Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vasile Valeriu Lupu | -- | 1605 | 2023-09-11 20:56:39 | | | |
| 2 | Wendy Huang | Meta information modification | 1605 | 2023-09-12 03:19:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pantazi, A.C.; Kassim, M.A.K.; Nori, W.; Tuta, L.A.; Mihai, C.M.; Chisnoiu, T.; Balasa, A.L.; Mihai, L.; Lupu, A.; Frecus, C.E.; et al. Relationship between Gut Microbiota and CKD and ESKD. Encyclopedia. Available online: https://encyclopedia.pub/entry/49038 (accessed on 07 February 2026).
Pantazi AC, Kassim MAK, Nori W, Tuta LA, Mihai CM, Chisnoiu T, et al. Relationship between Gut Microbiota and CKD and ESKD. Encyclopedia. Available at: https://encyclopedia.pub/entry/49038. Accessed February 07, 2026.
Pantazi, Alexandru Cosmin, Mustafa Ali Kassim Kassim, Wassan Nori, Liliana Ana Tuta, Cristina Maria Mihai, Tatiana Chisnoiu, Adriana Luminita Balasa, Larisia Mihai, Ancuta Lupu, Corina Elena Frecus, et al. "Relationship between Gut Microbiota and CKD and ESKD" Encyclopedia, https://encyclopedia.pub/entry/49038 (accessed February 07, 2026).
Pantazi, A.C., Kassim, M.A.K., Nori, W., Tuta, L.A., Mihai, C.M., Chisnoiu, T., Balasa, A.L., Mihai, L., Lupu, A., Frecus, C.E., Lupu, V.V., Chirila, S.I., Badescu, A.G., Hangan, L., & Cambrea, S.C. (2023, September 11). Relationship between Gut Microbiota and CKD and ESKD. In Encyclopedia. https://encyclopedia.pub/entry/49038
Pantazi, Alexandru Cosmin, et al. "Relationship between Gut Microbiota and CKD and ESKD." Encyclopedia. Web. 11 September, 2023.
Copy Citation
A microbiota describes all microorganisms that colonize the epidermis, respiratory tract, genital system, and especially the gastrointestinal tract. In a healthy state, gut microbiota (GM) plays several critical roles in our bodies, such as helping to metabolize nutrients, preserving the structural soundness of the gut’s mucosal barrier, moderating immune responses, and providing defense against harmful pathogens. The gut microbiota (GM) plays a vital role in human health, with increasing evidence linking its imbalance to chronic kidney disease (CKD) and end-stage kidney disease (ESKD).
gut microbiota
chronic kidney disease
end-stage kidney disease
dysbiosis
inflammation
complications
short-chain fatty acids
1. Introduction
Microbes colonize every surface of the human body, but an increasing proportion of microbes inhabit the intestine. Consequently, gut microbiota (GM) is regarded as a “forgotten organ”. In a healthy state, GM plays several critical roles in our bodies, such as helping to metabolize nutrients, preserving the structural soundness of the gut’s mucosal barrier, moderating immune responses, and providing defense against harmful pathogens [1][2]. A microbiota describes all microorganisms that colonize the epidermis, respiratory tract, genital system, and especially the gastrointestinal tract. GM is constantly evolving and displaying a wide diversity within the same person and in comparison, to others [3]. GM connects with vital organs, including the brain, bone marrow, cardiovascular system (CVS), kidney, body’s immune system, and the central nervous system, and has been seen as a potential cause for a variety of diseases in the aforementioned organs [4][5][6][7][8][9][10]. GM activates immune cells derived from bone marrow, resulting in a low-grade inflammatory reaction that affects the brain and kidneys via circulation [11]. Simultaneously, peripheral stimuli affect the brain and modulate neural inputs to the kidney, intestine, and lymphoid organs [11]. This bidirectional relationship lends credence to the notion that GM modulation is an innovative method for the management of kidney diseases [12][13].
Dysbiosis is an imbalance or perturbation in the GMs composition that results in a proliferation of harmful bacteria like Enterobacteriaceae or a reduction in beneficial bacteria like Bifidobacterium and Lactobacillus [14]. For individuals dealing with ongoing kidney conditions, such as chronic kidney disease (CKD) and terminal kidney failure, often referred to as end-stage kidney disease (ESKD), the harmonious and mutually advantageous connection is disrupted, leading to an imbalance known as dysbiosis [15]. The consequences of this dysbiosis go beyond the gut and impact the kidneys via the so-called gut–kidney axis [13]. One of these adverse outcomes is the overproduction of uremic toxins such as indoxyl sulfate and p-cresyl sulfate, which are derived from bacterial metabolism of dietary amino acids [16]. In healthy individuals, these toxins are efficiently excreted by the kidneys, but in CKD and ESKD, their clearance is significantly reduced, leading to a high plasma concentration of these toxins [17]. Increasing evidence confirms that dysbiosis by itself contributes to CKD development and progression [18].
CKD and ESKD affect roughly 10 percent of the world’s population and impose a substantial financial burden on the healthcare system [19]. Owing to an insufficient understanding of both the origin and the bodily responses associated with CKD, there have not been any significant advances in decades, despite efforts to slow the progression of CKD [20][21].
2. How do CKD and ESKD Contribute to Disturbed GM?
CKD cases have common dietary restrictions, like low protein intake and avoiding foods rich in potassium and phosphorus [22][23][24], which affect the composition of GM [25]. Building-up of waste products (uremia toxin) in the blood owing to impaired kidney function directly affects the GM and leads to dysbiosis [26]. Prescribed Medications: Patients are often on antibiotics, immunosuppressants, and phosphate binders [27][28]. Collectively, they can disrupt the balance of GM [29]. Patients with ESKD often require dialysis, which involves filtering waste products from the bloodstream via a machine or peritoneal dialysis fluid [30]. Dialysis by itself can impact GM composition [31]. A state of systemic inflammation associated with CKD and ESKD may alter the GM composition and function [32]. Chronic kidney disease patients often suffer altered intestinal motility, leading to constipation or diarrhea. These bowel changes can impact the GM [33] (Figure 1).
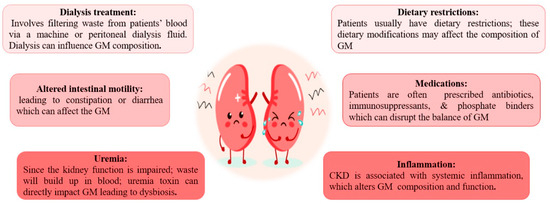
Figure 1. Causes of altered gut microbiota in patients with chronic kidney disease and end-stage kidney disease.
3. How Does Disturbed GM Impact CKD and ESKD Progression?
It is important to note that these causes may interact with each other, leading to a complex interplay between gut dysbiosis and chronic renal disease progression. As renal function declines, the capacity to eliminate toxins decreases, leading to a detrimental cycle of gut dysbiosis and exacerbating uremia [34]. Reduced microbial diversity has been linked to an increase in disease severity and deteriorating health outcomes [35]. Some GM can transform specific toxins into perilous byproducts, which intensify renal damage and induce widespread inflammation within the body [36]. GM plays a pivotal role in the processes of nutrient metabolism and energy extraction [37]. However, when dysbiosis occurs, it can have detrimental effects on nutrient assimilation and metabolism, leading to conditions such as malnutrition or an imbalanced energy equilibrium [38]. The presence of altered gut microbiota leads to the disruption of the intestinal barrier function, which permits the passage of microbial components and harmful substances into the bloodstream [39]. This, commonly referred to as “Leaky gut syndrome” or “endotoxemia”, subsequently initiates a systemic inflammatory response [40]. Dysbiosis and the associated modification of GM can result in an impaired immune response, making the host more susceptible to infections and inflammatory diseases [41][42].
4. How Does Disturbed GM Contribute to CKD- and ESKD-Related Complications?
There is growing data indicating a connection between dysbiosis and complications associated with CKD, including high blood pressure, cardiovascular incidents, disorders related to minerals and bones (MBD), and cognitive impairments.
- ❖
-
CKD- and ESKD-related cardiovascular disease
Several studies find that diverse mechanisms play a role in the development and progression of cardiovascular disease, a major mortality cause among those patients [43][44]. These include increased reactive oxygen species (ROS) production, leukocyte activation, pro-inflammatory cytokines production, myocyte hypertrophy, and dyslipidemia. This relationship between the digestive tract and the heart is known as the gut–heart axis [45][46]. Lin et al. [47] found an association between elevated pCS levels and increased CVS mortality in CKD patients. Conversely, low TMAO was associated with a 1.7-fold greater risk of severe CVS events [48].
- ❖
-
Cognitive psychiatric disorders
Cognitive psychiatric disorders are prevalent among CKD patients and are associated with an increase in morbidity and mortality [49]. The gut–brain axis promotes dysregulation of the hypothalamus–pit axis [50]. The contribution of gut-microbiota-derived toxins to cognitive dysfunction is conveyed through mechanisms like direct toxicity or other potential influences, such as oxidative stress, inflammation, dysfunction of endothelial cells, and vascular calcification [51]. Lin et al. [52] demonstrated in a study involving 260 hemodialytic cases that the circulating free form of IS is substantially associated with decreased cognitive function, especially in the memory domain, mental manipulation, and language ability.
- ❖
-
CKD—disorder of bone and minerals
This syndrome was recently renamed to encompass biochemical, skeletal, and CVS pathogenesis in addition to bone disease [53]. It was suggested that elevated GM-derived toxins contribute to the onset of bone abnormalities in CKD [54]. Previous research has shown that increased levels of IS can impede the function of osteoblasts and have a restraining effect on osteoclasts and parathyroid hormone, which may consequently affect the bone remodeling process in patients with CKD [55][56].
5. How Does Disturbed GM Affect the Production of Key Metabolic Intermediates Such as Short-Chain Fatty Acids?
Multifaceted interactions characterize the relationship between GM and the health of individuals with CKD. Entities such as GM are responsible for the production of key metabolic intermediates, such as short-chain fatty acids (SCFAs), via the process of fermenting dietary fiber [57][58]. Compromised renal function has the potential to disturb the equilibrium of these entities and metabolic pathways, thereby potentially exacerbating CKD and disease progression [57][58]. SCFAs were intimately linked to diverse physiological processes, such as immune function, inflammation, and metabolism [57]. SCFAs are a class of organic compounds with short carbon chains (2 to 6 carbons, typically). The intestinal GM produces them along with other complex carbohydrates [57].
The principal SCFAs synthesized are acetate, propionate, and butyrate. SCFAs role has been extensively investigated in patients with CKD and may be summarized as energy metabolism, modulating immunity, maintaining gut integrity, and CVS wellbeing [59].
First, SCFAs once absorbed into the circulation act as a host’s energy source. They are, presumably, influencing insulin sensitivity and weight management through their effect on glucose and lipid metabolism [60]. Second, SCFAs stimulate the production of regulatory T cells (Tregs) and other immune cells that assist in maintaining immune homeostasis and reducing excessive inflammation [61]. Thus, SCFAs modulate immunity and affect the equilibrium between pro-inflammatory and anti-inflammatory responses [61]. For that, reduced SCFA production tends to impair the immune system, amplify inflammation, impair immunological function, and contribute to the advancement of chronic kidney disease (CKD) [61]. Third, butyrate was shown to improve the intestinal barrier’s integrity [62]. It stimulates the production of mucins and tight junction proteins, which are crucial for maintaining the gut barrier integrity. This effect is vital in avoiding the translocation of toxins and bacterial products into the circulation, thereby reducing systemic inflammation [62]. Fourth, SCFAs have been linked with cardiovascular health [63]. They affect blood pressure regulation, lipid metabolism, and endothelial function [63]. All of these are relevant factors in CKD patients, who suffer from an increased risk of cardiovascular complications and form a significant cause of mortality [63].
The impact of short-chain fatty acids within the setting of chronic kidney disease is intricate and diverse. It is essential to note that this relationship is still the subject of active research, and the precise mechanisms by which SCFAs influence CKD have not been fully elucidated [64]. In addition, interventions targeting the intestinal microbiota and SCFA production are being investigated as potential therapeutic strategies for managing the progression of CKD; however, additional research is warranted to establish their efficacy, safety, and possibly lead to innovative methods for treating CKD and its complications.
References
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota Medicine: Towards Clinical Revolution. J. Transl. Med. 2022, 20, 111.
- Pantazi, A.C.; Mihai, C.M.; Balasa, A.L.; Chisnoiu, T.; Lupu, A.; Frecus, C.E.; Mihai, L.; Ungureanu, A.; Kassim, M.A.K.; Andrusca, A.; et al. Relationship between Gut Microbiota and Allergies in Children: A Literature Review. Nutrients 2023, 15, 2529.
- Nori, W.; Akram, N.N.; Mueen Al-kaabi, M. Probiotics in Women and Pediatrics Health; A Narrative Review. Al-Anbar Med. J. 2023, 19, 10–16.
- Lupu, V.V.; Adam Raileanu, A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12, 1158.
- Pantazi, A.C.; Balasa, A.L.; Mihai, C.M.; Chisnoiu, T.; Lupu, V.V.; Kassim, M.A.K.; Mihai, L.; Frecus, C.E.; Chirila, S.I.; Lupu, A.; et al. Development of Gut Microbiota in the First 1000 Days after Birth and Potential Interventions. Nutrients 2023, 15, 3647.
- Lupu, V.V.; Ghiciuc, C.M.; Stefanescu, G.; Mihai, C.M.; Popp, A.; Sasaran, M.O.; Bozomitu, L.; Starcea, I.M.; Adam Raileanu, A.; Lupu, A. Emerging Role of the Gut Microbiome in Post-Infectious Irritable Bowel Syndrome: A Literature Review. World J. Gastroenterol. 2023, 29, 3241–3256.
- Bozomitu, L.; Miron, I.; Adam Raileanu, A.; Lupu, A.; Paduraru, G.; Marcu, F.M.; Buga, A.M.L.; Rusu, D.C.; Dragan, F.; Lupu, V.V. The Gut Microbiome and Its Implication in the Mucosal Digestive Disorders. Biomedicines 2022, 10, 3117.
- Lupu, V.V.; Trandafir, L.M.; Raileanu, A.A.; Mihai, C.M.; Morariu, I.D.; Starcea, I.M.; Mocanu, A.; Butnariu, L.I.; Stoleriu, G.; Salaru, D.L.; et al. Advances in Understanding the Human Gut Microbiota and Its Implication in Pediatric Celiac Disease—A Narrative Review. Nutrients 2023, 15, 2499.
- Lupu, A.; Jechel, E.; Mihai, C.M.; Mitrofan, E.C.; Fotea, S.; Starcea, I.M.; Ioniuc, I.; Mocanu, A.; Ghica, D.C.; Popp, A.; et al. The Footprint of Microbiome in Pediatric Asthma—A Complex Puzzle for a Balanced Development. Nutrients 2023, 15, 3278.
- Lupu, V.V.; Butnariu, L.I.; Fotea, S.; Morariu, I.D.; Badescu, M.C.; Starcea, I.M.; Salaru, D.L.; Popp, A.; Dragan, F.; Lupu, A.; et al. The Disease with a Thousand Faces and the Human Microbiome—A Physiopathogenic Intercorrelation in Pediatric Practice. Nutrients 2023, 15, 3359.
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-Microbiota-Targeted Diets Modulate Human Immune Status. Cell 2021, 184, 4137–4153.e14.
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The Gut Microbiota and the Brain–Gut–Kidney Axis in Hypertension and Chronic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 442–456.
- Suganya, K.; Son, T.; Kim, K.-W.; Koo, B.-S. Impact of Gut Microbiota: How It Could Play Roles beyond the Digestive System on Development of Cardiovascular and Renal Diseases. Microb. Pathog. 2021, 152, 104583.
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066.
- Chen, T.-H.; Cheng, C.-Y.; Huang, C.-K.; Ho, Y.-H.; Lin, J.-C. Exploring the Relevance between Gut Microbiota-Metabolites Profile and Chronic Kidney Disease with Distinct Pathogenic Factor. Microbiol. Spectr. 2023, 11, e02805-22.
- Stanford, J.; Charlton, K.; Stefoska-Needham, A.; Zheng, H.; Bird, L.; Borst, A.; Fuller, A.; Lambert, K. Associations Among Plant-Based Diet Quality, Uremic Toxins, and Gut Microbiota Profile in Adults Undergoing Hemodialysis Therapy. J. Ren. Nutr. 2021, 31, 177–188.
- Lin, X.; Liang, W.; Li, L.; Xiong, Q.; He, S.; Zhao, J.; Guo, X.; Xiang, S.; Zhang, P.; Wang, H.; et al. The Accumulation of Gut Microbiome–Derived Indoxyl Sulfate and P-Cresyl Sulfate in Patients with End-Stage Renal Disease. J. Ren. Nutr. 2022, 32, 578–586.
- Feng, Z.; Wang, T.; Dong, S.; Jiang, H.; Zhang, J.; Raza, H.K.; Lei, G. Association between Gut Dysbiosis and Chronic Kidney Disease: A Narrative Review of the Literature. J. Int. Med. Res. 2021, 49, 030006052110532.
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765.
- Kassim, M.A.K.; Pantazi, A.C.; Nori, W.; Tuta, L.A.; Balasa, A.L.; Mihai, C.M.; Mihai, L.; Frecus, C.E.; Lupu, V.V.; Lupu, A.; et al. Non-Pharmacological Interventions for Pain Management in Hemodialysis: A Narrative Review. J. Clin. Med. 2023, 12, 5390.
- Carney, E.F. The Impact of Chronic Kidney Disease on Global Health. Nat. Rev. Nephrol. 2020, 16, 251.
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low–Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176.
- Arnold, R.; Pianta, T.J.; Pussell, B.A.; Kirby, A.; O’Brien, K.; Sullivan, K.; Holyday, M.; Cormack, C.; Kiernan, M.C.; Krishnan, A.V. Randomized, Controlled Trial of the Effect of Dietary Potassium Restriction on Nerve Function in CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 1569–1577.
- Russo, D.; Bellasi, A.; Pota, A.; Russo, L.; Di Iorio, B. Effects of Phosphorus-Restricted Diet and Phosphate-Binding Therapy on Outcomes in Patients with Chronic Kidney Disease. J. Nephrol. 2015, 28, 73–80.
- Bolte, L.A.; Vila, A.V.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.; Fu, J.; et al. Long-Term Dietary Patterns Are Associated with pro-Inflammatory and Anti-Inflammatory Features of the Gut Microbiome. Gut 2021, 70, 1287–1298.
- Beker, B.M.; Colombo, I.; Gonzalez-Torres, H.; Musso, C.G. Decreasing Microbiota-Derived Uremic Toxins to Improve CKD Outcomes. Clin. Kidney J. 2022, 15, 2214–2219.
- Biruete, A.; Hill Gallant, K.M.; Lindemann, S.R.; Wiese, G.N.; Chen, N.X.; Moe, S.M. Phosphate Binders and Nonphosphate Effects in the Gastrointestinal Tract. J. Ren. Nutr. 2020, 30, 4–10.
- Pergola, P.E.; Rosenbaum, D.P.; Yang, Y.; Chertow, G.M. A Randomized Trial of Tenapanor and Phosphate Binders as a Dual-Mechanism Treatment for Hyperphosphatemia in Patients on Maintenance Dialysis (AMPLIFY). J. Am. Soc. Nephrol. 2021, 32, 1465–1473.
- Kim, S.M.; Song, I.H. The Clinical Impact of Gut Microbiota in Chronic Kidney Disease. Korean J. Intern. Med. 2020, 35, 1305–1316.
- Chakraborty, S.; Ghosh, S.; Banerjea, A.; De, R.; Hazra, A.; Mandal, S. Prescribing Patterns of Medicines in Chronic Kidney Disease Patients on Maintenance Hemodialysis. Indian. J. Pharmacol. 2016, 48, 586.
- Luo, D.; Zhao, W.; Lin, Z.; Wu, J.; Lin, H.; Li, Y.; Song, J.; Zhang, J.; Peng, H. The Effects of Hemodialysis and Peritoneal Dialysis on the Gut Microbiota of End-Stage Renal Disease Patients, and the Relationship Between Gut Microbiota and Patient Prognoses. Front. Cell Infect. Microbiol. 2021, 11, 579386.
- Luo, M.; Cai, J.; Luo, S.; Hong, X.; Xu, L.; Lin, H.; Chen, X.; Fu, W. Causal Effects of Gut Microbiota on the Risk of Chronic Kidney Disease: A Mendelian Randomization Study. Front. Cell Infect. Microbiol. 2023, 13, 1142140.
- Ikee, R.; Yano, K.; Tsuru, T. Constipation in Chronic Kidney Disease: It Is Time to Reconsider. Ren. Replace. Ther. 2019, 5, 51.
- Joossens, M.; Faust, K.; Gryp, T.; Nguyen, A.T.L.; Wang, J.; Eloot, S.; Schepers, E.; Dhondt, A.; Pletinck, A.; Vieira-Silva, S.; et al. Gut Microbiota Dynamics and Uraemic Toxins: One Size Does Not Fit All. Gut 2019, 68, 2257–2260.
- Wehedy, E.; Shatat, I.F.; Al Khodor, S. The Human Microbiome in Chronic Kidney Disease: A Double-Edged Sword. Front. Med. 2022, 8, 790783.
- Lohia, S.; Vlahou, A.; Zoidakis, J. Microbiome in Chronic Kidney Disease (CKD): An Omics Perspective. Toxins 2022, 14, 176.
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24.
- Kambale, R.M.; Ntagazibwa, J.N.; Kasengi, J.B.; Zigashane, A.B.; Francisca, I.N.; Mashukano, B.N.; Amani Ngaboyeka, G.; Bahizire, E.; Zech, F.; Bindels, L.B.; et al. Probiotics for Children with Uncomplicated Severe Acute Malnutrition (PruSAM Study): A Randomized Controlled Trial in the Democratic Republic of Congo. Am. J. Clin. Nutr. 2023, 117, 976–984.
- Yang, J.; Lim, S.Y.; Ko, Y.S.; Lee, H.Y.; Oh, S.W.; Kim, M.G.; Cho, W.Y.; Jo, S.K. Intestinal Barrier Disruption and Dysregulated Mucosal Immunity Contribute to Kidney Fibrosis in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2019, 34, 419–428.
- Wang, H.; Wang, G.; Banerjee, N.; Liang, Y.; Du, X.; Boor, P.J.; Hoffman, K.L.; Khan, M.F. Aberrant Gut Microbiome Contributes to Intestinal Oxidative Stress, Barrier Dysfunction, Inflammation and Systemic Autoimmune Responses in MRL/Lpr Mice. Front. Immunol. 2021, 12, 651191.
- Koshida, K.; Ito, M.; Yakabe, K.; Takahashi, Y.; Tai, Y.; Akasako, R.; Kimizuka, T.; Takano, S.; Sakamoto, N.; Haniuda, K.; et al. Dysfunction of Foxp3+ Regulatory T Cells Induces Dysbiosis of Gut Microbiota via Aberrant Binding of Immunoglobulins to Microbes in the Intestinal Lumen. Int. J. Mol. Sci. 2023, 24, 8549.
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135.
- Lim, K.; McGregor, G.; Coggan, A.R.; Lewis, G.D.; Moe, S.M. Cardiovascular Functional Changes in Chronic Kidney Disease: Integrative Physiology, Pathophysiology and Applications of Cardiopulmonary Exercise Testing. Front. Physiol. 2020, 11, 572355.
- Li, X.; Lindholm, B. Cardiovascular Risk Prediction in Chronic Kidney Disease. Am. J. Nephrol. 2022, 53, 730–739.
- Onal, E.M.; Afsar, B.; Covic, A.; Vaziri, N.D.; Kanbay, M. Gut Microbiota and Inflammation in Chronic Kidney Disease and Their Roles in the Development of Cardiovascular Disease. Hypertens. Res. 2019, 42, 123–140.
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.-S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The Role of Reactive Oxygen Species in the Pathophysiology of Cardiovascular Diseases and the Clinical Significance of Myocardial Redox. Ann. Transl. Med. 2017, 5, 326.
- Lin, C.-J.; Wu, V.; Wu, P.-C.; Wu, C.-J. Meta-Analysis of the Associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with Cardiovascular Events and All-Cause Mortality in Patients with Chronic Renal Failure. PLoS ONE 2015, 10, e0132589.
- Kanitsoraphan, C.; Rattanawong, P.; Charoensri, S.; Senthong, V. Trimethylamine N-Oxide and Risk of Cardiovascular Disease and Mortality. Curr. Nutr. Rep. 2018, 7, 207–213.
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947.
- Watanabe, I.; Tatebe, J.; Namba, S.; Koizumi, M.; Yamazaki, J.; Morita, T. Activation of Aryl Hydrocarbon Receptor Mediates Indoxyl Sulfate-Induced Monocyte Chemoattractant Protein-1 Expression in Human Umbilical Vein Endothelial Cells. Circ. J. 2013, 77, 224–230.
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S. The Impact of Gut Microbiota on Kidney Function and Pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419.
- Lin, Y.-T.; Wu, P.-H.; Liang, S.-S.; Mubanga, M.; Yang, Y.-H.; Hsu, Y.-L.; Kuo, M.-C.; Hwang, S.-J.; Kuo, P.-L. Protein-Bound Uremic Toxins Are Associated with Cognitive Function among Patients Undergoing Maintenance Hemodialysis. Sci. Rep. 2019, 9, 20388.
- Kwon, Y.E.; Choi, H.Y.; Kim, S.; Ryu, D.-R.; Oh, H.J. Fracture Risk in Chronic Kidney Disease: A Korean Population-Based Cohort Study. Kidney Res. Clin. Pract. 2019, 38, 220–228.
- Park, J.S.; Choi, H.I.; Bae, E.H.; Ma, S.K.; Kim, S.W. Paricalcitol Attenuates Indoxyl Sulfate-Induced Apoptosis through the Inhibition of MAPK, Akt, and NF-KB Activation in HK-2 Cells. Korean J. Intern. Med. 2019, 34, 146–155.
- Goto, S.; Fujii, H.; Hamada, Y.; Yoshiya, K.; Fukagawa, M. Association Between Indoxyl Sulfate and Skeletal Resistance in Hemodialysis Patients. Ther. Apher. Dial. 2010, 14, 417–423.
- Nii-Kono, T.; Iwasaki, Y.; Uchida, M.; Fujieda, A.; Hosokawa, A.; Motojima, M.; Yamato, H.; Kurokawa, K.; Fukagawa, M. Indoxyl Sulfate Induces Skeletal Resistance to Parathyroid Hormone in Cultured Osteoblastic Cells. Kidney Int. 2007, 71, 738–743.
- Magliocca, G.; Mone, P.; Di Iorio, B.R.; Heidland, A.; Marzocco, S. Short-Chain Fatty Acids in Chronic Kidney Disease: Focus on Inflammation and Oxidative Stress Regulation. Int. J. Mol. Sci. 2022, 23, 5354.
- Mertowska, P.; Mertowski, S.; Wojnicka, J.; Korona-Głowniak, I.; Grywalska, E.; Błażewicz, A.; Załuska, W. A Link between Chronic Kidney Disease and Gut Microbiota in Immunological and Nutritional Aspects. Nutrients 2021, 13, 3637.
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277.
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356.
- Liu, X.; Shao, J.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.; Liu, Z.; He, D.; Li, C.; Zhang, X. Regulation of Short-Chain Fatty Acids in the Immune System. Front. Immunol. 2023, 14, 1186892.
- Zhang, Y.; Zhu, X.; Yu, X.; Novák, P.; Gui, Q.; Yin, K. Enhancing Intestinal Barrier Efficiency: A Novel Metabolic Diseases Therapy. Front. Nutr. 2023, 10, 1120168.
- Wu, Y.; Xu, H.; Tu, X.; Gao, Z. The Role of Short-Chain Fatty Acids of Gut Microbiota Origin in Hypertension. Front. Microbiol. 2021, 12, 730809.
- Zheng, L.; Luo, M.; Zhou, H.; Chen, J. Natural Products from Plants and Microorganisms: Novel Therapeutics for Chronic Kidney Disease via Gut Microbiota Regulation. Front. Pharmacol. 2023, 13, 1068613.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
434
Revisions:
2 times
(View History)
Update Date:
12 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




