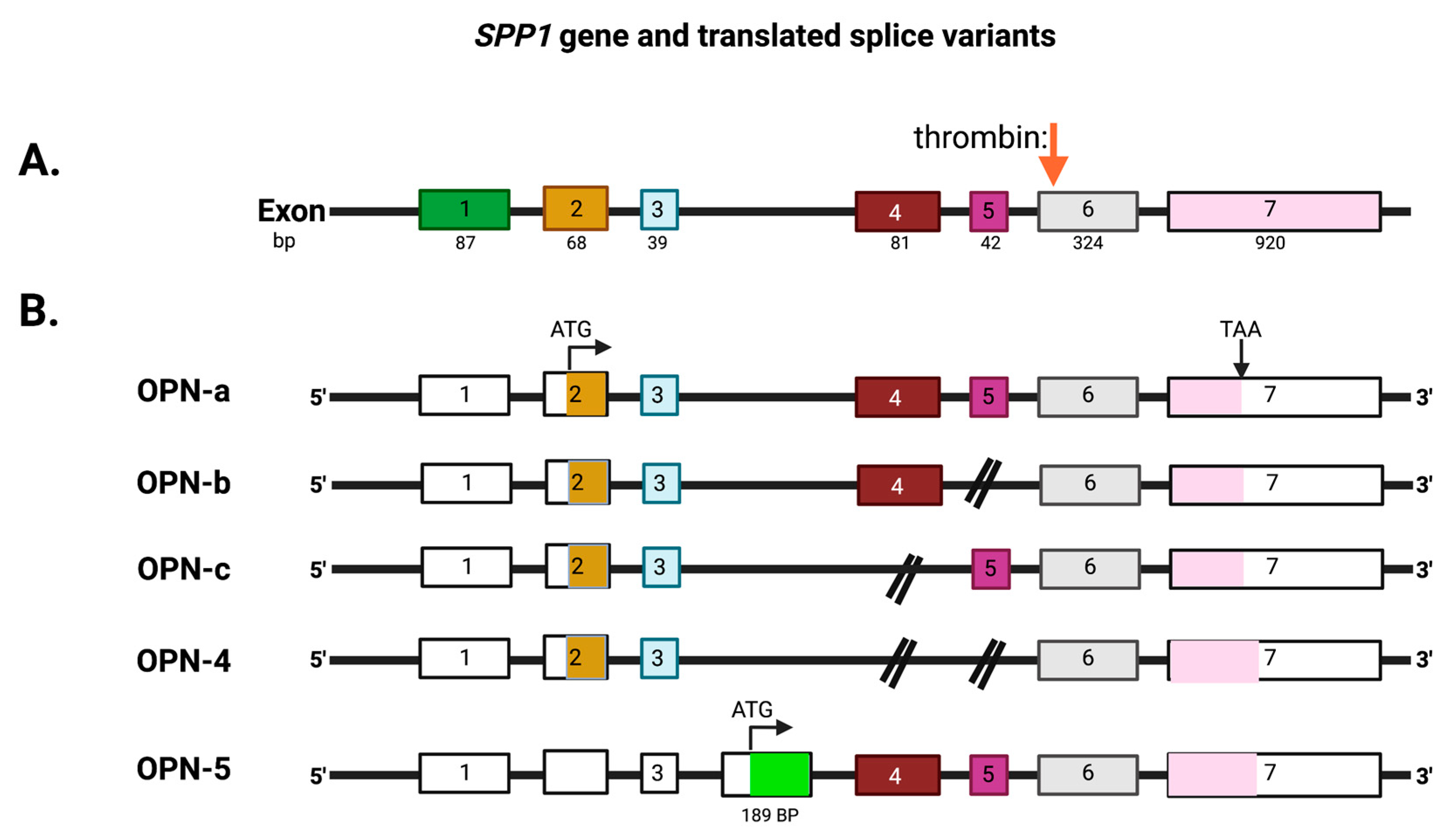
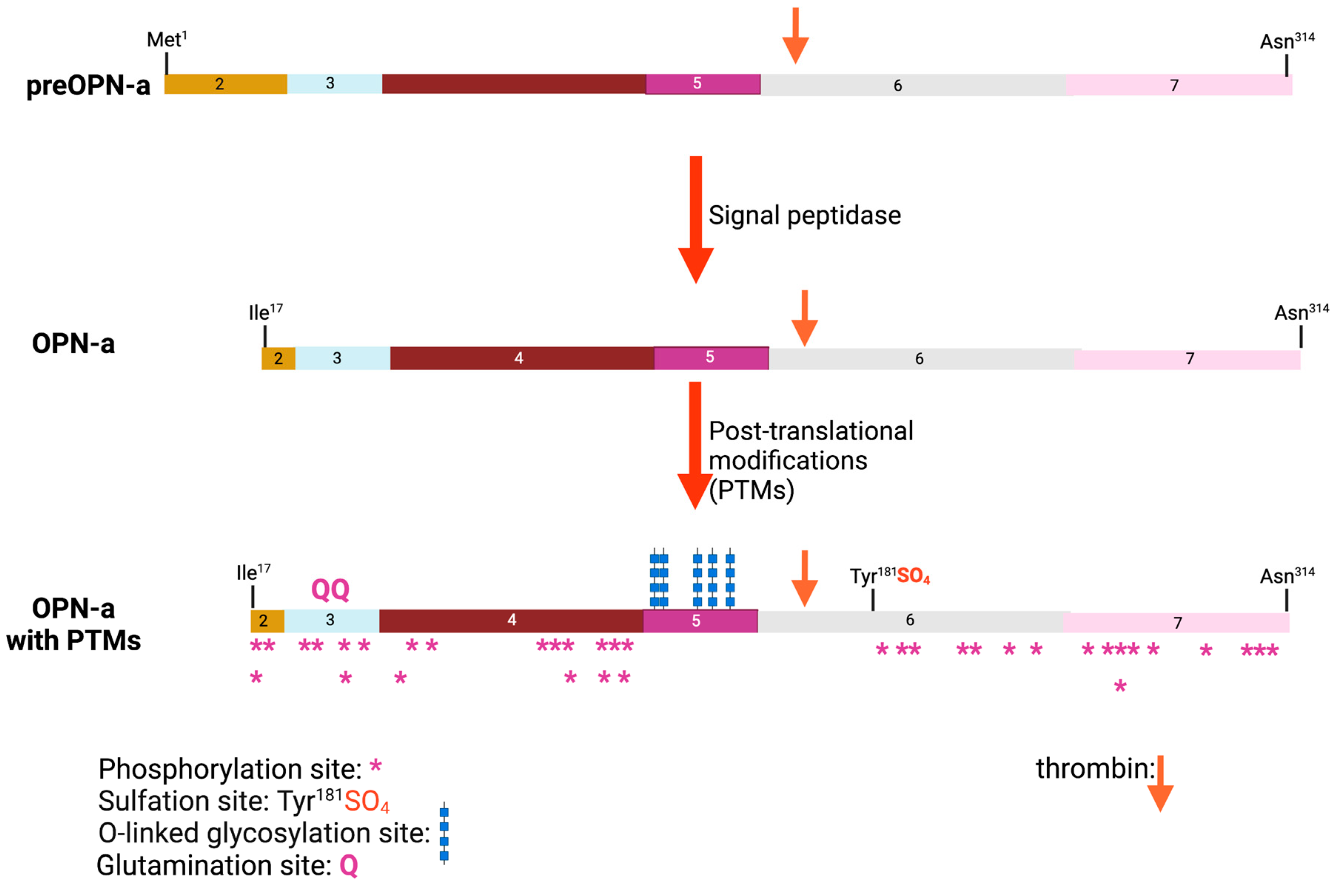
2. The Role of Osteopontin in Cancer
3. Increased Expression of Osteopontin and Cancer
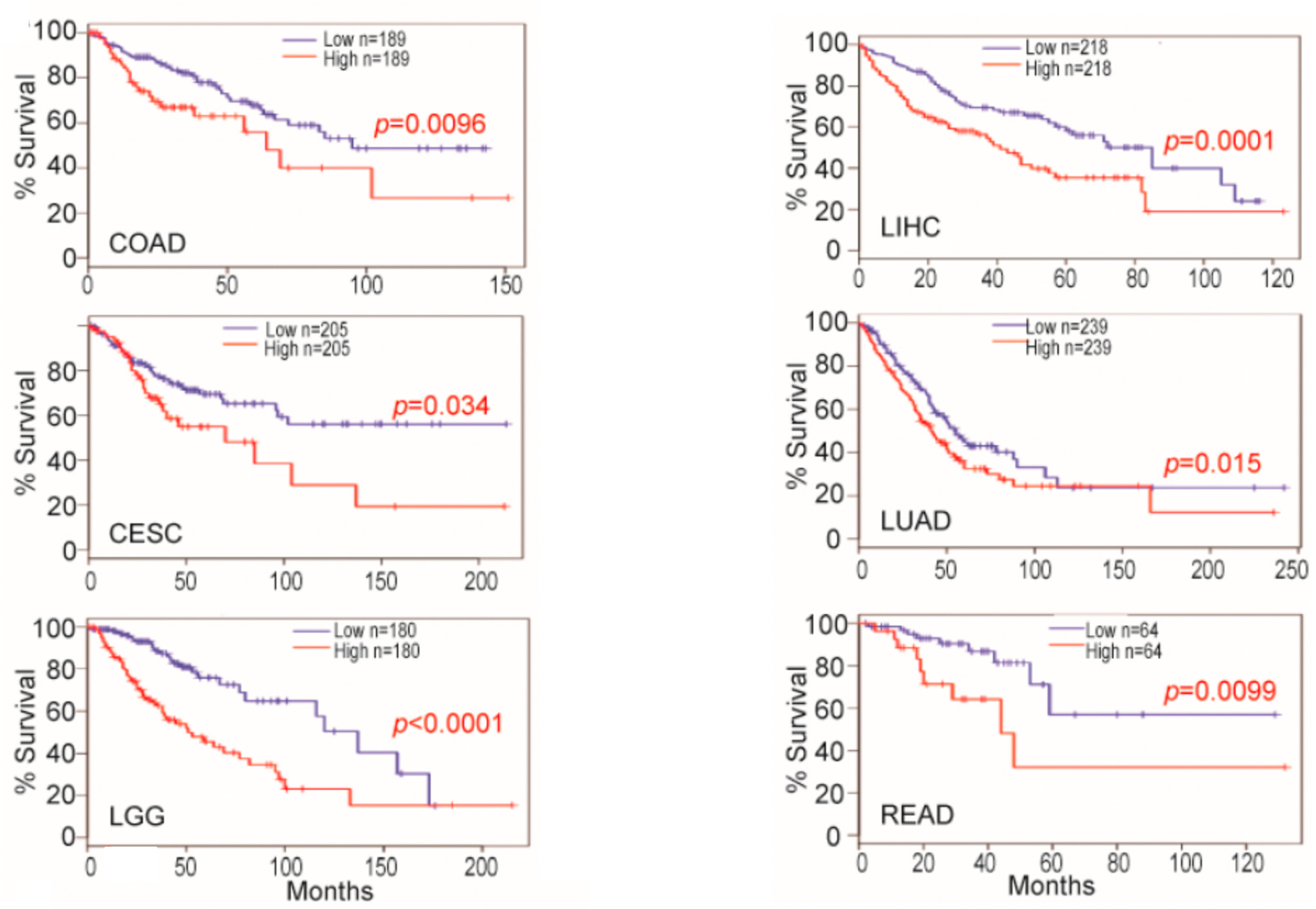
4. Osteopontin mRNA Expression in Tumor Cells and Tumor-Associated Cells
5. Osteopontin in Tumor Cell Culture Models: Its Expression and Its Effects
6. Therapies Targeting Osteopontin in Cancer
References
- Patarca, R.; Freeman, G.J.; Singh, R.P.; Wei, F.Y.; Durfee, T.; Blattner, F.; Regnier, D.C.; Kozak, C.A.; Mock, B.A.; Morse, H.C., 3rd; et al. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J. Exp. Med. 1989, 170, 145–161.
- Franzen, A.; Heinegard, D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem. J. 1985, 232, 715–724.
- Zohar, R.; Lee, W.; Arora, P.; Cheifetz, S.; McCulloch, C.; Sodek, J. Single cell analysis of intracellular osteopontin in osteogenic cultures of fetal rat calvarial cells. J. Cell. Physiol. 1997, 170, 88–100.
- Oldberg, A.; Franzen, A.; Heinegard, D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc. Natl. Acad. Sci. USA 1986, 83, 8819–8823.
- Uede, T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol. Int. 2011, 61, 265–280.
- Jiang, R.; Lonnerdal, B. Osteopontin in human milk and infant formula affects infant plasma osteopontin concentrations. Pediatr. Res. 2019, 85, 502–505.
- Liaw, L.; Birk, D.E.; Ballas, C.B.; Whitsitt, J.S.; Davidson, J.M.; Hogan, B.L. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J. Clin. Investig. 1998, 101, 1468–1478.
- Jiang, R.; Lonnerdal, B. Evaluation of Bioactivities of Bovine Milk Osteopontin Using a Knockout Mouse Model. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 125–131.
- Jiang, R.; Lo, J.; Prell, C.; Lonnerdal, B. Milk osteopontin promotes intestinal development by up-regulating the expression of integrin alphavbeta3 and CD44. FASEB J. 2023, 37, e22988.
- Jiang, R.; Tran, M.; Lonnerdal, B. Recombinant Bovine and Human Osteopontin Generated by Chlamydomonas reinhardtii Exhibit Bioactivities Similar to Bovine Milk Osteopontin When Assessed in Mouse Pups Fed Osteopontin-Deficient Milk. Mol. Nutr. Food Res. 2021, 65, e2000644.
- Moorman, H.R.; Poschel, D.; Klement, J.D.; Lu, C.; Redd, P.S.; Liu, K. Osteopontin: A Key Regulator of Tumor Progression and Immunomodulation. Cancers 2020, 12, 3379.
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic. Acids Res. 2022, 50, D988–D995.
- Liu, Y.N.; Kang, B.B.; Chen, J.H. Transcriptional regulation of human osteopontin promoter by C/EBPalpha and AML-1 in metastatic cancer cells. Oncogene 2004, 23, 278–288.
- Vietor, I.; Kurzbauer, R.; Brosch, G.; Huber, L.A. TIS7 regulation of the beta-catenin/Tcf-4 target gene osteopontin (OPN) is histone deacetylase-dependent. J. Biol. Chem. 2005, 280, 39795–39801.
- Wai, P.Y.; Mi, Z.; Gao, C.; Guo, H.; Marroquin, C.; Kuo, P.C. Ets-1 and runx2 regulate transcription of a metastatic gene, osteopontin, in murine colorectal cancer cells. J. Biol. Chem. 2006, 281, 18973–18982.
- Cabiati, M.; Salvadori, C.; Sapio, A.; Burchielli, S.; Carlucci, L.; Moscato, S.; Sabatino, L.; Caselli, C.; Mattii, L.; Del Ry, S. Aging and biomarkers: Transcriptional levels evaluation of Osteopontin/miRNA-181a axis in hepatic tissue of rats in different age ranges. Exp. Gerontol. 2020, 133, 110879.
- Marisetty, A.; Wei, J.; Kong, L.Y.; Ott, M.; Fang, D.; Sabbagh, A.; Heimberger, A.B. MiR-181 Family Modulates Osteopontin in Glioblastoma Multiforme. Cancers 2020, 12, 3813.
- Zhang, J.; Guo, H.; Mi, Z.; Gao, C.; Bhattacharya, S.; Li, J.; Kuo, P.C. EF1A1-actin interactions alter mRNA stability to determine differential osteopontin expression in HepG2 and Hep3B cells. Exp. Cell Res. 2009, 315, 304–312.
- Arjomandi, M.; Galanter, J.M.; Choudhry, S.; Eng, C.; Hu, D.; Beckman, K.; Chapela, R.; Rodriguez-Santana, J.R.; Rodriguez-Cintron, W.; Ford, J.; et al. Polymorphism in Osteopontin Gene (SPP1) Is Associated with Asthma and Related Phenotypes in a Puerto Rican Population. Pediatr. Allergy Immunol. Pulmonol. 2011, 24, 207–214.
- Gazal, S.; Sacre, K.; Allanore, Y.; Teruel, M.; Goodall, A.H.; Tohma, S.; Alfredsson, L.; Okada, Y.; Xie, G.; Constantin, A.; et al. Identification of secreted phosphoprotein 1 gene as a new rheumatoid arthritis susceptibility gene. Ann. Rheum. Dis. 2015, 74, e19.
- Glas, J.; Seiderer, J.; Bayrle, C.; Wetzke, M.; Fries, C.; Tillack, C.; Olszak, T.; Beigel, F.; Steib, C.; Friedrich, M.; et al. The role of osteopontin (OPN/SPP1) haplotypes in the susceptibility to Crohn’s disease. PLoS ONE 2011, 6, e29309.
- Amar, A.; Afzal, A.; Hameed, A.; Ahmad, M.; Khan, A.R.; Najma, H.; Abid, A.; Khaliq, S. Osteopontin promoter polymorphisms and risk of urolithiasis: A candidate gene association and meta-analysis study. BMC Med. Genet. 2020, 21, 172.
- Konya, E.; Umekawa, T.; Iguchi, M.; Kurita, T. The role of osteopontin on calcium oxalate crystal formation. Eur. Urol. 2003, 43, 564–571.
- Bastos, A.; Gomes, A.V.P.; Silva, G.R.; Emerenciano, M.; Ferreira, L.B.; Gimba, E.R.P. The Intracellular and Secreted Sides of Osteopontin and Their Putative Physiopathological Roles. Int. J. Mol. Sci. 2023, 24, 2942.
- Shinohara, M.L.; Kim, H.J.; Kim, J.H.; Garcia, V.A.; Cantor, H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc. Natl. Acad. Sci. USA 2008, 105, 7235–7239.
- Leavenworth, J.W.; Verbinnen, B.; Yin, J.; Huang, H.; Cantor, H. A p85alpha-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat. Immunol. 2015, 16, 96–106.
- Zduniak, K.; Ziolkowski, P.; Ahlin, C.; Agrawal, A.; Agrawal, S.; Blomqvist, C.; Fjallskog, M.L.; Weber, G.F. Nuclear osteopontin-c is a prognostic breast cancer marker. Br. J. Cancer 2015, 112, 729–738.
- Zhao, K.; Zhang, M.; Zhang, L.; Wang, P.; Song, G.; Liu, B.; Wu, H.; Yin, Z.; Gao, C. Intracellular osteopontin stabilizes TRAF3 to positively regulate innate antiviral response. Sci. Rep. 2016, 6, 23771.
- Dong, Q.Z.; Zhang, X.F.; Zhao, Y.; Jia, H.L.; Zhou, H.J.; Dai, C.; Sun, H.J.; Qin, Y.; Zhang, W.D.; Ren, N.; et al. Osteopontin promoter polymorphisms at locus -443 significantly affect the metastasis and prognosis of human hepatocellular carcinoma. Hepatology 2013, 57, 1024–1034.
- Lee, T.Y.; Lin, J.T.; Wu, C.C.; Yu, C.C.; Wu, M.S.; Lee, T.C.; Chen, H.P.; Wu, C.Y. Osteopontin promoter polymorphisms are associated with susceptibility to gastric cancer. J. Clin. Gastroenterol. 2013, 47, e55–e59.
- Zhao, F.; Chen, X.; Meng, T.; Hao, B.; Zhang, Z.; Zhang, G. Genetic polymorphisms in the osteopontin promoter increases the risk of distance metastasis and death in Chinese patients with gastric cancer. BMC Cancer 2012, 12, 477.
- Wang, J.L.; Nong, L.G.; Tang, Y.J.; Wei, Y.S.; Yang, F.L.; Wang, C.F. Correlation between OPN gene polymorphisms and the risk of nasopharyngeal carcinoma. Med. Oncol. 2014, 31, 20.
- Chen, J.; Wu, Q.; Lu, Y.; Xu, T.; Huang, Y.; Ribas, J.; Ni, X.; Hu, G.; Huang, F.; Zhou, L.; et al. SPP1 promoter polymorphisms and glioma risk in a Chinese Han population. J. Hum. Genet. 2010, 55, 456–461.
- Chen, X.G.; Godbey, W.T. The potential of the human osteopontin promoter and single-nucleotide polymorphisms for targeted cancer gene therapy. Curr. Gene Ther. 2015, 15, 82–92.
- Giacopelli, F.; Marciano, R.; Pistorio, A.; Catarsi, P.; Canini, S.; Karsenty, G.; Ravazzolo, R. Polymorphisms in the osteopontin promoter affect its transcriptional activity. Physiol. Genom. 2004, 20, 87–96.
- Briones-Orta, M.A.; Avendano-Vazquez, S.E.; Aparicio-Bautista, D.I.; Coombes, J.D.; Weber, G.F.; Syn, W.K. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 93–108.
- Briones-Orta, M.A.; Avendano-Vazquez, S.E.; Ivette Aparicio-Bautista, D.; Coombes, J.D.; Weber, G.F.; Syn, W.K. Prediction of transcription factor bindings sites affected by SNPs located at the osteopontin promoter. Data Brief 2017, 14, 538–542.
- Beninati, S.; Senger, D.R.; Cordella-Miele, E.; Mukherjee, A.B.; Chackalaparampil, I.; Shanmugam, V.; Singh, K.; Mukherjee, B.B. Osteopontin: Its transglutaminase-catalyzed posttranslational modifications and cross-linking to fibronectin. J. Biochem. 1994, 115, 675–682.
- Prince, C.W.; Dickie, D.; Krumdieck, C.L. Osteopontin, a substrate for transglutaminase and factor XIII activity. Biochem. Biophys. Res. Commun. 1991, 177, 1205–1210.
- Gimba, E.R.; Tilli, T.M. Human osteopontin splicing isoforms: Known roles, potential clinical applications and activated signaling pathways. Cancer Lett. 2013, 331, 11–17.
- Gimba, E.R.P.; Brum, M.C.M.; Nestal De Moraes, G. Full-length osteopontin and its splice variants as modulators of chemoresistance and radioresistance (Review). Int. J. Oncol. 2019, 54, 420–430.
- An, Y.; Fnu, G.; Xie, C.; Weber, G.F. Meta-analysis of Osteopontin splice variants in cancer. BMC Cancer 2023, 23, 373.
- Weber, G.F. The Phylogeny of Osteopontin-Analysis of the Protein Sequence. Int. J. Mol. Sci. 2018, 19, 2557.
- Das, S.; Samant, R.S.; Shevde, L.A. Hedgehog signaling induced by breast cancer cells promotes osteoclastogenesis and osteolysis. J. Biol. Chem. 2011, 286, 9612–9622.
- Zunich, S.M.; Douglas, T.; Valdovinos, M.; Chang, T.; Bushman, W.; Walterhouse, D.; Iannaccone, P.; Lamm, M.L. Paracrine sonic hedgehog signalling by prostate cancer cells induces osteoblast differentiation. Mol. Cancer 2009, 8, 12.
- Wei, R.; Wong, J.P.C.; Kwok, H.F. Osteopontin—A promising biomarker for cancer therapy. J. Cancer 2017, 8, 2173–2183.
- Chiodoni, C.; Colombo, M.P.; Sangaletti, S. Matricellular proteins: From homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010, 29, 295–307.
- Lamort, A.S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells 2019, 8, 815.
- Shevde, L.A.; Samant, R.S. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 37, 131–141.
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930.
- Atai, N.A.; Bansal, M.; Lo, C.; Bosman, J.; Tigchelaar, W.; Bosch, K.S.; Jonker, A.; De Witt Hamer, P.C.; Troost, D.; McCulloch, C.A.; et al. Osteopontin is up-regulated and associated with neutrophil and macrophage infiltration in glioblastoma. Immunology 2011, 132, 39–48.
- Rohde, F.; Rimkus, C.; Friederichs, J.; Rosenberg, R.; Marthen, C.; Doll, D.; Holzmann, B.; Siewert, J.R.; Janssen, K.P. Expression of osteopontin, a target gene of de-regulated Wnt signaling, predicts survival in colon cancer. Int. J. Cancer 2007, 121, 1717–1723.
- Klement, J.D.; Poschel, D.B.; Lu, C.; Merting, A.D.; Yang, D.; Redd, P.S.; Liu, K. Osteopontin Blockade Immunotherapy Increases Cytotoxic T Lymphocyte Lytic Activity and Suppresses Colon Tumor Progression. Cancers 2021, 13, 1006.
- Sun, J.; Chen, X.; Wang, Y. Comparison of the diagnostic value of CEA combined with OPN or DKK1 in non-small cell lung cancer. Oncol. Lett. 2020, 20, 3046–3052.
- Xu, C.; Yuan, Q.; Wang, W.; Chi, C.; Zhang, Q.; Li, L.; Yang, R.; Wang, Y. Prognostic significance of serum osteopontin levels in small cell lung cancer. BMC Pulm. Med. 2020, 20, 235.
- Ji, X.; Liu, Y.; Mei, F.; Li, X.; Zhang, M.; Yao, B.; Wu, R.; You, J.; Pei, F. SPP1 overexpression is associated with poor outcomes in ALK fusion lung cancer patients without receiving targeted therapy. Sci. Rep. 2021, 11, 14031.
- Creaney, J.; Yeoman, D.; Musk, A.W.; de Klerk, N.; Skates, S.J.; Robinson, B.W. Plasma versus serum levels of osteopontin and mesothelin in patients with malignant mesothelioma—Which is best? Lung Cancer 2011, 74, 55–60.
- Kerenidi, T.; Kazakou, A.P.; Lada, M.; Tsilioni, I.; Daniil, Z.; Gourgoulianis, K.I. Clinical Significance of Circulating Osteopontin Levels in Patients With Lung Cancer and Correlation With VEGF and MMP-9. Cancer Investig. 2016, 34, 385–392.
- Rong, W.; Zhang, Y.; Yang, L.; Feng, L.; Wei, B.; Wu, F.; Wang, L.; Gao, Y.; Cheng, S.; Wu, J.; et al. Post-surgical resection prognostic value of combined OPN, MMP7, and PSG9 plasma biomarkers in hepatocellular carcinoma. Front. Med. 2019, 13, 250–258.
- Sun, T.; Li, P.; Sun, D.; Bu, Q.; Li, G. Prognostic value of osteopontin in patients with hepatocellular carcinoma: A systematic review and meta-analysis. Medicine 2018, 97, e12954.
- Cabiati, M.; Gaggini, M.; De Simone, P.; Del Ry, S. Data mining of key genes expression in hepatocellular carcinoma: Novel potential biomarkers of diagnosis prognosis or progression. Clin. Exp. Metastasis 2022, 39, 589–602.
- Cabiati, M.; Gaggini, M.; Cesare, M.M.; Caselli, C.; De Simone, P.; Filipponi, F.; Basta, G.; Gastaldelli, A.; Del Ry, S. Osteopontin in hepatocellular carcinoma: A possible biomarker for diagnosis and follow-up. Cytokine 2017, 99, 59–65.
- Cabiati, M.; Di Giorgi, N.; Salvadori, C.; Finamore, F.; Del Turco, S.; Cecchettini, A.; Rocchiccioli, S.; Del Ry, S. Transcriptional level evaluation of osteopontin/miRNA-181a axis in hepatocellular carcinoma cell line-secreted extracellular vesicles. Pathol. Res. Pract. 2022, 238, 154088.
- Anborgh, P.H.; Lee, D.J.; Stam, P.F.; Tuck, A.B.; Chambers, A.F. Role of osteopontin as a predictive biomarker for anti-EGFR therapy in triple-negative breast cancer. Expert Opin. Ther. Targets 2018, 22, 727–734.
- Bellahcene, A.; Castronovo, V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am. J. Pathol. 1995, 146, 95–100.
- Singhal, H.; Bautista, D.S.; Tonkin, K.S.; O’Malley, F.P.; Tuck, A.B.; Chambers, A.F.; Harris, J.F. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1997, 3, 605–611.
- Tuck, A.B.; O’Malley, F.P.; Singhal, H.; Tonkin, K.S.; Harris, J.F.; Bautista, D.; Chambers, A.F. Osteopontin and p53 expression are associated with tumor progression in a case of synchronous, bilateral, invasive mammary carcinomas. Arch. Pathol. Lab. Med. 1997, 121, 578–584.
- Lindahl, G.; Rzepecka, A.; Dabrosin, C. Increased Extracellular Osteopontin Levels in Normal Human Breast Tissue at High Risk of Developing Cancer and Its Association With Inflammatory Biomarkers in situ. Front. Oncol. 2019, 9, 746.
- Gothlin Eremo, A.; Lagergren, K.; Othman, L.; Montgomery, S.; Andersson, G.; Tina, E. Evaluation of SPP1/osteopontin expression as predictor of recurrence in tamoxifen treated breast cancer. Sci. Rep. 2020, 10, 1451.
- Wang, Y.D.; Chen, H.; Liu, H.Q.; Hao, M. Correlation between ovarian neoplasm and serum levels of osteopontin: A meta-analysis. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 11799–11808.
- Gao, W.; Liu, D.; Sun, H.; Shao, Z.; Shi, P.; Li, T.; Yin, S.; Zhu, T. SPP1 is a prognostic related biomarker and correlated with tumor-infiltrating immune cells in ovarian cancer. BMC Cancer 2022, 22, 1367.
- Rani, S.; Sehgal, A.; Kaur, J.; Pandher, D.K.; Punia, R.S. Osteopontin as a Tumor Marker in Ovarian Cancer. J. Midlife Health 2022, 13, 200–205.
- Zhao, Y.; Huang, C. The role of osteopontin in the development and metastasis of melanoma. Melanoma Res. 2021, 31, 283–289.
- Kiss, T.; Ecsedi, S.; Vizkeleti, L.; Koroknai, V.; Emri, G.; Kovacs, N.; Adany, R.; Balazs, M. The role of osteopontin expression in melanoma progression. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 7841–7847.
- Abildgaard, S.K.; Vorum, H. Proteomics of uveal melanoma: A minireview. J. Oncol. 2013, 2013, 820953.
- Guarneri, C.; Bevelacqua, V.; Polesel, J.; Falzone, L.; Cannavo, P.S.; Spandidos, D.A.; Malaponte, G.; Libra, M. NF-kappaB inhibition is associated with OPN/MMP-9 downregulation in cutaneous melanoma. Oncol. Rep. 2017, 37, 737–746.
- Szasz, I.; Koroknai, V.; Kiss, T.; Vizkeleti, L.; Adany, R.; Balazs, M. Molecular alterations associated with acquired resistance to BRAFV600E targeted therapy in melanoma cells. Melanoma Res. 2019, 29, 390–400.
- Ouyang, X.; Huang, Y.; Jin, X.; Zhao, W.; Hu, T.; Wu, F.; Huang, J. Osteopontin promotes cancer cell drug resistance, invasion, and lactate production and is associated with poor outcome of patients with advanced non-small-cell lung cancer. Onco Targets Ther. 2018, 11, 5933–5941.
- Patel, V.; Szasz, I.; Koroknai, V.; Kiss, T.; Balazs, M. Molecular Alterations Associated with Acquired Drug Resistance during Combined Treatment with Encorafenib and Binimetinib in Melanoma Cell Lines. Cancers 2021, 13, 6058.
- Chen, J.; Hou, C.; Zheng, Z.; Lin, H.; Lv, G.; Zhou, D. Identification of Secreted Phosphoprotein 1 (SPP1) as a Prognostic Factor in Lower-Grade Gliomas. World Neurosurg. 2019, 130, e775–e785.
- Sreekanthreddy, P.; Srinivasan, H.; Kumar, D.M.; Nijaguna, M.B.; Sridevi, S.; Vrinda, M.; Arivazhagan, A.; Balasubramaniam, A.; Hegde, A.S.; Chandramouli, B.A.; et al. Identification of potential serum biomarkers of glioblastoma: Serum osteopontin levels correlate with poor prognosis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1409–1422.
- Kohata, T.; Ito, S.; Masuda, T.; Furuta, T.; Nakada, M.; Ohtsuki, S. Laminin Subunit Alpha-4 and Osteopontin Are Glioblastoma-Selective Secreted Proteins That Are Increased in the Cerebrospinal Fluid of Glioblastoma Patients. J. Proteome Res. 2020, 19, 3542–3553.
- Yamaguchi, Y.; Shao, Z.; Sharif, S.; Du, X.Y.; Myles, T.; Merchant, M.; Harsh, G.; Glantz, M.; Recht, L.; Morser, J.; et al. Thrombin-cleaved fragments of osteopontin are overexpressed in malignant glial tumors and provide a molecular niche with survival advantage. J. Biol. Chem. 2013, 288, 3097–3111.
- Colin, C.; Baeza, N.; Bartoli, C.; Fina, F.; Eudes, N.; Nanni, I.; Martin, P.M.; Ouafik, L.; Figarella-Branger, D. Identification of genes differentially expressed in glioblastoma versus pilocytic astrocytoma using Suppression Subtractive Hybridization. Oncogene 2006, 25, 2818–2826.
- Noda, M.; Yoon, K.; Prince, C.W.; Butler, W.T.; Rodan, G.A. Transcriptional regulation of osteopontin production in rat osteosarcoma cells by type beta transforming growth factor. J. Biol. Chem. 1988, 263, 13916–13921.
- Brown, L.F.; Papadopoulos-Sergiou, A.; Berse, B.; Manseau, E.J.; Tognazzi, K.; Perruzzi, C.A.; Dvorak, H.F.; Senger, D.R. Osteopontin expression and distribution in human carcinomas. Am. J. Pathol. 1994, 145, 610–623.
- Craig, A.M.; Smith, J.H.; Denhardt, D.T. Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate in mouse epidermis. J. Biol. Chem. 1989, 264, 9682–9689.
- Rittling, S.R.; Novick, K.E. Osteopontin expression in mammary gland development and tumorigenesis. Cell Growth Differ. 1997, 8, 1061–1069.
- Ue, T.; Yokozaki, H.; Kitadai, Y.; Yamamoto, S.; Yasui, W.; Ishikawa, T.; Tahara, E. Co-expression of osteopontin and CD44v9 in gastric cancer. Int. J. Cancer 1998, 79, 127–132.
- Das, S.; Harris, L.G.; Metge, B.J.; Liu, S.; Riker, A.I.; Samant, R.S.; Shevde, L.A. The hedgehog pathway transcription factor GLI1 promotes malignant behavior of cancer cells by up-regulating osteopontin. J. Biol. Chem. 2009, 284, 22888–22897.
- Wu, W.; Yang, H.; Wang, Z.; Zhang, Z.; Lu, X.; Yang, W.; Xu, X.; Jiang, Y.; Li, Y.; Fan, X.; et al. A Noncanonical Hedgehog Signaling Exerts a Tumor-Promoting Effect on Pancreatic Cancer Cells Via Induction of Osteopontin Expression. Cancer Biother. Radiopharm. 2021.
- Manda, K.R.; Tripathi, P.; Hsi, A.C.; Ning, J.; Ruzinova, M.B.; Liapis, H.; Bailey, M.; Zhang, H.; Maher, C.A.; Humphrey, P.A.; et al. NFATc1 promotes prostate tumorigenesis and overcomes PTEN loss-induced senescence. Oncogene 2016, 35, 3282–3292.
- Sathe, A.; Mason, K.; Grimes, S.M.; Zhou, Z.; Lau, B.T.; Bai, X.; Su, A.; Tan, X.; Lee, H.; Suarez, C.J.; et al. Colorectal Cancer Metastases in the Liver Establish Immunosuppressive Spatial Networking between Tumor-Associated SPP1+ Macrophages and Fibroblasts. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2023, 29, 244–260.
- Chen, K.; Wang, Q.; Liu, X.; Wang, F.; Ma, Y.; Zhang, S.; Shao, Z.; Yang, Y.; Tian, X. Single Cell RNA-Seq Identifies Immune-Related Prognostic Model and Key Signature-SPP1 in Pancreatic Ductal Adenocarcinoma. Genes 2022, 13, 1760.
- Li, X.; Zhang, Q.; Chen, G.; Luo, D. Multi-Omics Analysis Showed the Clinical Value of Gene Signatures of C1QC(+) and SPP1(+) TAMs in Cervical Cancer. Front. Immunol. 2021, 12, 694801.
- Yang, L.; Zhang, Z.; Sun, Y.; Pang, S.; Yao, Q.; Lin, P.; Cheng, J.; Li, J.; Ding, G.; Hui, L.; et al. Integrative analysis reveals novel driver genes and molecular subclasses of hepatocellular carcinoma. Aging 2020, 12, 23849–23871.
- Xie, L.; Ning, Z.; Hua, Y.; Wang, P.; Meng, Z. Single-cell transcriptome analysis revealed the immune profile of PD-1 blockade in gallbladder carcinoma liver metastasis. Hepatol. Commun. 2023, 7, e0131.
- Gabrusiewicz, K.; Rodriguez, B.; Wei, J.; Hashimoto, Y.; Healy, L.M.; Maiti, S.N.; Thomas, G.; Zhou, S.; Wang, Q.; Elakkad, A.; et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight 2016, 1, e85841.
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173.
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. A J. Virtual Libr. 2008, 13, 453–461.
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78.
- Yang, Q.; Zhang, H.; Wei, T.; Lin, A.; Sun, Y.; Luo, P.; Zhang, J. Single-Cell RNA Sequencing Reveals the Heterogeneity of Tumor-Associated Macrophage in Non-Small Cell Lung Cancer and Differences Between Sexes. Front. Immunol. 2021, 12, 756722.
- Araujo, J.M.; Prado, A.; Cardenas, N.K.; Zaharia, M.; Dyer, R.; Doimi, F.; Bravo, L.; Pinillos, L.; Morante, Z.; Aguilar, A.; et al. Repeated observation of immune gene sets enrichment in women with non-small cell lung cancer. Oncotarget 2016, 7, 20282–20292.
- Leader, A.M.; Grout, J.A.; Maier, B.B.; Nabet, B.Y.; Park, M.D.; Tabachnikova, A.; Chang, C.; Walker, L.; Lansky, A.; Le Berichel, J.; et al. Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell 2021, 39, 1594–1609.e12.
- Martinez-Zayas, G.; Almeida, F.A.; Yarmus, L.; Steinfort, D.; Lazarus, D.R.; Simoff, M.J.; Saettele, T.; Murgu, S.; Dammad, T.; Duong, D.K.; et al. Predicting Lymph Node Metastasis in Non-small Cell Lung Cancer: Prospective External and Temporal Validation of the HAL and HOMER Models. Chest 2021, 160, 1108–1120.
- Dong, B.; Wu, C.; Huang, L.; Qi, Y. Macrophage-Related SPP1 as a Potential Biomarker for Early Lymph Node Metastasis in Lung Adenocarcinoma. Front. Cell Dev. Biol. 2021, 9, 739358.
- Jain, S.; Rick, J.W.; Joshi, R.S.; Beniwal, A.; Spatz, J.; Gill, S.; Chang, A.C.; Choudhary, N.; Nguyen, A.T.; Sudhir, S.; et al. Single-cell RNA sequencing and spatial transcriptomics reveal cancer-associated fibroblasts in glioblastoma with protumoral effects. J. Clin. Investig. 2023, 133, e147087.
- Ozato, Y.; Kojima, Y.; Kobayashi, Y.; Hisamatsu, Y.; Toshima, T.; Yonemura, Y.; Masuda, T.; Kagawa, K.; Goto, Y.; Utou, M.; et al. Spatial and single-cell transcriptomics decipher the cellular environment containing HLA-G+ cancer cells and SPP1+ macrophages in colorectal cancer. Cell Rep. 2023, 42, 111929.
- Butti, R.; Kumar, T.V.S.; Nimma, R.; Banerjee, P.; Kundu, I.G.; Kundu, G.C. Osteopontin Signaling in Shaping Tumor Microenvironment Conducive to Malignant Progression. Adv. Exp. Med. Biol. 2021, 1329, 419–441.
- Robertson, B.W.; Bonsal, L.; Chellaiah, M.A. Regulation of Erk1/2 activation by osteopontin in PC3 human prostate cancer cells. Mol. Cancer 2010, 9, 260.
- Chen, Y.J.; Wei, Y.Y.; Chen, H.T.; Fong, Y.C.; Hsu, C.J.; Tsai, C.H.; Hsu, H.C.; Liu, S.H.; Tang, C.H. Osteopontin increases migration and MMP-9 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J. Cell. Physiol. 2009, 221, 98–108.
- Zhang, H.; Guo, M.; Chen, J.H.; Wang, Z.; Du, X.F.; Liu, P.X.; Li, W.H. Osteopontin knockdown inhibits alphav, beta3 integrin-induced cell migration and invasion and promotes apoptosis of breast cancer cells by inducing autophagy and inactivating the PI3K/Akt/mTOR pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 33, 991–1002.
- Robertson, B.W.; Chellaiah, M.A. Osteopontin induces beta-catenin signaling through activation of Akt in prostate cancer cells. Exp. Cell Res. 2010, 316, 1–11.
- Fu, Y.; Zhang, Y.; Lei, Z.; Liu, T.; Cai, T.; Wang, A.; Du, W.; Zeng, Y.; Zhu, J.; Liu, Z.; et al. Abnormally activated OPN/integrin alphaVbeta3/FAK signalling is responsible for EGFR-TKI resistance in EGFR mutant non-small-cell lung cancer. J. Hematol. Oncol. 2020, 13, 169.
- Wang, X.; Zhang, F.; Yang, X.; Xue, M.; Li, X.; Gao, Y.; Liu, L. Secreted Phosphoprotein 1 (SPP1) Contributes to Second-Generation EGFR Tyrosine Kinase Inhibitor Resistance in Non-Small Cell Lung Cancer. Oncol. Res. 2019, 27, 871–877.
- Wang, Y.J.; Wang, Q.W.; Yu, D.H.; Song, C.K.; Guo, Z.X.; Liu, X.P.; Chen, C.; Yao, J.; Wang, A.F.; Hu, W.D. Osteopontin improves sensitivity to tyrosine kinase inhibitor in lung adenocarcinoma in vitro by promoting epidermal growth factor receptor phosphorylation. J. Cancer Res. Clin. Oncol. 2021, 147, 3245–3254.
- Ellert-Miklaszewska, A.; Wisniewski, P.; Kijewska, M.; Gajdanowicz, P.; Pszczolkowska, D.; Przanowski, P.; Dabrowski, M.; Maleszewska, M.; Kaminska, B. Tumour-processed osteopontin and lactadherin drive the protumorigenic reprogramming of microglia and glioma progression. Oncogene 2016, 35, 6366–6377.
- Ding, Q.; Stewart, J., Jr.; Prince, C.W.; Chang, P.L.; Trikha, M.; Han, X.; Grammer, J.R.; Gladson, C.L. Promotion of malignant astrocytoma cell migration by osteopontin expressed in the normal brain: Differences in integrin signaling during cell adhesion to osteopontin versus vitronectin. Cancer Res. 2002, 62, 5336–5343.
- Tuck, A.B.; Arsenault, D.M.; O’Malley, F.P.; Hota, C.; Ling, M.C.; Wilson, S.M.; Chambers, A.F. Osteopontin induces increased invasiveness and plasminogen activator expression of human mammary epithelial cells. Oncogene 1999, 18, 4237–4246.
- Irby, R.B.; McCarthy, S.M.; Yeatman, T.J. Osteopontin regulates multiple functions contributing to human colon cancer development and progression. Clin. Exp. Metastasis 2004, 21, 515–523.
- Chae, S.; Jun, H.O.; Lee, E.G.; Yang, S.J.; Lee, D.C.; Jung, J.K.; Park, K.C.; Yeom, Y.I.; Kim, K.W. Osteopontin splice variants differentially modulate the migratory activity of hepatocellular carcinoma cell lines. Int. J. Oncol. 2009, 35, 1409–1416.
- Guo, H.; Cai, C.Q.; Schroeder, R.A.; Kuo, P.C. Osteopontin is a negative feedback regulator of nitric oxide synthesis in murine macrophages. J. Immunol. 2001, 166, 1079–1086.
- Guo, H.; Marroquin, C.E.; Wai, P.Y.; Kuo, P.C. Nitric oxide-dependent osteopontin expression induces metastatic behavior in HepG2 cells. Dig. Dis. Sci. 2005, 50, 1288–1298.
- Ikeguchi, M.; Ueta, T.; Yamane, Y.; Hirooka, Y.; Kaibara, N. Inducible nitric oxide synthase and survivin messenger RNA expression in hepatocellular carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 3131–3136.
- Ortiz-Martinez, F.; Sanmartin, E.; Pomares-Navarro, E.; Perez-Balaguer, A.; Andres, L.; Sanchez-Paya, J.; Aranda, F.I.; Lerma, E.; Peiro, G. Osteopontin Regulates VEGFA and ICAM-1 mRNA Expression in Breast Carcinoma. Am. J. Clin. Pathol. 2015, 143, 812–822.
- Wu, X.L.; Lin, K.J.; Bai, A.P.; Wang, W.X.; Meng, X.K.; Su, X.L.; Hou, M.X.; Dong, P.D.; Zhang, J.J.; Wang, Z.Y.; et al. Osteopontin knockdown suppresses the growth and angiogenesis of colon cancer cells. World J. Gastroenterol. 2014, 20, 10440–10448.
- Gupta, A.; Zhou, C.Q.; Chellaiah, M.A. Osteopontin and MMP9: Associations with VEGF Expression/Secretion and Angiogenesis in PC3 Prostate Cancer Cells. Cancers 2013, 5, 617–638.
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422.
- Diaz, A.H.; Rodgers, G.M.; Gilreath, J.A. Enoxaparin once daily vs. twice daily dosing for the treatment of venous thromboembolism in cancer patients: A literature summary. J. Oncol. Pharm. Pract. 2012, 18, 264–270.
- Schulze, E.B.; Hedley, B.D.; Goodale, D.; Postenka, C.O.; Al-Katib, W.; Tuck, A.B.; Chambers, A.F.; Allan, A.L. The thrombin inhibitor Argatroban reduces breast cancer malignancy and metastasis via osteopontin-dependent and osteopontin-independent mechanisms. Breast Cancer Res. Treat. 2008, 112, 243–254.
- Niers, T.M.; Bruggemann, L.W.; GL, V.A.N.S.; Liu, R.D.; Versteeg, H.H.; Buller, H.R.; CJ, V.A.N.N.; Reitsma, P.H.; Spek, C.A.; Richel, D.J. Long-term thrombin inhibition promotes cancer cell extravasation in a mouse model of experimental metastasis. J. Thromb. Haemost. 2009, 7, 1595–1597.
- Kahale, L.A.; Matar, C.F.; Tsolakian, I.; Hakoum, M.B.; Barba, M.; Yosuico, V.E.; Terrenato, I.; Sperati, F.; Schunemann, H.; Akl, E.A. Oral anticoagulation in people with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst. Rev. 2021, 10, CD006466.
- Chiasakul, T.; Zwicker, J.I. The impact of warfarin on overall survival in cancer patients. Thromb. Res. 2022, 213, S113–S119.
- Prandoni, P.; Lensing, A.W.; Piccioli, A.; Bernardi, E.; Simioni, P.; Girolami, B.; Marchiori, A.; Sabbion, P.; Prins, M.H.; Noventa, F.; et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002, 100, 3484–3488.
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427.
- Viallard, C.; Larrivee, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426.
- Farge, D.; Debourdeau, P.; Beckers, M.; Baglin, C.; Bauersachs, R.M.; Brenner, B.; Brilhante, D.; Falanga, A.; Gerotzafias, G.T.; Haim, N.; et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J. Thromb. Haemost. 2013, 11, 56–70.
- Lin, R.J.; Green, D.L.; Shah, G.L. Therapeutic Anticoagulation in Patients with Primary Brain Tumors or Secondary Brain Metastasis. Oncologist 2018, 23, 468–473.
- Maeda, N.; Ohashi, T.; Chagan-Yasutan, H.; Hattori, T.; Takahashi, Y.; Harigae, H.; Hasegawa, H.; Yamada, Y.; Fujii, M.; Maenaka, K.; et al. Osteopontin-integrin interaction as a novel molecular target for antibody-mediated immunotherapy in adult T-cell leukemia. Retrovirology 2015, 12, 99.
- Dai, J.; Li, B.; Shi, J.; Peng, L.; Zhang, D.; Qian, W.; Hou, S.; Zhao, L.; Gao, J.; Cao, Z.; et al. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol. Immunother. 2010, 59, 355–366.
- Shojaei, F.; Scott, N.; Kang, X.; Lappin, P.B.; Fitzgerald, A.A.; Karlicek, S.; Simmons, B.H.; Wu, A.; Lee, J.H.; Bergqvist, S.; et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J. Exp. Clin. Cancer Res. 2012, 31, 26.
- Kon, S.; Yokosaki, Y.; Maeda, M.; Segawa, T.; Horikoshi, Y.; Tsukagoshi, H.; Rashid, M.M.; Morimoto, J.; Inobe, M.; Shijubo, N.; et al. Mapping of functional epitopes of osteopontin by monoclonal antibodies raised against defined internal sequences. J. Cell. Biochem. 2002, 84, 420–432.
- Yamamoto, N.; Nakashima, T.; Torikai, M.; Naruse, T.; Morimoto, J.; Kon, S.; Sakai, F.; Uede, T. Successful treatment of collagen-induced arthritis in non-human primates by chimeric anti-osteopontin antibody. Int. Immunopharmacol. 2007, 7, 1460–1470.
- Boumans, M.J.; Houbiers, J.G.; Verschueren, P.; Ishikura, H.; Westhovens, R.; Brouwer, E.; Rojkovich, B.; Kelly, S.; den Adel, M.; Isaacs, J.; et al. Safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of the monoclonal antibody ASK8007 blocking osteopontin in patients with rheumatoid arthritis: A randomised, placebo controlled, proof-of-concept study. Ann. Rheum. Dis. 2012, 71, 180–185.
- Farrokhi, V.; Chabot, J.R.; Neubert, H.; Yang, Z. Assessing the Feasibility of Neutralizing Osteopontin with Various Therapeutic Antibody Modalities. Sci. Rep. 2018, 8, 7781.
- Wu, J.; Pungaliya, P.; Kraynov, E.; Bates, B. Identification and quantification of osteopontin splice variants in the plasma of lung cancer patients using immunoaffinity capture and targeted mass spectrometry. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2012, 17, 125–133.




