Microbes colonize every surface of the human body, but an increasing proportion of microbes inhabit the intestine. Consequently, gut microbiota (GM) is regarded as a “forgotten organ”. In a healthy state, GM plays several critical roles in our bodies, such as helping to metabolize nutrients, preserving the structural soundness of the gut’s mucosal barrier, moderating immune responses, and providing defense against harmful pathogens [
1,
2]. A microbiota describes all microorganisms that colonize the epidermis, respiratory tract, genital system, and especially the gastrointestinal tract. GM is constantly evolving and displaying a wide diversity within the same person and in comparison, to others [
3]. GM connects with vital organs, including the brain, bone marrow, cardiovascular system (CVS), kidney, body’s immune system, and the central nervous system, and has been seen as a potential cause for a variety of diseases in the aforementioned organs [
4,
5,
6,
7,
8,
9,
10]. GM activates immune cells derived from bone marrow, resulting in a low-grade inflammatory reaction that affects the brain and kidneys via circulation [
11]. Simultaneously, peripheral stimuli affect the brain and modulate neural inputs to the kidney, intestine, and lymphoid organs [
11]. This bidirectional relationship lends credence to the notion that GM modulation is an innovative method for the management of kidney diseases [
12,
13].
Dysbiosis is an imbalance or perturbation in the GMs composition that results in a proliferation of harmful bacteria like
Enterobacteriaceae or a reduction in beneficial bacteria like
Bifidobacterium and
Lactobacillus [
14]. For individuals dealing with ongoing kidney conditions, such as chronic kidney disease (CKD) and terminal kidney failure, often referred to as end-stage kidney disease (ESKD), the harmonious and mutually advantageous connection is disrupted, leading to an imbalance known as dysbiosis [
15]. The consequences of this dysbiosis go beyond the gut and impact the kidneys via the so-called gut–kidney axis [
13]. One of these adverse outcomes is the overproduction of uremic toxins such as indoxyl sulfate and p-cresyl sulfate, which are derived from bacterial metabolism of dietary amino acids [
16]. In healthy individuals, these toxins are efficiently excreted by the kidneys, but in CKD and ESKD, their clearance is significantly reduced, leading to a high plasma concentration of these toxins [
17]. Increasing evidence confirms that dysbiosis by itself contributes to CKD development and progression [
18].
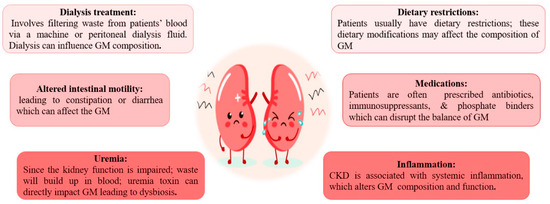
CKD cases have common dietary restrictions, like low protein intake and avoiding foods rich in potassium and phosphorus [
24,
25,
26], which affect the composition of GM [
27]. Building-up of waste products (uremia toxin) in the blood owing to impaired kidney function directly affects the GM and leads to dysbiosis [
28]. Prescribed Medications: Patients are often on antibiotics, immunosuppressants, and phosphate binders [
29,
30]. Collectively, they can disrupt the balance of GM [
31]. Patients with ESKD often require dialysis, which involves filtering waste products from the bloodstream via a machine or peritoneal dialysis fluid [
32]. Dialysis by itself can impact GM composition [
33]. A state of systemic inflammation associated with CKD and ESKD may alter the GM composition and function [
34]. Chronic kidney disease patients often suffer altered intestinal motility, leading to constipation or diarrhea. These bowel changes can impact the GM [
35] (
Figure 1).
Figure 1. Causes of altered gut microbiota in patients with chronic kidney disease and end-stage kidney disease.
3. How Does Disturbed GM Impact CKD and ESKD Progression?
It is important to note that these causes may interact with each other, leading to a complex interplay between gut dysbiosis and chronic renal disease progression. As renal function declines, the capacity to eliminate toxins decreases, leading to a detrimental cycle of gut dysbiosis and exacerbating uremia [
36]. Reduced microbial diversity has been linked to an increase in disease severity and deteriorating health outcomes [
37]. Some GM can transform specific toxins into perilous byproducts, which intensify renal damage and induce widespread inflammation within the body [
38]. GM plays a pivotal role in the processes of nutrient metabolism and energy extraction [
39]. However, when dysbiosis occurs, it can have detrimental effects on nutrient assimilation and metabolism, leading to conditions such as malnutrition or an imbalanced energy equilibrium [
40]. The presence of altered gut microbiota leads to the disruption of the intestinal barrier function, which permits the passage of microbial components and harmful substances into the bloodstream [
41]. This, commonly referred to as “Leaky gut syndrome” or “endotoxemia”, subsequently initiates a systemic inflammatory response [
42]. Dysbiosis and the associated modification of GM can result in an impaired immune response, making the host more susceptible to infections and inflammatory diseases [
43,
44].
4. How Does Disturbed GM Contribute to CKD- and ESKD-Related Complications?
There is growing data indicating a connection between dysbiosis and complications associated with CKD, including high blood pressure, cardiovascular incidents, disorders related to minerals and bones (MBD), and cognitive impairments.
- ❖
-
CKD- and ESKD-related cardiovascular disease
Several studies find that diverse mechanisms play a role in the development and progression of cardiovascular disease, a major mortality cause among those patients [
45,
46]. These include increased reactive oxygen species (ROS) production, leukocyte activation, pro-inflammatory cytokines production, myocyte hypertrophy, and dyslipidemia. This relationship between the digestive tract and the heart is known as the gut–heart axis [
47,
48]. Lin et al. [
49] found an association between elevated pCS levels and increased CVS mortality in CKD patients. Conversely, low TMAO was associated with a 1.7-fold greater risk of severe CVS events [
50].
- ❖
-
Cognitive psychiatric disorders
Cognitive psychiatric disorders are prevalent among CKD patients and are associated with an increase in morbidity and mortality [
51]. The gut–brain axis promotes dysregulation of the hypothalamus–pit axis [
52]. The contribution of gut-microbiota-derived toxins to cognitive dysfunction is conveyed through mechanisms like direct toxicity or other potential influences, such as oxidative stress, inflammation, dysfunction of endothelial cells, and vascular calcification [
53]. Lin et al. [
54] demonstrated in a study involving 260 hemodialytic cases that the circulating free form of IS is substantially associated with decreased cognitive function, especially in the memory domain, mental manipulation, and language ability.
- ❖
-
CKD—disorder of bone and minerals
This syndrome was recently renamed to encompass biochemical, skeletal, and CVS pathogenesis in addition to bone disease [
55]. It was suggested that elevated GM-derived toxins contribute to the onset of bone abnormalities in CKD [
56]. Previous research has shown that increased levels of IS can impede the function of osteoblasts and have a restraining effect on osteoclasts and parathyroid hormone, which may consequently affect the bone remodeling process in patients with CKD [
57,
58].
5. How Does Disturbed GM Affect the Production of Key Metabolic Intermediates Such as Short-Chain Fatty Acids?
Multifaceted interactions characterize the relationship between GM and the health of individuals with CKD. Entities such as GM are responsible for the production of key metabolic intermediates, such as short-chain fatty acids (SCFAs), via the process of fermenting dietary fiber [
59,
60]. Compromised renal function has the potential to disturb the equilibrium of these entities and metabolic pathways, thereby potentially exacerbating CKD and disease progression [
59,
60]. SCFAs were intimately linked to diverse physiological processes, such as immune function, inflammation, and metabolism [
59]. SCFAs are a class of organic compounds with short carbon chains (2 to 6 carbons, typically). The intestinal GM produces them along with other complex carbohydrates [
59].
The principal SCFAs synthesized are acetate, propionate, and butyrate. SCFAs role has been extensively investigated in patients with CKD and may be summarized as energy metabolism, modulating immunity, maintaining gut integrity, and CVS wellbeing [
61].
First, SCFAs once absorbed into the circulation act as a host’s energy source. They are, presumably, influencing insulin sensitivity and weight management through their effect on glucose and lipid metabolism [
62]. Second, SCFAs stimulate the production of regulatory T cells (Tregs) and other immune cells that assist in maintaining immune homeostasis and reducing excessive inflammation [
63]. Thus, SCFAs modulate immunity and affect the equilibrium between pro-inflammatory and anti-inflammatory responses [
63]. For that, reduced SCFA production tends to impair the immune system, amplify inflammation, impair immunological function, and contribute to the advancement of chronic kidney disease (CKD) [
63]. Third, butyrate was shown to improve the intestinal barrier’s integrity [
64]. It stimulates the production of mucins and tight junction proteins, which are crucial for maintaining the gut barrier integrity. This effect is vital in avoiding the translocation of toxins and bacterial products into the circulation, thereby reducing systemic inflammation [
64]. Fourth, SCFAs have been linked with cardiovascular health [
65]. They affect blood pressure regulation, lipid metabolism, and endothelial function [
65]. All of these are relevant factors in CKD patients, who suffer from an increased risk of cardiovascular complications and form a significant cause of mortality [
65].
The impact of short-chain fatty acids within the setting of chronic kidney disease is intricate and diverse. It is essential to note that this relationship is still the subject of active research, and the precise mechanisms by which SCFAs influence CKD have not been fully elucidated [
66]. In addition, interventions targeting the intestinal microbiota and SCFA production are being investigated as potential therapeutic strategies for managing the progression of CKD; however, additional research is warranted to establish their efficacy, safety, and possibly lead to innovative methods for treating CKD and its complications.