Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ali Athafah Tomah | -- | 2028 | 2023-09-11 21:47:51 | | | |
| 2 | Camila Xu | Meta information modification | 2028 | 2023-09-12 03:16:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tomah, A.A.; Zhang, Z.; Alamer, I.S.A.; Khattak, A.A.; Ahmed, T.; Hu, M.; Wang, D.; Xu, L.; Li, B.; Wang, Y. Trichogenic Nanoparticles of Trichoderma. Encyclopedia. Available online: https://encyclopedia.pub/entry/49039 (accessed on 07 February 2026).
Tomah AA, Zhang Z, Alamer ISA, Khattak AA, Ahmed T, Hu M, et al. Trichogenic Nanoparticles of Trichoderma. Encyclopedia. Available at: https://encyclopedia.pub/entry/49039. Accessed February 07, 2026.
Tomah, Ali Athafah, Zheng Zhang, Iman Sabah Abd Alamer, Arif Ali Khattak, Temoor Ahmed, Minjun Hu, Daoze Wang, Lihui Xu, Bin Li, Yanli Wang. "Trichogenic Nanoparticles of Trichoderma" Encyclopedia, https://encyclopedia.pub/entry/49039 (accessed February 07, 2026).
Tomah, A.A., Zhang, Z., Alamer, I.S.A., Khattak, A.A., Ahmed, T., Hu, M., Wang, D., Xu, L., Li, B., & Wang, Y. (2023, September 11). Trichogenic Nanoparticles of Trichoderma. In Encyclopedia. https://encyclopedia.pub/entry/49039
Tomah, Ali Athafah, et al. "Trichogenic Nanoparticles of Trichoderma." Encyclopedia. Web. 11 September, 2023.
Copy Citation
Trichoderma is the asexual stage of the filamentous Hypocrea genus belonging to the Ascomycota fungi division. The species of this genus are free-living saprophytic fungi found in all soils, with an average presence in temperate and tropical soils of nearly 101-103 culturable propagules per gram.
Trichoderma spp.
cell-free culture filtrate
mycosynthesis
nanoparticles
1. Introduction (General Properties of Trichoderma)
Although the genus Trichoderma was first discovered by Persoon in 1794, it was not until Weindling published his first full paper on Trichoderma lignorum in 1934 that its role as a biological control agent was realized. Weindling’s paper showed that this species can control plant diseases, and this discovery led to a renewed interest in Trichoderma as a potential biocontrol agent [1]. Trichoderma is the asexual stage of the filamentous Hypocrea genus belonging to the Ascomycota fungi division. The species of this genus are free-living saprophytic fungi found in all soils, with an average presence in temperate and tropical soils of nearly 101-103 culturable propagules per gram [2][3]. These fungi reproduce asexually by the production of conidia and chlamydospores and in wild habitats by ascospores [4]. The genus Trichoderma is one of the most frequently isolated soil microorganisms. It has several useful properties, including being non-pathogenic to humans and plants, environmentally friendly, easy to isolate and culture, capable of rapid growth on a variety of inexpensive organic substrates, and able to produce a wide range of proteins, enzymes, and secondary metabolites [5][6][7]. The species of this genus are genetically quite diverse, with differences in capabilities among strains. These fungi have been widely used as biocontrol agents. Some Trichoderma spp. are known to control plant diseases through a variety of mechanisms, either indirectly (by competing for nutrients and space, modifying the environmental conditions, or promoting plant growth and plant defense mechanisms and antibiosis) or directly (by mechanisms such as mycoparasitism or the synergistic action of several mechanisms) [8]. Trichoderma spp. produce a variety of antibiotics and secondary metabolites that play an important role in inhibiting pathogens. The antibiotic’s mechanism is via low-molecular-weight diffusible organic compounds excreted by Trichoderma that inhibit the growth of the pathogen. The bioactive compounds secreted by Trichoderma are natural compounds that are chemically different, non-polar, and low molecular mass (less than 3000 Daltons) [9][10] and include polyketides, alkaloids, terpenoids, non-ribosomally biosynthesized peptides (NRPs), and metabolites of mixed biogenesis [10][11]. The compounds, which are typically composed of 5–20 amino acid residues, have a high content of α-aminoisobutyric acid (Aib) with an acylated N-terminus and a complex C-terminus that may consist of a free or methoxy-substituted 2-amino alcohol, amine, amide, free amino acid or sugar alcohol [12][13].
The antibiotic production of over 180 secondary metabolites, representing different classes of chemical compounds exhibiting biocontrol activity, has been reported for isolates of Trichoderma [10][14]. For example, gliovirin is produced from Gliocladium virens and has antimicrobial active against of Pythium ultimum [15], while gliotoxin from T. virens [16] inhibits the mycelium of both P. ultimum and Rhizoctonia solani [17]. Some species produce types of peptaibols (linear peptides), such as trichokonin VI from T. pseudokoningii SMF2, which has a wide antimicrobial spectrum against several bacteria, yeasts, and filamentous fungi [18], and trichorzianine from T. harzianum, which exhibits antibacterial activity against S. aureus [19], while koninginin A and B (polyketides group) are secreted by T. koningii [20][21]. Pyrone 6-PP was first discovered in a culture broth of T. viride [22] and is classified as a volatile organic compound. The 6-PP compound displayed good antifungal activity against Aspergillus flavus, Penicillium expansum, and Fusarium acuminatum [23]. Other antifungal compounds isolated from Trichoderma spp., belonging to different chemical classes, have been used in plant protection as an environmentally friendly and efficient management tool against a variety of phytopathogens.
These metabolites can be either overproduced or combined with other metals to create new formulations that are more efficient for use in several fields, including in the control of plant diseases. Biomolecules can bind to metals through their proteins and amino acid residues, forming a coating on the surface of the NPs. This coating, known as capping, can increase the stability of the NPs and prevent them from aggregating [24][25]. Subsequent stabilization can also be provided by free amino groups or cysteine residues or through the electrostatic attraction resulting from negative carboxyl groups provided by mycelial cell wall enzymes present in the filtrate. Furthermore, the ability of the thiol (-SH) group to form disulfides—perhaps the most important bridging structure in nature—with cysteine subunits of endogenous proteins renders them unique among the functional groups utilized in nanotechnology to form thiolated disulfide bonds that tightly bind with noble metals, leading to the formation of stable NPs [24][26][27]. For example, FTIR analysis confirmed that a gliotoxin bioactive compound secreted by T. virens is responsible for capping and reducing Ag+ and the biosynthesis of silver NPs through binding of each of the negative carboxyl groups, sulfur, and oxygen from the hydroxyl groups of gliotoxin with silver [28].
2. Trichogenic Nanoparticles
The term “mycosynthesis” was first used to describe the synthesis of NPs using the fungus F. acuminatum by Ingle et al. [29]. Since then, the term “Myconanotechnology” has expanded to include literature studies that describe NPs synthesized using fungi [30]. The term “Trichogenic” has recently been used to describe the biogenic synthesis of selenium NPs using six species of Trichoderma [31].
Among the most important fungi that are used in biological control against phytopathogens, the Trichoderma species have the potential to be used to synthesize NPs on a large scale by using an environmentally friendly production process. According to the inventory list conducted by Cai and Druzhinina [32], there are around 460 species with valid names in the Trichoderma genus that have been deposited in public updated databases available on the International Subcommission on Taxonomy of Trichoderma website (www.trichoderma.info, accessed on 25 February 2022). However, and from a thorough inventory of literature studies, researchers found that only about 13 species belonging to the Trichoderma genus show the ability for NP synthesis, including T. harzianum, T. asperellum, T. viride, T. atroviride, T. virens, T. longibrachiatum, T. pseudokoningii, T. reesei, T. koningii, T. brevicompactum, T. citrinoviride, T. hamatum, and T. gamsii. These species are known to be applied globally as biological control agents against different plant pathogens. Although the number of Trichoderma species has been increasing, research on the synthesis of NPs using this genus is still in its early stages. More research is needed to explore the potential of Trichoderma species for nanoparticle synthesis.
The first report on the use of Trichoderma in the synthesis of silver NPs by Mukherjee [33] dates back to 2008. This strategy has attracted much more attention in the most recent decade as the synthesis mechanism for various NPs using diverse metals such as silver (Ag), gold (Au), copper (Cu), zinc (Zn), and so on. NPs of both silver and gold are considered more secure in contrast to other metallic NPs [34]. Through a survey of the literature published from the years 2008 to 2021, more than 100 research papers published in the approved journals adopted the use of Trichoderma in the synthesis of NPs from different metals. Using the percentage equation [(M/N) × 100)], where M means the number of studies that used Trichoderma in the synthesis of NPs from a specific metal and N means the number of research studies that used Trichoderma in the synthesis of NPs from different minerals, the proportional analysis shows that silver metal occupied the leading position in the synthesis of NPs by the Trichoderma species, which was 54% of the total of other metals (Figure 1A). On the other hand, most of the Trichoderma spp. applied in biological control were suggested to have the capacity to biosynthesize NPs. Five different species of Trichoderma, viz., T. asperellum, T. harzianum, T. longibrachiatum, T. pseudokoningii, and T. virens, led in the production of silver NPs (AgNPs) [35]. Spherical AgNPs with sizes from 2 to 15 nm were formed using cell filtrates of T. inhamatum [36]. Production of silver NPs was achieved through extracellular reduction by six isolates of T. virens, and a single and aggregated form was obtained, which was uniform in shape and had a size of 8–60 nm [37]. Meanwhile, six species belonging to the Trichoderma genus, viz., T. asperellum, T. harzianum, T. atroviride, T. virens, T. longibrachiatum, and T. brevicompactum, were successful in the biogenic synthesis of selenium NPs (SeNPs) [31].
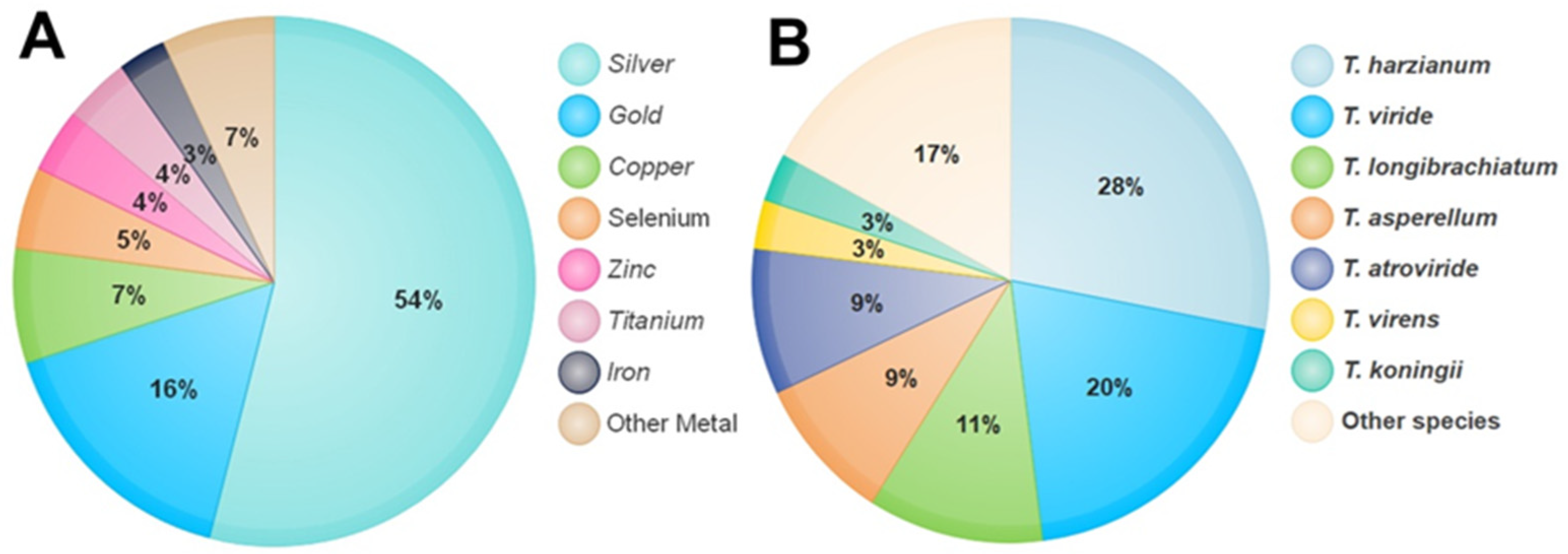
Figure 1. The percentage of metals used in the synthesis of NPs by Trichoderma species (A). Percentage of Trichoderma species used in the synthesis of NPs (B).
Also, using the extracellular filtrate of T. viride to yield of AuNPs, a mixture of spheres, triangles, hexagons, and rod shapes with sizes 20–30 nm were obtained, with a few as big as 120 nm [38]. Meanwhile, the AuNPs obtained using the T. asperellum biomass were well dispersed, with diverse shapes such as spheres, triangles, and hexagons [39].
A T. hamatum cell-free filtrate is used for the synthesis of AuNPs of dimensions 5–30 nm and with diverse shapes such as spheres, pentagons, and hexagons [40]. Additionally, magnesium oxide (MgO) NPs at a size ranging from 45.12 to 95.37 nm were obtained using the extracellular approach of T. viride [41], zinc oxide (ZnO) NPs with a mean size of 30.34 nm were biosynthesized using the fungal mycelial water extract derived from T. harzianum [42], copper oxide (CuO) NPs with crystalline and spherical-shaped particles with an average size of 110 nm were produced using a cell-free extract of T. asperellum [43], and the biosynthesis of cadmium sulfide (CdS) NPs with a size range of 3–8 nm and a spherical morphology has been already reported using the fungal biomass of T. harzianum [44]. Titanium oxide (TiO2) NPs of highly irregular size (10–400 nm) and triangular, pentagonal, spherical, and rod-like shapes were synthesized using TiO2 with the extract of T. citrinoviride as a reducing agent [45]. A literature survey of research papers published from 2008 to 2021 in approved journals revealed that more than 100 studies had adopted the use of Trichoderma in the synthesis of NPs. Using the percentage equation [(T/N) × 100)], where T means the number of studies that used one species of Trichoderma in the synthesis of NPs, while N means the number of research that used all different types of Trichoderma in the synthesis of NPs, the proportional analysis shows the potential of most Trichoderma species in the biosynthesis of NPs by the reduction of a wide range of diverse metals (Figure 1B).
Among the interesting metals, selenium (Se), and its applications in biomedicine, agriculture, and environmental health, has become of great research interest in recent decades. Trichoderma has been used in the formation of nanoproducts by selenium metal due to its ability to reduce selenite and convert it into less toxic derivatives [46].
Selenium (Se) NPs are gaining importance in the field of medicine owing to their antibacterial and anticancer properties [47]. SeNPs at a size of 20–220 nm and spherical and pseudo-spherical Se particles were synthesized by the SeO2 reduction process in the Trichoderma spp. WL-Go culture broth and the pure product were obtained and characterized through filtration of the culture broth supernatant [48]. SeNPs, as plant biostimulants, were obtained using the filtrate of Trichoderma strains to mediate the reduction of selenium (Se) ions, and their effects on different stages of Vigna radiata plant growth were observed. Besides the growth improvement and protection of the plants from phytopathogens, SeNPs were found to be much less toxic than Se selenite in the tested seeds [49]. Inoculated plants with biosynthesized SeNPs from T. atroviride exhibited a significant level of protection of 72.9% against late blight of tomato caused by P. infestans, where the defense responses noted included an accumulation of lignin, callose, and hydrogen peroxide that supported the cellular defense mechanism, while the biochemical defense mechanism collaborated by elevating the levels of lipoxygenase (LOX), phenylalanine lyase (PAL), β-1,3-glucanase (GLU), superoxide dismutase (SOD) in plants [50]. The selenium (Se) NPs synthesized using the Trichoderma sp. fungus culture filtrate showed great effectiveness as a larvicidal and antifeedant agent at a concentration of 100 µg/mL for 48 h, and they can be used for the control of Spodoptera litura larvae that infect peanut and castor plants [51]. Acknowledging these Se advantages, the synthesis of SeNPs via Trichoderma is preferred as it offers a safer, more eco-friendly, and less toxic alternative compared with other metals. Additionally, biologically synthesized SeNPs tend to be more stable as they have a natural coating of organic material on their surface, which will prevent the aggregation of the NPs over a long period of time [51].
References
- Weindling, R. Studies on a lethal principle effective in the parasitic action of Trichoderma lignorum on Rhizoctonia solani and other soil fungi. Phytopathology 1934, 24, 1153–1179.
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56.
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759.
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2013, 51, 105–129.
- Mukhopadhyay, R.; Kumar, D. Trichoderma: A beneficial antifungal agent and insights into its mechanism of biocontrol potential. Egypt. J. Biol. Pest. Control 2020, 30, 133.
- Abdel-Fattah, G.M.; Shabana, Y.M.; Ismail, A.E.; Rashad, Y.M. Trichoderma harzianum: A biocontrol agent against Bipolaris oryzae. Mycopathologia 2007, 164, 81–89.
- Dubey, S.C.; Aradhika, T.; Dureja, P.; Grover, A. Characterization of secondary metabolites and enzymes produced by Trichoderma species and their efficacy against plant pathogenic fungi. Indian J. Agric. Sci. 2011, 81, 455–461.
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codon, A.C. Biocontrol mechanisms of Trichoderma strains. Int. J. Microbiol. 2004, 7, 249–260.
- Demain, A.L.; Fang, A. The natural functions of secondary metabolites. In History of Modern Biotechnology I; Fiechter, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–39.
- Reino, J.L.; Guerrero, R.F.; Hernandez-Galan, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123.
- Almassi, F.; Ghisalberti, E.L.; Narbey, M.J.; Sivasithamparam, K. New antibiotics from strains of Trichoderma harzianum. J. Nat. Prod. 1991, 54, 396–402.
- Ayers, S.; Ehrmann, B.M.; Adcock, A.F.; Kroll, D.J.; Carcache de Blanco, E.J.; Shen, Q.; Swanson, S.M.; Falkinham, J.O.; Wani, M.C.; Mitchell, S.M. Peptaibols from two unidentified fungi of the order Hypocreales with cytotoxic, antibiotic, and anthelmintic activities. J. Pept. Sci. 2012, 18, 500–510.
- Whitmore, L.; Chugh, J.K.; Snook, C.F.; Wallace, B.A. The peptaibol database: A sequence and structure resource. J. Pept. Sci. 2003, 9, 663–665.
- Gams, W.; Bissett, J. Morphology and identification of Trichoderma. In Trichoderma and Gliocladium; Basic Biology, Taxonomy and Genetics; Kubicek, C.P., Harman, G.E., Eds.; Taylor & Francis: London, UK, 1998; Volume 1, pp. 3–34.
- Howell, C.R.; Stipanovic, R.D. Gliovirin, a new antibiotic from Gliocladium virens, and its role in the biological control of Pythium ultimum. Can. J. Microbiol. 1983, 29, 321–324.
- Scharf, D.H.; Brakhage, A.A.; Mukherjee, P.K. Gliotoxin–bane or boon? Environ. Microbiol. 2016, 18, 1096–1109.
- Harris, A.R.; Lumsden, R.D. Interactions of Gliocladium virens with Rhizoctonia solani and Pythium ultimum in non-sterile potting medium. Biocontrol. Sci. Technol. 1997, 7, 37–48.
- Xiao-Yan, S.; Qing-Tao, S.; Shu-Tao, X.; Xiu-Lan, C.; Cai-Yun, S.; Yu-Zhong, Z. Broad-spectrum antimicrobial activity and high stability of Trichokonins from Trichoderma koningii SMF2 against plant pathogens. FEMS Microbiol. Lett. 2006, 260, 119–125.
- Goulard, C.; Hlimi, S.; Rebuffat, S.; Bodo, B.; Trichorzins, H.A. MA, antibiotic peptides from Trichoderma harzianum. I. Fermentation, isolation and biological properties. J. Antibiot. 1995, 48, 1248–1253.
- Cutler, H.G.; Himmelsbach, D.S.; Arrendale, R.F.; Cole, P.D.; Cox, R.H. Koninginin A: A novel plant growth regulator from Trichoderma koningii. Agric. Biol. Chem. 1989, 53, 2605–2611.
- Cutler, H.G.; Himmelsbach, D.S.; Yagen, B.; Arrendale, R.F.; Jacyno, J.M.; Cole, P.D.; Cox, R.H. Koninginin B: A biologically active congener of koninginin A from Trichoderma koningii. J. Agric. Food Chem. 1991, 39, 977–980.
- Collins, R.P.; Halim, A.F. Characterization of the major aroma constituent of the fungus Trichoderma viride. J. Agric. Food Chem. 1972, 20, 437–438.
- Ismaiel, A.A.; Ali, D.M. Antimicrobial properties of 6-pentyl-α-pyrone produced by endophytic strains of Trichoderma koningii and its effect on aflatoxin B1 production. Biologia 2017, 72, 1403–1415.
- Ahmed, T.; Noman, M.; Gardea-Torresdey, J.L.; White, J.C.; Li, B. Dynamic interplay between nano-enabled agrochemicals and the plant-associated microbiome. Trends Plant Sci. 2023; in press.
- Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf. B Biointerfaces 2012, 96, 69–74.
- Husseiny, S.M.; Salah, T.A.; Anter, H.A. Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. Beni Suef Univ. J. Basic Appl. Sci. 2015, 4, 225–231.
- Hock, N.; Racaniello, G.F.; Aspinall, S.; Denora, N.; Khutoryanskiy, V.V.; Bernkop-Schnürch, A. Thiolated Nanoparticles for Biomedical Applications: Mimicking the Workhorses of Our Body. Adv. Sci. 2022, 9, 2102451.
- Tomah, A.A.; Abd Alamer, I.S.; Li, B.; Zhang, J.-Z. Mycosynthesis of Silver Nanoparticles Using Screened Trichoderma Isolates and Their Antifungal Activity against Sclerotinia sclerotiorum. Nanomaterials 2020, 10, 1955.
- Ingle, A.; Gade, A.; Pierrat, S.; Sonnichsen, C.; Rai, M. Mycosynthesis of silver NPs using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr. Nanosci. 2008, 4, 141–144.
- Rai, P.R.; Cool, C.D.; King, J.A.C.; Stevens, T.; Burns, N.; Winn, R.A.; Kasper, M.; Voelkel, N.F. The cancer paradigm of severe pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2008, 178, 558–564.
- Nandini, B.; Hariprasad, P.; Prakash, H.S.; Shetty, H.S.; Geetha, N. Trichogenic-selenium nanoparticles enhance disease suppressive ability of Trichoderma against downy mildew disease caused by Sclerospora graminicola in pearl millet. Sci. Rep. 2017, 7, 2612.
- Cai, F.; Druzhinina, I.S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021, 107, 1–69.
- Mukherjee, P.; Roy, M.; Mandal, B.P.; Dey, G.; Mukherjee, P.; Ghatak, J.; Tyagi, A.K.; Kale, S.P. Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology 2008, 19, 075103.
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851.
- Devi, T.P.; Kulanthaivel, S.; Kamil, D.; Borah, J.L.; Prabhakaran, N.; Srinivasa, N. Biosynthesis of silver nanoparticles from Trichoderma species. Indian J. Exp. Biol. 2013, 51, 543–547.
- Hussein, M. Silver tolerance and silver nanoparticle biosynthesis by Neoscytalidium novaehollandae and Trichoderma inhamatum. Eur. J. Biol. Res. 2016, 6, 28–35.
- Peeran, M.F.; Deeba, K.; Lakshman, P. Extracellular myco-synthesis of silver nanoparticles from and Trichoderma virens and Metarhizzium anisopliae. J. Mycol. Plant Pathol. 2017, 47, 424–429.
- Mishra, A.; Kumari, M.; Pandey, S.; Chaudhry, V.; Gupta, K.C.; Nautiyal, C.S. Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Bioresour. Technol. 2014, 166, 235–242.
- Qu, Y.; Shen, W.; Pei, X.; Ma, F.; You, S.; Li, S.; Wang, J.; Zhou, J. Biosynthesis of gold nanoparticles by Trichoderma sp. WL-Go for azo dyes decolorization. J. Environ. Sci. 2017, 56, 79–86.
- Abdel-Kareem, M.M.; Zohri, A.A. Extracellular mycosynthesis of gold nanoparticles using Trichoderma hamatum: Optimization, characterization and antimicrobial activity. Lett. Appl. Microbiol. 2018, 67, 465–475.
- Alrabadi, N.I.; Thalij, K.M.; Hussein, E.I.; Al-Trad, B.M. Antibacterial Activity of Ag and MgO Nanoparticles Synthesized By Trichoderma viride. J. Appl. Environ. Biol. Sci. 2017, 7, 94–101.
- Saravanakumar, K.; Jeevithan, E.; Hu, X.; Chelliah, R.; Oh, D.; Wang, M. Enhanced anti-lung carcinoma and anti-biofilm activity of fungal molecules mediated biogenic zinc oxide nanoparticles conjugated with β-D-glucan from barley. J. Photochem. Photobiol. B 2020, 203, 111728.
- Saravanakumar, K.; Shanmugam, S.; Varukattu, N.B.; MubarakAli, D.; Kathiresan, K.; Wang, M. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J. Photochem. Photobiol. B 2019, 190, 103–109.
- Bhadwal, A.S.; Tripathi, R.M.; Gupta, R.K.; Kumar, N.; Singh, R.P.; Shrivastav, A. Biogenic synthesis and photocatalytic activity of CdS nanoparticles. RSC Adv. 2014, 4, 9484–9490.
- Arya, S.; Sonawane, H.; Math, S.; Tambade, P.; Chaskar, M.; Shinde, D. Biogenic titanium nanoparticles (TiO2NPs) from Trichoderma citrinoviride extract: Synthesis, characterization and antibacterial activity against extremely drug-resistant Pseudomonas aeruginosa. Int. Nano Lett. 2020, 26, 35–42.
- Gharieb, M.M.; Wilkinson, S.C.; Gadd, G.M. Reduction of selenium oxyanions by unicellular, polymorphic and filamentous fungi: Cellular location of reduced selenium and implications for tolerance. J. Ind. Microbiol. 1995, 14, 300–311.
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566.
- Diko, C.; Zhang, H.; Lian, S.; Fan, S.; Li, Z.; Qu, Y. Optimal synthesis conditions and characterization of selenium nanoparticles in Trichoderma sp. WL-Go culture broth. Mater. Chem. Phys. 2020, 246, 122583.
- Bărbieru, O.; Dimitriu, L.; Călin, M.; Răut, I.; Constantinescu-Aruxandei, D.; Oancea, F. Plant Biostimulants Based on Selenium Nanoparticles Biosynthesized by Trichoderma Strains. Proceedings 2019, 29, 95.
- Joshi, S.M.; De Britto, S.; Jogaiah, S. Myco-engineered selenium nanoparticles elicit resistance against tomato late blight disease by regulating differential expression of cellular, biochemical and defense responsive genes. J. Biotechnol. 2021, 325, 196–206.
- Arunthirumeni, M.; Veerammal, V.; Shivakumar, M. Biocontrol Efficacy of Mycosynthesized Selenium Nanoparticle Using Trichoderma sp. on Insect Pest Spodoptera litura. J. Clust. Sci. 2021, 33, 1645–1653.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
677
Revisions:
2 times
(View History)
Update Date:
13 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




