1. Introduction
It is estimated that more than 26 million adults suffer from heart failure (HF) worldwide, with the prevalence rates growing rapidly
[1]. According to the current literature, 35–50% of patients with HF experience frequent rehospitalizations within 6 months of discharge, thus deteriorating their prognosis and incurring billions in costs
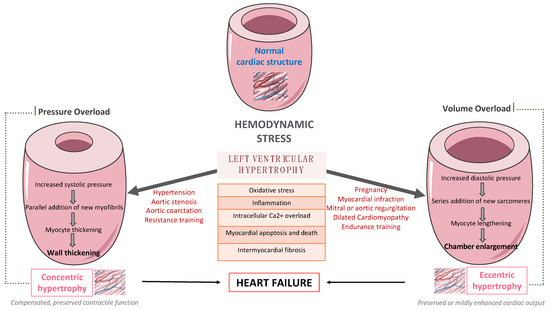
[2]. Left ventricular hypertrophy (LVH), characterized by an increased left ventricular (LV) mass and cardiomyocyte hypertrophy, mainly increases the cardiovascular risk. Hypertension, type 2 diabetes (T2D), chronic kidney disease, and aortic stenosis are considered major risk factors for LVH
[3]. Furthermore, LVH has been associated with an increased risk for LV dysfunction, HF, arrythmias, stroke, and sudden cardiac death
[4].
LV remodeling is a dynamic process, which is characterized by changes in ventricular size, shape, and wall thickness, thus altering myocardial geometry and function
[5]. It is considered as a negative prognostic factor in patients with HF. More specifically, obesity and hypertension cause an increase in systemic pressure, afterload, and wall stress, thus leading to the development of LVH
[6]. Up to 60% of patients with hypertension may present with signs of an increased LV mass on echocardiography. Mild to moderate hypertension and LVH are commonly accompanied by varying degrees of impaired LV diastolic filling with normal or mild increased systolic performance at rest, as well as diminished coronary vasodilator capacity. Nevertheless, LVH might evolve to overt systolic and diastolic dysfunction, thus leading to the development and progression of heart failure with reduced eject fraction (HFrEF) or heart failure with preserved eject fraction (HFpEF), respectively, as presented in
Figure 1.
Figure 1. The pathway from left ventricular hypertrophy to heart failure.
Many complex and multifactorial mechanisms resulting in transcriptional, signaling, structural, and electrophysiological changes are involved in this process of LV remodeling. More specifically, oxidative stress and altered intracellular calcium metabolism provoke cardiomyocyte hypertrophy, thus resulting in impaired contraction and relaxation, as well as an increased risk for fatal ventricular arrythmias and sudden cardiac death
[7]. Of note, interstitial and replacement fibrosis play a pivotal role in the progression of LV remodeling.
On the one hand, LV remodeling predicts adverse clinical outcomes, and possible regression seems to limit them, thus improving patient prognosis. On the other hand, short-term improvement in LV remodeling mediated by novel pharmaceutical agents and medical devices is associated with long-term improvement of clinical outcomes among patients with LV dysfunction
[8]. More specifically, empagliflozin, a sodium–glucose co-transporter-2 inhibitor (SGLT-2i), ameliorates LV remodeling at 6 months
[9] and cardiovascular outcomes after 2 years of treatment
[10]. Similarly, sacubitril–valsartan, an angiotensin receptor–neprilysin inhibitor (ARNI), improves LV remodeling at 12 months
[11] and clinical outcomes after 2 years of treatment
[12]. Furthermore, the beneficial impact of exercise training on primary and secondary prevention of many clinical conditions, such as cardiovascular disease (CVD), T2D, as well as obesity, has been well-established so far. Recent studies highlight that exercise training enhances functional capacity, muscle strength and endurance, cardiac function, and cardiac-related biomarkers
[13] among patients with established coronary artery disease (CAD)
[14] or HF
[15], thus improving substantially their cardiovascular prognosis, survival rates, and need for rehospitalization.
2. The Effect of Exercise Training on Left Ventricular Remodeling among Patients with Cardiometabolic Risk Factors
2.1. In Patients with Hypertension
According to the current literature, exercise training leads to a substantial reduction in resting systolic and diastolic blood pressure, as well as in LVH among hypertensive patients
[16][17][37,38]. Additionally, recent studies indicate that moderate and regular physical activity reduces significantly total peripheral resistance
[18][39]. Exercise-mediated hemodynamic changes include also an increased cardiac output along with the redistribution of blood flow to muscular territories. So far, there is evidence that exercise training may reduce LV hypertrophy in parallel with systolic and diastolic blood pressure improvement
[19][40].
Turner et al. were among the first to report that exercise training may induce regression of LVH and LV concentric remodeling among patients with mild or moderate hypertension
[20][41]. Specifically, exercise training improved aerobic efficacy by 16% and decreased substantially systolic and diastolic blood pressure, LV wall thickness, as well as LV mass index. Of note, LVH regression was mainly attributed to the reduction in the systolic blood pressure. Indeed, exercise training improves significantly systolic and diastolic blood pressure among patients with mild or moderate hypertension.
Furthermore, low-fit individuals with hypertension seem to have a higher LV mass index when compared to the moderate and high-fit individuals
[17][38]. In this randomized controlled trial, 16 weeks of aerobic exercise led to a substantial regression of LV mass and thickness of the interventricular septum, which were mainly attributed to a linear reduction in systolic and diastolic blood pressure
[17][38]. Similarly, regular exercise training results in lowering blood pressure, LV mass index, as well as exercise capacity among patients with borderline or mild hypertension
[19][40]. An exercise-mediated decreased posterior wall and an intraventricular septal thickness are also found in hypertensive patients
[21][42]. Furthermore, there is also evidence that a 1-MET increase in workload offers a 42% reduction in the risk of LVH
[22][43]. It is important to note that regular physical activity seems to prevent the development of LVH among hypertensive patients at stage 1
[23][44]. More specifically, patients in the physically active group were less likely to develop LVH when compared to those following a sedentary lifestyle, after a median follow-up of 8.3 years.
2.2. In Patients with Type 2 Diabetes
So far, there is evidence that exercise training may improve both LV systolic and diastolic function in patients with diabetes, resulting in favorable changes in stroke volume, LVEF, end-systolic volumes, as well as LV filling
[24][45]. Both endurance and combined endurance and resistance exercise training positively affect cardiovascular parameters among patients with T2D
[25][46]. According to a randomized clinical trial, high intensity intermittent training (HIIT) seems to improve substantially cardiac function and structure among patients with T2D, resulting finally in a positive cardiac remodeling
[26][47]. In more details, this type of 12-month exercise interventional program ameliorated both LV mass and systolic function when compared to standard care. Of note, these changes were accompanied by modest improvements in glycemic control.
Furthermore, Otten et al. reported that supervised exercise training (3 weeks/hour) paired with a paleolithic diet (based on vegetables, fruits, berries, nuts, seafood, eggs, fish, and lean meat with a high restriction of dairy products, cereals, legumes, refined fats, added sugar and salt) resulted in favorable metabolic and cardiac changes with a decrease in triglycerides levels and LV mass to end-diastolic volume ratio and an increase in LVEDV and stroke volume, among overweight and obese patients with T2D
[27][48]. Similarly, exercise training seems to also improve diastolic function among patients with T2D. In a randomized clinical trial, a 12-week supervised aerobic exercise training program improved diastolic function in the absence of any major effects on LV remodeling, perfusion, or aortic stiffening, among asymptomatic young patients with T2D
[28][49]. Interestingly, exercise-mediated favorable changes in a LV remodeling index seem to be the best predictor of improvement in LV diastolic function after the lifestyle intervention program, including increased physical activity among patients with T2D and CAD
[29][50].
2.3. In patients with Type Obesity
Physical activity improves LVH among patients with obesity and hypertension. In more details, higher physical activity was associated with a reduction in the LV mass index and an improvement in LVH, as well as cardiac biomarkers, such as N-terminal pro-atrial natriuretic peptide (NT-pro BNP) and a mid-regional sequence of pro-A-type natriuretic peptide (MR pro-ANP)
[30][51]. Similarly, a decrease in triglycerides levels and LV mass to end-diastolic volume ratio and an increase in LVEDV and stroke volume are reported among overweight and obese patients with T2D who participated in supervised exercise training programs
[27][48]. Of note, the beneficial effects of exercise training on the reduction in LV mass are apparent regardless of whether the obese patients are normotensive or hypertensive. More specifically, Himeno et al. found that mild exercise together with mild hypocaloric intake resulted in significant weight and LV mass reduction among obese patients, after a 12-week intervention period
[31][52]. Indeed, LV mass was significantly decreased among obese patients regardless of the presence of hypertension or not, whereas significant changes were found in the systolic, diastolic, and mean blood pressure. These data provide evidence that an exercise-mediated reduction in LV mass is not only attributed to a reduction in blood pressure and weight loss, but also to further mechanisms, such as improved cardiac autonomous function
[32][53], myocardial metabolism and metabolic flexibility, as well as reduced LV stiffness
[33][54].
Main studies evaluating the effect of exercise training on LV remodeling in patients with cardiometabolic risk factors, such as hypertension, T2D, and obesity.
2.4. In Patients with Coronary Artery Disease
LV remodeling following acute myocardial infraction (AMI) is a complex process characterized by fibroblast proliferation, collagen deposition, scar formation, as well as ventricular expansion, resulting in LV dysfunction and HF, thus negatively affecting long-term prognosis in these patients
[34][55]. The beneficial effect of exercise training on cardiovascular mortality and morbidity, functional capacity, and quality of life in patients with AMI is quite well documented so far
[35][56]. Plenty of studies demonstrated that exercise training affects favorably LVH and LV remodeling. Interestingly, exercise training might reverse LVH to a normal status or at least undergo concentric remodeling. Moreover, there is evidence that training, especially aerobic, improves LVEF and decreases end-diastolic volume (EDV) and end-systolic volume (ESV)
[36][57]. According to a large meta-analysis, exercise training leads to an increase in LVEF, as well as reduction in ESV and EDV in clinically stable post-MI patients
[37][58]. Of note, the greatest benefits in LVEF, ESV, and EDV are occurring when exercise training starts earlier following MI and lasts longer than 3 months, with each week of training delay requiring one additional month of training to achieve the same level of improvement in LV remodeling and the comparable reduction in volumes. Moreover, a decrease in plasma NT-pro-BNP and an increase in peak early mitral flow velocity have been also observed post-training
[38][59]. Additionally, there is evidence that systematic exercise and participation in cardiac rehabilitation programs may significantly improve the cardiorespiratory function, exercise ability, and quality of life in patients with ischemic and nonobstructive coronary arteries (INOCA)
[39][60]. Nevertheless, prolonged endurance exercise training seems to also have detrimental effects on LV systolic and diastolic function.
Exercise training started early after STEMI reduces stress-induced hypoperfusion and improves LV function and contractility. Exercise-induced changes in myocardial perfusion and function is associated with the absence of unfavorable LV remodeling and with an improvement of cardiovascular functional capacity
[40][61]. The beneficial effect of exercise on LV remodeling and cardiopulmonary rehabilitation in LV dysfunction among post-MI patients is also verified by Zhang et al. In this large meta-analysis, it was reported that the greatest benefit of exercise on LV remodeling and cardiopulmonary capacity rehabilitation, as assessed by peak oxygen uptake (VO2), was observed when exercise was initiated in the acute phase after MI, without an increase in the incidence of MACEs
[41][62]. Indeed, during the healing phase after acute MI, the beneficial effects of exercise training on LVEF, LVESD, and peak VO2 weakened compared to the acute phase. Even HIIT seems to improve exercise capacity and quality of life without any detrimental effects on LV remodeling
[37][42][58,63]. These data imply that secondary prevention along with cardiac rehabilitation programs should be initiated early to achieve the maximal anti-remodeling benefit.
2.5. In patients with Heart Failure
Obesity and physical inactivity are considered major lifestyle risk factors for the development and progression of HFpEF
[43][34]. Indeed, low fitness has been associated with a greater risk of HFpEF than HFrEF, whereas patients with HFpEF demonstrate impaired peak VO2 and cardiorespiratory fitness (CRF), which encompasses exercise intolerance, when compared to healthy individuals, deteriorating substantially their prognosis
[44][64].
Physical activity and fitness substantially reduce the risk of developing HF and improve the cardiovascular prognosis among patients with established HF
[45][65]. Interestingly, for every 1-MET improvement in functional capacity, the risk of HF is reduced by 17%
[46][66]. Participation in exercise-based cardiac rehabilitation programs seems to increase exercise capacity by up to 25% and improves the New York Heart Association’s (NYHA) functional status, as well as LV remodeling and hypertrophy. Of note, there is evidence that the beneficial effect of training programs on symptoms, CRF, left ventricular diastolic and systolic function, as well as quality of life and HF-related hospitalizations are also apparent among patients with HFpEF and HFrEF
[47][67]. Moreover, it is also reported that even endurance training reverses adverse cardiac remodeling, and reduces ESV and EDV, thus improving both systolic and diastolic dysfunction in patients with HFrEF
[48][68]. Similarly, high-intensity training beneficially affects exercise capacity and quality of life, with no detrimental changes in LV remodeling among patients with HFrEF
[49][69]. As a result, patients with higher levels of physical activity experience less adverse cardiac events
[50][70].
According to the current literature, exercise training has been associated with a substantial improvement in LV diastolic function
[45][65]. Moreover, it has been reported that exercise reduces LV volumes, which are surrogate markers of LV concentric remodeling or LVH, in patients with HFpEF, whereas exercise training is also correlated with improved measures of CRF. These data demonstrate the pathophysiologic role of LV concentric remodeling contributing to impaired CRF and exercise intolerance in patients with HFpEF and imply that the exercise-meditated improvement in LV remodeling may improve CRF in patients with HFpEF, thus providing novel therapeutical implications
[51][71].