
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Evanthia Bletsa | -- | 2291 | 2023-09-08 15:53:25 | | | |
| 2 | Fanny Huang | Meta information modification | 2291 | 2023-09-11 08:03:40 | | | | |
| 3 | Fanny Huang | -5 word(s) | 2286 | 2023-09-12 07:19:13 | | |
Video Upload Options
Left ventricular (LV) remodeling is a dynamic process, which is characterized by changes in ventricular size, shape, and wall thickness, thus altering myocardial geometry and function, and is considered as a negative prognostic factor in patients with heart failure (HF). Hypertension, type 2 diabetes (T2D), and obesity are strongly correlated with the development and the progression of LV remodeling, LV hypertrophy, and LV systolic and/or diastolic dysfunction. Indeed, the beneficial impact of exercise training on primary and secondary prevention of cardiovascular disease (CVD) has been well-established. Exercise training enhances functional capacity, muscle strength and endurance, cardiac function, and cardiac-related biomarkers among patients with established coronary artery disease (CAD) or HF, thus substantially improving their cardiovascular prognosis, survival rates, and need for rehospitalization.
1. Introduction
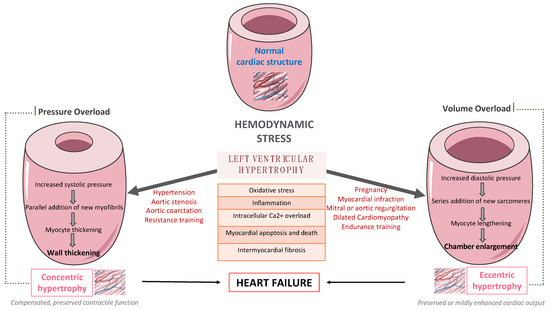
2. The Effect of Exercise Training on Left Ventricular Remodeling among Patients with Cardiometabolic Risk Factors
2.1. In Patients with Hypertension
2.2. In Patients with Type 2 Diabetes
2.3. In patients with Type Obesity
2.4. In Patients with Coronary Artery Disease
2.5. In patients with Heart Failure
References
- Mortality, G.B.D.; Causes of Death, C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171.
- Gupta, A.; Fonarow, G.C. The Hospital Readmissions Reduction Program-learning from failure of a healthcare policy. Eur. J. Heart Fail. 2018, 20, 1169–1174.
- Stein, E.J.; Fearon, W.F.; Elmariah, S.; Kim, J.B.; Kapadia, S.; Kumbhani, D.J.; Gillam, L.; Whisenant, B.; Quader, N.; Zajarias, A.; et al. Left Ventricular Hypertrophy and Biomarkers of Cardiac Damage and Stress in Aortic Stenosis. J. Am. Heart Assoc. 2022, 11, e023466.
- Lorell, B.H.; Carabello, B.A. Left ventricular hypertrophy: Pathogenesis, detection, and prognosis. Circulation 2000, 102, 470–479.
- Sharpe, N. Left ventricular remodeling: Pathophysiology and treatment. Heart Fail. Monit. 2003, 4, 55–61.
- Lovic, D.; Narayan, P.; Pittaras, A.; Faselis, C.; Doumas, M.; Kokkinos, P. Left ventricular hypertrophy in athletes and hypertensive patients. J. Clin. Hypertens. (Greenwich) 2017, 19, 413–417.
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407.
- Kramer, D.G.; Trikalinos, T.A.; Kent, D.M.; Antonopoulos, G.V.; Konstam, M.A.; Udelson, J.E. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: A meta-analytic approach. J. Am. Coll. Cardiol. 2010, 56, 392–406.
- Santos-Gallego, C.G.; Vargas-Delgado, A.P.; Requena-Ibanez, J.A.; Garcia-Ropero, A.; Mancini, D.; Pinney, S.; Macaluso, F.; Sartori, S.; Roque, M.; Sabatel-Perez, F.; et al. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 243–255.
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424.
- Januzzi, J.L., Jr.; Prescott, M.F.; Butler, J.; Felker, G.M.; Maisel, A.S.; McCague, K.; Camacho, A.; Pina, I.L.; Rocha, R.A.; Shah, A.M.; et al. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA 2019, 322, 1085–1095.
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004.
- Glenney, S.S.; Brockemer, D.P.; Ng, A.C.; Smolewski, M.A.; Smolgovskiy, V.M.; Lepley, A.S. Effect of Exercise Training on Cardiac Biomarkers in At-Risk Populations: A Systematic Review. J. Phys. Act. Health 2017, 14, 968–989.
- Rauch, B.; Davos, C.H.; Doherty, P.; Saure, D.; Metzendorf, M.I.; Salzwedel, A.; Voller, H.; Jensen, K.; Schmid, J.P.; Cardiac Rehabilitation Section; et al. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: A systematic review and meta-analysis of randomized and non-randomized studies—The Cardiac Rehabilitation Outcome Study (CROS). Eur. J. Prev. Cardiol. 2016, 23, 1914–1939.
- Lewinter, C.; Doherty, P.; Gale, C.P.; Crouch, S.; Stirk, L.; Lewin, R.J.; LeWinter, M.M.; Ades, P.A.; Kober, L.; Bland, J.M. Exercise-based cardiac rehabilitation in patients with heart failure: A meta-analysis of randomised controlled trials between 1999 and 2013. Eur. J. Prev. Cardiol. 2015, 22, 1504–1512.
- Zanettini, R.; Bettega, D.; Agostoni, O.; Ballestra, B.; del Rosso, G.; di Michele, R.; Mannucci, P.M. Exercise training in mild hypertension: Effects on blood pressure, left ventricular mass and coagulation factor VII and fibrinogen. Cardiology 1997, 88, 468–473.
- Kokkinos, P.F.; Narayan, P.; Colleran, J.A.; Pittaras, A.; Notargiacomo, A.; Reda, D.; Papademetriou, V. Effects of regular exercise on blood pressure and left ventricular hypertrophy in African-American men with severe hypertension. N. Engl. J. Med. 1995, 333, 1462–1467.
- Korsager Larsen, M.; Matchkov, V.V. Hypertension and physical exercise: The role of oxidative stress. Medicina 2016, 52, 19–27.
- Pitsavos, C.; Chrysohoou, C.; Koutroumbi, M.; Aggeli, C.; Kourlaba, G.; Panagiotakos, D.; Michaelides, A.; Stefanadis, C. The impact of moderate aerobic physical training on left ventricular mass, exercise capacity and blood pressure response during treadmill testing in borderline and mildly hypertensive males. Hell. J. Cardiol. 2011, 52, 6–14.
- Turner, M.J.; Spina, R.J.; Kohrt, W.M.; Ehsani, A.A. Effect of endurance exercise training on left ventricular size and remodeling in older adults with hypertension. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M245–M251.
- Lou, M.; Zong, X.F.; Wang, L.L. Curative treatment of hypertension by physical exercise. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3320–3326.
- Kokkinos, P.; Pittaras, A.; Narayan, P.; Faselis, C.; Singh, S.; Manolis, A. Exercise capacity and blood pressure associations with left ventricular mass in prehypertensive individuals. Hypertension 2007, 49, 55–61.
- Palatini, P.; Visentin, P.; Dorigatti, F.; Guarnieri, C.; Santonastaso, M.; Cozzio, S.; Pegoraro, F.; Bortolazzi, A.; Vriz, O.; Mos, L.; et al. Regular physical activity prevents development of left ventricular hypertrophy in hypertension. Eur. Heart J. 2009, 30, 225–232.
- Gusso, S.; Pinto, T.; Baldi, J.C.; Derraik, J.G.B.; Cutfield, W.S.; Hornung, T.; Hofman, P.L. Exercise Training Improves but Does Not Normalize Left Ventricular Systolic and Diastolic Function in Adolescents With Type 1 Diabetes. Diabetes Care 2017, 40, 1264–1272.
- Verboven, M.; Van Ryckeghem, L.; Belkhouribchia, J.; Dendale, P.; Eijnde, B.O.; Hansen, D.; Bito, V. Effect of Exercise Intervention on Cardiac Function in Type 2 Diabetes Mellitus: A Systematic Review. Sports Med. 2019, 49, 255–268.
- Cassidy, S.; Thoma, C.; Hallsworth, K.; Parikh, J.; Hollingsworth, K.G.; Taylor, R.; Jakovljevic, D.G.; Trenell, M.I. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: A randomised controlled trial. Diabetologia 2016, 59, 56–66.
- Otten, J.; Andersson, J.; Stahl, J.; Stomby, A.; Saleh, A.; Waling, M.; Ryberg, M.; Hauksson, J.; Svensson, M.; Johansson, B.; et al. Exercise Training Adds Cardiometabolic Benefits of a Paleolithic Diet in Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2019, 8, e010634.
- Gulsin, G.S.; Swarbrick, D.J.; Athithan, L.; Brady, E.M.; Henson, J.; Baldry, E.; Argyridou, S.; Jaicim, N.B.; Squire, G.; Walters, Y.; et al. Effects of Low-Energy Diet or Exercise on Cardiovascular Function in Working-Age Adults With Type 2 Diabetes: A Prospective, Randomized, Open-Label, Blinded End Point Trial. Diabetes Care 2020, 43, 1300–1310.
- Piche, M.E.; Poirier, P.; Marette, A.; Mathieu, P.; Levesque, V.; Bibeau, K.; Larose, E.; Despres, J.P. Benefits of 1-Year Lifestyle Modification Program on Exercise Capacity and Diastolic Function Among Coronary Artery Disease Men With and Without Type 2 Diabetes. Metab. Syndr. Relat. Disord. 2019, 17, 149–159.
- Kamimura, D.; Loprinzi, P.D.; Wang, W.; Suzuki, T.; Butler, K.R.; Mosley, T.H.; Hall, M.E. Physical Activity Is Associated With Reduced Left Ventricular Mass in Obese and Hypertensive African Americans. Am. J. Hypertens. 2017, 30, 617–623.
- Himeno, E.; Nishino, K.; Nakashima, Y.; Kuroiwa, A.; Ikeda, M. Weight reduction regresses left ventricular mass regardless of blood pressure level in obese subjects. Am. Heart J. 1996, 131, 313–319.
- Voulgari, C.; Pagoni, S.; Vinik, A.; Poirier, P. Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism 2013, 62, 609–621.
- Carbone, S.; Del Buono, M.G.; Ozemek, C.; Lavie, C.J. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog. Cardiovasc. Dis. 2019, 62, 327–333.
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling--concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582.
- Taylor, R.S.; Brown, A.; Ebrahim, S.; Jolliffe, J.; Noorani, H.; Rees, K.; Skidmore, B.; Stone, J.A.; Thompson, D.R.; Oldridge, N. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am. J. Med. 2004, 116, 682–692.
- Giallauria, F.; Galizia, G.; Lucci, R.; D’Agostino, M.; Vitelli, A.; Maresca, L.; Orio, F.; Vigorito, C. Favourable effects of exercise-based Cardiac Rehabilitation after acute myocardial infarction on left atrial remodeling. Int. J. Cardiol. 2009, 136, 300–306.
- Haykowsky, M.; Scott, J.; Esch, B.; Schopflocher, D.; Myers, J.; Paterson, I.; Warburton, D.; Jones, L.; Clark, A.M. A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: Start early and go longer for greatest exercise benefits on remodeling. Trials 2011, 12, 92.
- Giallauria, F.; De Lorenzo, A.; Pilerci, F.; Manakos, A.; Lucci, R.; Psaroudaki, M.; D’Agostino, M.; Del Forno, D.; Vigorito, C. Reduction of N terminal-pro-brain (B-type) natriuretic peptide levels with exercise-based cardiac rehabilitation in patients with left ventricular dysfunction after myocardial infarction. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 625–632.
- Wen, Y.; Zhang, X.; Lan, W.; Zhao, S.; Qi, Q.; Yang, L. Effects of Cardiac Rehabilitation on Cardiac Function and Quality of Life in Patients with Ischemic Nonobstructive Coronary Artery Disease and Diabetes Mellitus. Biomed. Res. Int. 2022, 2022, 3487107.
- Giallauria, F.; Acampa, W.; Ricci, F.; Vitelli, A.; Torella, G.; Lucci, R.; Del Prete, G.; Zampella, E.; Assante, R.; Rengo, G.; et al. Exercise training early after acute myocardial infarction reduces stress-induced hypoperfusion and improves left ventricular function. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 315–324.
- Zhang, Y.M.; Lu, Y.; Tang, Y.; Yang, D.; Wu, H.F.; Bian, Z.P.; Xu, J.D.; Gu, C.R.; Wang, L.S.; Chen, X.J. The effects of different initiation time of exercise training on left ventricular remodeling and cardiopulmonary rehabilitation in patients with left ventricular dysfunction after myocardial infarction. Disabil. Rehabil. 2016, 38, 268–276.
- Wisloff, U.; Stoylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, O.; Haram, P.M.; Tjonna, A.E.; Helgerud, J.; Slordahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094.
- Pandey, A.; LaMonte, M.; Klein, L.; Ayers, C.; Psaty, B.M.; Eaton, C.B.; Allen, N.B.; de Lemos, J.A.; Carnethon, M.; Greenland, P.; et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1129–1142.
- Pandey, A.; Allen, N.B.; Ayers, C.; Reis, J.P.; Moreira, H.T.; Sidney, S.; Rana, J.S.; Jacobs, D.R., Jr.; Chow, L.S.; de Lemos, J.A.; et al. Fitness in Young Adulthood and Long-Term Cardiac Structure and Function: The CARDIA Study. JACC Heart Fail. 2017, 5, 347–355.
- Pandey, A.; Cornwell, W.K., 3rd; Willis, B.; Neeland, I.J.; Gao, A.; Leonard, D.; DeFina, L.; Berry, J.D. Body Mass Index and Cardiorespiratory Fitness in Mid-Life and Risk of Heart Failure Hospitalization in Older Age: Findings From the Cooper Center Longitudinal Study. JACC Heart Fail. 2017, 5, 367–374.
- Pandey, A.; Patel, M.; Gao, A.; Willis, B.L.; Das, S.R.; Leonard, D.; Drazner, M.H.; de Lemos, J.A.; DeFina, L.; Berry, J.D. Changes in mid-life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: The Cooper Center Longitudinal Study. Am. Heart J. 2015, 169, 290–297 e291.
- Edwards, J.J.; O’Driscoll, J.M. Exercise Training in Heart failure with Preserved and Reduced Ejection Fraction: A Systematic Review and Meta-Analysis. Sports Med. Open 2022, 8, 76.
- Sandri, M.; Kozarez, I.; Adams, V.; Mangner, N.; Hollriegel, R.; Erbs, S.; Linke, A.; Mobius-Winkler, S.; Thiery, J.; Kratzsch, J.; et al. Age-related effects of exercise training on diastolic function in heart failure with reduced ejection fraction: The Leipzig Exercise Intervention in Chronic Heart Failure and Aging (LEICA) Diastolic Dysfunction Study. Eur. Heart J. 2012, 33, 1758–1768.
- Haykowsky, M.J.; Timmons, M.P.; Kruger, C.; McNeely, M.; Taylor, D.A.; Clark, A.M. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am. J. Cardiol. 2013, 111, 1466–1469.
- Hegde, S.M.; Claggett, B.; Shah, A.M.; Lewis, E.F.; Anand, I.; Shah, S.J.; Sweitzer, N.K.; Fang, J.C.; Pitt, B.; Pfeffer, M.A.; et al. Physical Activity and Prognosis in the TOPCAT Trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist). Circulation 2017, 136, 982–992.
- Heizer, J.; Carbone, S.; Billingsley, H.E.; BW, V.A.N.T.; Arena, R.; Abbate, A.; Canada, J.M. Left ventricular concentric remodeling and impaired cardiorespiratory fitness in patients with heart failure and preserved ejection fraction. Minerva Cardiol. Angiol. 2021, 69, 438–445.




