Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nikolay K Shakhpazyan | -- | 3251 | 2023-09-08 09:38:21 | | | |
| 2 | Camila Xu | Meta information modification | 3251 | 2023-09-08 09:48:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shakhpazyan, N.K.; Mikhaleva, L.M.; Bedzhanyan, A.L.; Sadykhov, N.K.; Midiber, K.Y.; Konyukova, A.K.; Kontorschikov, A.S.; Maslenkina, K.S.; Orekhov, A.N. Long Non-Coding RNAs in Colorectal Cancer Microenvironment. Encyclopedia. Available online: https://encyclopedia.pub/entry/48952 (accessed on 13 January 2026).
Shakhpazyan NK, Mikhaleva LM, Bedzhanyan AL, Sadykhov NK, Midiber KY, Konyukova AK, et al. Long Non-Coding RNAs in Colorectal Cancer Microenvironment. Encyclopedia. Available at: https://encyclopedia.pub/entry/48952. Accessed January 13, 2026.
Shakhpazyan, Nikolay K., Liudmila M. Mikhaleva, Arcady L. Bedzhanyan, Nikolay K. Sadykhov, Konstantin Y. Midiber, Alexandra K. Konyukova, Andrey S. Kontorschikov, Ksenia S. Maslenkina, Alexander N. Orekhov. "Long Non-Coding RNAs in Colorectal Cancer Microenvironment" Encyclopedia, https://encyclopedia.pub/entry/48952 (accessed January 13, 2026).
Shakhpazyan, N.K., Mikhaleva, L.M., Bedzhanyan, A.L., Sadykhov, N.K., Midiber, K.Y., Konyukova, A.K., Kontorschikov, A.S., Maslenkina, K.S., & Orekhov, A.N. (2023, September 08). Long Non-Coding RNAs in Colorectal Cancer Microenvironment. In Encyclopedia. https://encyclopedia.pub/entry/48952
Shakhpazyan, Nikolay K., et al. "Long Non-Coding RNAs in Colorectal Cancer Microenvironment." Encyclopedia. Web. 08 September, 2023.
Copy Citation
Colorectal cancer (CRC), encompassing cancers of the colon and rectum, is one of the most prevalent malignancies worldwide and a leading cause of cancer-related death. Long non-coding RNAs (lncRNAs) are RNA molecules that exceed 200 nucleotides in length and are characterized by their lack of classical protein-coding capacity. In CRC, lncRNAs are known to notably modulate fundamental cellular mechanisms and reciprocal interactions, thereby shaping the immune landscape of the TME.
colorectal cancers (CRCs)
immune cells
tumor environment (TME)
long non-coding RNAs (lncRNAs)
1. Introduction
Colorectal cancer (CRC), encompassing cancers of the colon and rectum, is one of the most prevalent malignancies worldwide and a leading cause of cancer-related death [1]. This complex disease, arising from a blend of genetic and environmental factors, manifests as a substantial challenge in oncology due to late-stage diagnosis and resistance to conventional therapies. Understanding the intricate web of CRC requires a deep exploration of its underlying mechanisms, including the significant role of inflammation.
Inflammation is pivotal in CRC pathogenesis, from the inception of aberrant crypt foci to advanced metastatic disease [2][3][4][5]. It not only fuels the initiation and progression of CRC but also fundamentally shapes the tumor microenvironment and the immune response to the tumor [6]. This intertwining of inflammation and cancer has unveiled new frontiers in treatment, including the potential role of long non-coding RNAs (lncRNAs).
Recent evidence has revealed lncRNAs, non-coding RNAs exceeding 200 nucleotides in length, as key players in various biological processes, including inflammation and cancer [7]. Dysregulated lncRNAs are increasingly being recognized as significant contributors to the pathogenesis of CRC immune evasion, and the response to therapy. Moreover, lncRNAs appear to be intricately connected to the inflammatory processes driving CRC, marking them as potential predictive biomarkers for treatment response [8].
The interest in lncRNAs extends to their potential role in enhancing the efficacy of existing treatments, such as immune checkpoint blockade therapies like anti-PD-1/PD-L1 [9][10]. While these therapies have shown promise, their effectiveness varies between individuals, and many patients remain unresponsive. The exploration of lncRNAs in this context might unveil novel avenues for identifying the patients who are most likely to benefit from these immunotherapies and open doors for innovative therapeutic targets [11].
2. The Fundamentals of Long Non-Coding RNAs
Long non-coding RNAs (lncRNAs) are RNA molecules that exceed 200 nucleotides in length and are characterized by their lack of classical protein-coding capacity. These critical biomolecules have recently been brought to the forefront of molecular biology due to their essential roles in regulating several biological processes such as gene expression, cellular differentiation, and development [11].
To further elucidate the intriguing nature of lncRNAs, it is vital to understand their genomic classification, which can provide insight into their nature and, to some extent, their function. As shown in Table 1, lncRNAs can be classified according to the genomic locations of their genes.
Table 1. The classification of long non-coding RNAs (lncRNAs) according to the genomic locations of Their genes.
| Classification | Description |
|---|---|
| Sense lncRNAs | Transcribed from the same strand as a protein-coding gene and may overlap entirely or partially with the gene [12]. |
| Antisense lncRNAs | Transcribed from the opposite strand of a protein-coding gene and may overlap with exons or introns [13]. |
| Intronic lncRNAs | Located within the introns of a protein-coding gene but transcribed independently [14]. |
| Intergenic lncRNAs | Situated between protein-coding genes and do not overlap with them. Also known as long intergenic non-coding RNAs (lincRNAs) [15]. |
| Bidirectional lncRNAs | Transcribed in close proximity to a protein-coding gene but in the opposite direction [16]. |
| Enhancer lncRNAs (eRNAs) | Associated with enhancer regions and may regulate the activity of enhancers, influencing gene expression [17]. |
This classification is valuable for understanding potential regulatory roles and interactions within cellular processes. While some lncRNAs may function in close conjunction with neighboring protein-coding genes, others might exert influence over more distant genomic regions or engage in complex interactions that are not solely dictated by their location [12]. Further research is needed to fully grasp the diverse functions of lncRNAs, considering factors such as cell type, developmental stage, and post-transcriptional modifications.
Expanding upon their roles, lncRNAs also play significant roles in various cellular processes, including gene expression regulation, RNA interactions, RNA–protein interactions, structural roles, and signaling regulation. An overview of these functions and mechanisms is presented in Table 2.
Table 2. Overview of long non-coding RNA (lncRNA) functions and mechanisms in cellular processes.
| Broad Function | Specific Mechanism | Description |
|---|---|---|
| Gene Expression Regulation | Transcriptional Control | Involves the activation/repression of transcription, enhancer activity, RNA polymerase interference, chromatin remodeling, histone modification, and DNA methylation [18][19][20]. |
| Post-transcriptional Control | Includes the regulation of splicing, mRNA stability, and translation [21][22]. | |
| RNA Interactions | miRNA Sponging | lncRNAs may sequester miRNAs away from their target mRNAs [23]. |
| RNA-RNA Interactions | Includes base pairing with other RNAs, affecting function or stability [24]. | |
| RNA–Protein Interactions | Scaffolding and Sequestration | lncRNAs can act as scaffolds for protein complexes or sequester proteins away from functional locations. May overlap with gene expression regulation and RNA interactions [25][26]. |
| Structural Roles | Nuclear Architecture | Contributes to the organization of nuclear structures. May have indirect effects on gene regulation [27]. |
| Signaling Regulation | Pathway Modulation | Involves interactions with signaling molecules or pathway components, potentially impacting various cellular processes, including gene expression, growth, and stress [28][29]. |
The multifaceted nature of lncRNAs and the context-dependent specificity of their functions may lead to real-world scenarios in which the complexities extend beyond this categorization [18][19][20][21][22][23][24][25][26][27][28][29].
Shifting the focus to the broader context of oncogenesis, lncRNAs modulate key cellular processes like proliferation, apoptosis, migration, and invasion. They can potentially instigate the transformation of normal cells into malignant ones [30][31][32]. Moreover, lncRNAs significantly shape the anti-tumor immune landscape by interacting with DNA, RNA, and proteins and by modulating the expression of immune response genes [33].
The mechanisms through which lncRNAs function are as diverse as their roles. For instance, lncRNAs like HOTAIR can promote or hinder the assembly of transcriptional machinery at gene promoters, interacting with the Polycomb Repressive Complex 2 (PRC2) and thereby silencing target genes [34]. Certain lncRNAs also influence post-transcriptional processes, such as mRNA splicing, stability, and translation with MALAT1 [35][36][37]. Furthermore, lncRNAs can function as molecular scaffolds or as molecular decoys, like the lncRNA GAS5 does [38][39].
Delving into their role in cellular communication, lncRNAs play a critical role within the tumor microenvironment. They can be encapsulated into extracellular vesicles such as exosomes and secreted from cells, altering various cellular functions inside recipient cells. This intercellular lncRNA exchange has been associated with aspects of tumor biology like immune modulation, angiogenesis, metastasis, and therapy resistance [40].
In conclusion, this chapter provides general information about this versatile and complex class of biomolecules. The insights laid out will serve as a foundation for the forthcoming discussion on the role of lncRNAs in processes associated with oncogenesis in colorectal cancer. The text is intended to offer a comprehensive yet concise overview, setting the stage for a deeper exploration of the topic.
It would be valuable to mention the role of the sources of information when investigating issues connected with lncRNAs. Various lncRNA–disease association data resources have played an instrumental role in capturing associations with diseases, including CRC. LncRNADisease, now upgraded to LncRNADisease v2.0, includes associations with 529 diseases [41]. Lnc2Cancer is a manually curated database focusing specifically on lncRNA–cancer associations [42], while MNDR covers a wide array of ncRNA–disease associations in mammals [43]. Additionally, resources like LNCipedia [44], lncRNAWiki [45], and lncRNome [46] offer sequence and annotation information, complementing the understanding of the attributes of lncRNAs. Comprehensive platforms such as LncTarD 2.0 have further enhanced the capacity to discern the properties of cancer stem cells and potential clinical applications [47]. Community collaboration is a salient feature, exemplified by databases like lncRNAWiki, which integrate data from various sources [45].
3. Interplay of lncRNAs and Consensus Molecular Subtypes (CMSs) in CRC
The Consensus Molecular Subtypes (CMSs) of CRC offer an invaluable framework for delving into the intricate molecular landscape of this heterogeneous disease [48][49][50]. The distinct biological profiles of CMS1 (MSI immune), CMS2 (canonical), CMS3 (metabolic), and CMS4 (mesenchymal) each carry implications for diagnosis, prognosis, and therapeutic intervention (Table 3).
Table 3. Typical molecular genetic alterations associated with the CMSs of CRC.
| CMS Subtype | Typical Molecular Genetic Alterations |
|---|---|
| CMS1 (MSI Immune) | High microsatellite instability (MSI-H), DNA mismatch repair (MMR) deficiency, hypermutated phenotype, and a high neoantigen load. |
| CMS2 (Canonical) | Chromosomal instability, a high level of somatic copy number alterations, the activation of the WNT and MYC signaling pathways, and mutations in APC and TP53. |
| CMS3 (Metabolic) | Microsatellite stable (MSS), metabolic dysregulation, KRAS mutations, and involvement in the PI3K/AKT signaling pathway and possibly others that affect metabolism, such as CDK2 signaling. |
| CMS4 (Mesenchymal) | Stromal invasion and involvement in the RAS/MAPK, Rb/E2F, CDK8/β-catenin, and Raf/ERK pathways. There is a focus on the epithelial-to-mesenchymal transition (EMT) and the regulation of pathways related to cell growth and migration. |
The CMS1 (MSI immune) subtype, characterized by high microsatellite instability (MSI-H), showcases a hypermutated phenotype that often incites robust immune responses. Within this subtype, both lncRNAs, HOTAIR and LINK-A, are involved in oncogenic activities, focusing on immune activation and hypermutation. HOTAIR, an antisense lncRNA, regulates gene expression by interacting with the Polycomb Repressive Complex 2 (PRC2) and LSD1, modulating H3K27 methylation and thereby affecting gene silencing. This has consequences for PTEN methylation and pathways like PI3K/p-AKT/p-MDM2/p53, and PI3K/AKT/mTOR in tumorigenesis [51][52][53][54]. LINK-A, an intergenic lncRNA, modulates pathways, attenuating PKA activity on TRIM71 and causing AKT hyperactivation, leading to tumorigenesis [55][56][57][58] (Table 4).
Table 4. Interactions and functions of lncRNAs in CRC CMSs.
| lncRNA | CMS Subtype | Main Characteristics | References |
|---|---|---|---|
| HOTAIR | CMS1 (MSI Immune) | Antisense lncRNA. Gene expression regulation. Oncogenic. Interacts with PRC2 and LSD1 to modulate H3K27 methylation, affecting gene silencing. Consequences: PTEN methylation, PI3K/p-AKT/p-MDM2/p53, and PI3K/AKT/mTOR pathways in tumorigenesis; regulates ASTN1, PCDHA1, and MUC5AC in metastasis. | [49][50][53][54] |
| LINK-A (LINC01139) | CMS1 (MSI Immune) | Intergenic lncRNA. Pathway modulation. Oncogenic. Facilitates crosstalk between the PIP3 and GPCR pathways, attenuating PKA activity on TRIM71, leading to the degradation of PLC and tumor suppressors Rb and p53. Directly binds to phosphatidylcholine, AKT, and PIP3, causing AKT hyperactivation and tumorigenesis. | [55][56][57][58] |
| CCAT1 | CMS2 (Canonical) | Intergenic lncRNA. Nuclear architecture; scaffolding. Oncogenic. Mediates chromosome looping with CTCF, affecting c-Myc promoter and promoting c-Myc expression. Acts as a ceRNA; serves as a scaffold for epigenetic complexes, with chromosome looping central to interaction. | [59][60] |
| CRNDE | CMS2 (Canonical) | Intergenic lncRNA. miRNA sponging; pathway modulation; transcriptional control. Oncogenic. Molecular sponge for miRNAs; promotes cell growth. Activates/inhibits the Wnt/β-catenin, PI3K/AKT/mTOR, Ras/MAPK, and Notch1 signaling pathways. Binds to EZH2. | [61][62][63] |
| lncRNA-ATB | CMS3 (Metabolic) | Intergenic lncRNA. miRNA sponging. Oncogenic. Interacts with miR-141-3p and miR-200c, influencing the CDK2 pathway, affecting EMT process, and contributing to cancer progression. | [64][65][66][67] |
| RP11-462C24.1 (RPL34-DT) | CMS3 (Metabolic) | Intergenic lncRNA. Pathway modulation; transcriptional control. Oncosuppressive. Upregulates HSP70; inhibits the PI3K/AKT signaling pathway. | [68][69] |
| H19 | CMS4 (Mesenchymal) | Intergenic lncRNA. RNA interactions; pathway modulation. Oncogenic. Promotes CRC progression by targeting RB with miR-675, sponging miR-200a and miR-138, leading to HMGA2 upregulation. Activates the RAS/MAPK, Rb/E2F, CDK8/β-catenin, and Raf/ERK pathways. | [70][71][72][73][74] |
| lincRNA-p21 (TP53COR1) | CMS4 (Mesenchymal) | Intergenic lncRNA. RNA–RNA interactions; RNA–protein interactions. Oncosuppressive. Interacts with the JUNB and CTNNB1 mRNAs, reducing translation. Antagonism via HuR. mTOR/lincRNA-p21 involved in carcinogenesis, progression, metastasis. Part of the p53 network. | [75][76][77][78] |
The CMS2 (canonical) subtype is marked by chromosomal instability and WNT and MYC signaling and is associated with the lncRNAs CCAT1 and CRNDE. These lncRNAs in this subtype are engaged in oncogenic mechanisms, such as miRNA sponging and pathway modulation. CCAT1, an intergenic lncRNA, might interact with EZH2 to mediate chromosome looping with CTCF, affecting the c-Myc promoter and leading to gene silencing [59][60]. CRNDE, another intergenic lncRNA, is involved in the Wnt/β-catenin and other pathways, promoting cell growth [61][62][63] (Table 4).
The CMS3 (metabolic) subtype, distinguished by metabolic dysregulation and prevalent KRAS mutations, includes lncRNAs like lncRNA-ATB and RP11-462C24.1. Both lncRNAs in this subtype are intergenic and contribute to metabolic reprogramming, either through oncogenic or oncosuppressive functions. lncRNA-ATB may induce the epithelial-to-mesenchymal transition (EMT) by sponging miR-200 family members, while RP11-462C24.1 is associated with oxidative phosphorylation regulation and the upregulation of HSP70 [64][65][66][67][68][69] (Table 4).
In the CMS4 (mesenchymal) subtype, which is known for TGF-β activation, stromal invasion, and angiogenesis, the lncRNAs H19 and lincRNA-p21 are vital. These lncRNAs are linked with EMT, angiogenesis, inflammation, and matrix remodeling. H19 can upregulate HMGA2 by sponging the let-7 microRNA, and lincRNA-p21 can induce EMT in response to TGF-β, augmenting the invasive and metastatic potential of cancer cells [70][71][72][73][74][75][76][78] (Table 4).
Interestingly, these lncRNAs not only play a variety of roles in cellular processes but also demonstrate subtype specificity in CRC. For instance, HOTAIR’s association with CMS1 and its potential role in immune evasion, or CCAT1′s correlation with CMS2 and its influence on cell proliferation and invasion, underline the versatility and specificity of lncRNAs. lncRNAs are emerging as potential therapeutic targets. Their distinct expression in different CMS subtypes could be exploited to develop personalized therapeutic strategies, such as inhibiting overexpressed lncRNAs like HOTAIR in CMS1 or CCAT1 in CMS2. Furthermore, lncRNAs like H19 in CMS4 have been associated with a poor prognosis, suggesting their potential utility as diagnostic or prognostic biomarkers [6].
Another fascinating aspect of lncRNAs is their influence on drug resistance in CRC, making them a crucial focus for improving treatment outcomes. Additionally, lncRNAs have been found to interact with microRNAs, influencing their function and contributing to complex regulatory networks. For instance, the interaction of H19 with the let-7 miRNA in CMS4 deregulates its targets, promoting the epithelial-to-mesenchymal transition and stemness [79]. As the field of lncRNA research continues to develop, it offers promising new possibilities for understanding the molecular intricacies of CRC subtypes and refining therapeutic strategies to enhance patient outcomes.
4. The Role of Immunity and Inflammation in CRC Tumor Stroma
LncRNAs have emerged as pivotal regulatory elements across a broad spectrum of biological processes. Their roles are especially conspicuous within the intricate orchestration of the tumor microenvironment (TME), a crucial aspect of cancer’s multifaceted architecture. In CRC, lncRNAs are known to notably modulate fundamental cellular mechanisms and reciprocal interactions, thereby shaping the immune landscape of the TME. Consequently, elucidating the influence of lncRNA-guided processes on inflammation, immune responses, and metabolic reprogramming within the TME is crucial. This section sheds light on the main orchestrators involved in shaping the immunoregulatory milieu surrounding tumor cells (Figure 1).
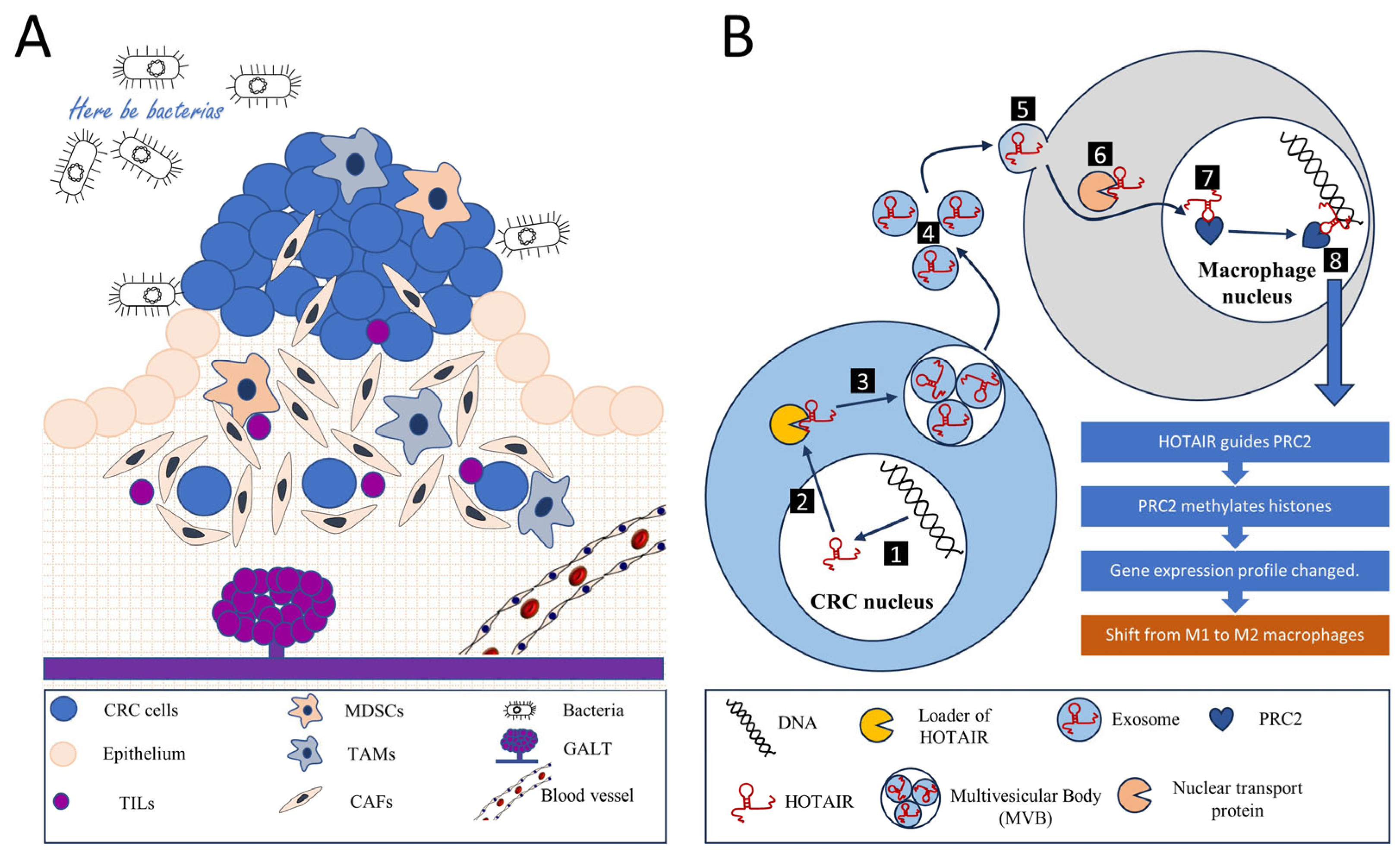
Figure 1. Tumor microenvironment and intercellular communications. (A) A colorectal cancer tumor is a complex, multicellular, and multi-dimensional entity. The tumor microenvironment encompasses not only tumor cells originating from the intestinal epithelium but also various components that help regulate metabolism, nutrition, and immune responses. These components include an extracellular matrix, a diverse population of tumor-infiltrating lymphocytes (TILs), myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and cancer-associated fibroblasts (CAFs), along with blood and lymphatic vessels. Another characteristic aspect of colorectal cancer is the presence of gut-associated lymphoid tissue (GALT). Additionally, bacteria play an essential role within the microenvironment. All these components interact to maintain a balance, thereby establishing a conducive environment for tumor growth. Among other forms of interaction, intercellular communication involving long non-coding RNAs (lncRNAs) is noteworthy. (B) The signaling mechanism of the long non-coding RNA (lncRNA) HOTAIR can serve as an example of intercellular communication through an lncRNA. In the nucleus of the tumor cell, the transcription and primary modification of the HOTAIR gene RNA occur (1), after which the mature lncRNA HOTAIR moves to the cytoplasm (2). Here, via a loader mechanism, HOTAIR is incorporated into a multivesicular body (MVB) where exosome particles loaded with HOTAIR are formed (3). These exosomes are then released into the extracellular environment (4). Their content is subsequently absorbed by macrophages via pinocytosis (5). Nuclear transport proteins guide HOTAIR into the macrophage’s nucleus (6), where this RNA interacts with the Polycomb Repressive Complex 2 (PRC2) chromatin remodeling complex (7). The HOTAIR-PRC2 complex identifies specific genomic regions (8), leading the PRC2 complex to methylate histones and silence a series of genes, thereby altering the gene expression profile of the macrophage. This results in a switch in macrophage polarization from M1 (anti-tumor) to M2 (pro-tumor), helping to sustain an environment conducive to tumor growth.
Inflammation plays a pivotal role in the TME of CRC. Cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs) significantly contribute to this process, secreting pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). Paradoxically, these cytokines can foster chronic inflammation, potentially promoting tumor progression [80]. Meanwhile, the ensuing inflammation can stimulate the recruitment and activation of immune cells capable of eliminating tumor cells.
The tumor stroma in CRC fosters the establishment of an immunosuppressive environment through various mechanisms. Notably, stromal cells, chiefly CAFs, secrete immunosuppressive factors such as transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10), which inhibit T cell activity while enhancing regulatory T cell (Treg) functions [81][82]. Similarly, TAMs secrete factors like TGF-β, IL-10, and programmed cell death ligand-1 (PD-L1), collectively inhibiting the function of cytotoxic T cells and promoting the activity of Tregs [83][84].
Moreover, chronic exposure to tumor antigens and inflammatory signals in the TME can drive a state of T cell exhaustion which is characterized by diminished effector functions and the persistent expression of inhibitory receptors, including programmed cell death protein-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). This state renders T cells less effective in eliminating cancer cells, thus facilitating immune evasion [85].
Another significant immunosuppressive mechanism in the TME involves the recruitment of regulatory immune cells. The tumor stroma can attract immunosuppressive cell types such as myeloid-derived suppressor cells (MDSCs) and Tregs, which impede the activity of cytotoxic T cells and natural killer (NK) cells, further facilitating tumor immune evasion [86][87].
Metabolic reprogramming is a defining feature of the TME. Both tumor cells and stromal cells can reshape the metabolic landscape of the TME, leading to conditions like hypoxia and nutrient deprivation. These conditions can adversely affect immune cell function and survival. For instance, tumor cells and CAFs can deplete vital nutrients like glucose and amino acids, thereby inhibiting T cell function [86].
CAFs, as primary stromal cells, play a crucial role in remodeling the extracellular matrix (ECM). This remodeling can construct a physical barrier that hinders immune cell infiltration and access to tumor cells. Therefore, understanding these mechanisms and interactions is vital for devising therapeutic strategies to counteract the immunosuppressive TME and boost antitumor immunity in CRC.
Within CRC, the TME can induce various forms of cell polarization to enhance the immunosuppressive state, thereby aiding the tumor in immune evasion. Macrophages within the TME often adopt an M2 polarization state known as “alternatively activated”. These M2 TAMs foster tissue repair, angiogenesis, and immune suppression by producing anti-inflammatory cytokines such as IL-10 and TGF-β and expressing high levels of immune checkpoint molecules like PD-L1, thereby inhibiting T cell function [87].
Conventional CD4+ T cells within the TME can be polarized into Tregs, identified via the expression of the transcription factor FOXP3 [83]. Tregs suppress the immune response by inhibiting the function of cytotoxic CD8+ T cells and other immune cells. Additionally, chronic exposure to antigens within the TME can instigate T cell exhaustion, which is characterized by upregulated inhibitory receptors like PD-1 and CTLA-4 and diminished effector functions [88].
MDSCs, a heterogeneous group of immature myeloid cells, can suppress the function of T cells through multiple mechanisms, including the production of immunosuppressive factors like arginase-1, nitric oxide, and reactive oxygen species [89][90]. Tumor-induced factors can also render dendritic cells (DCs) tolerogenic, diminishing their ability to activate T cells and potentially inducing Tregs or anergic T cells. These cellular polarization processes assist the colorectal tumor in establishing an immunosuppressive environment, thus contributing to immune evasion [91]. However, these processes also provide potential targets for therapeutic intervention, such as strategies aimed at reprogramming TAMs toward an M1 (pro-inflammatory and tumoricidal) phenotype, inhibiting Tregs, or reversing T cell exhaustion.
References
- Patel, S.G.; Karlitz, J.J.; Yen, T.; Lieu, C.H.; Boland, C.R. The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 2022, 7, 262–274.
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. European Crohn’s and Colitis Organisation and the European Society of Gastrointestinal and Abdominal Radiology . ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 2019, 13, 144–164.
- Nardone, O.M.; Zammarchi, I.; Santacroce, G.; Ghosh, S.; Iacucci, M. Inflammation-Driven Colorectal Cancer Associated with Colitis: From Pathogenesis to Changing Therapy. Cancers 2023, 15, 2389.
- Newman, P.; Muscat, J. Potential Role of Non-Steroidal Anti-Inflammatory Drugs in Colorectal Cancer Chemoprevention for Inflammatory Bowel Disease: An Umbrella Review. Cancers 2023, 15, 1102.
- Kobayashi, H.; Gieniec, K.A.; Lannagan, T.R.M.; Wang, T.; Asai, N.; Mizutani, Y.; Iida, T.; Ando, R.; Thomas, E.M.; Sakai, A.; et al. The Origin and Contribution of Cancer-Associated Fibroblasts in Colorectal Carcinogenesis. Gastroenterology 2022, 162, 890–906.
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667.
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447.
- Huang, M.; Ye, Y.; Chen, Y.; Zhu, J.; Xu, L.; Cheng, W.; Lu, X.; Yan, F. Identification and validation of an inflammation-related lncRNAs signature for improving outcomes of patients in colorectal cancer. Front. Genet. 2022, 13, 955240.
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520.
- Benelli, R.; Zocchi, M.R.; Poggi, A. Immune Checkpoint Receptor/Ligand Expression and Chemotherapy in Colorectal Cancer. Cancers 2023, 15, 914.
- Pan, X.; Li, C.; Feng, J. The role of LncRNAs in tumor immunotherapy. Cancer Cell Int. 2023, 23, 30.
- Perez, C.A.G.; Adachi, S.; Nong, Q.D.; Adhitama, N.; Matsuura, T.; Natsume, T.; Wada, T.; Kato, Y.; Watanabe, H. Sense-overlapping lncRNA as a decoy of translational repressor protein for dimorphic gene expression. PLoS Genet. 2021, 17, e1009683.
- Liu, B.; Xiang, W.; Liu, J.; Tang, J.; Wang, J.; Liu, B.; Long, Z.; Wang, L.; Yin, G.; Liu, J. The regulatory role of antisense lncRNAs in cancer. Cancer Cell Int. 2021, 21, 459.
- Tahira, A.C.; Kubrusly, M.S.; Faria, M.F.; Dazzani, B.; Fonseca, R.S.; Maracaja-Coutinho, V.; Verjovski-Almeida, S.; Machado, M.C.; Reis, E.M. Long noncoding intronic RNAs are differentially expressed in primary and metastatic pancreatic cancer. Mol. Cancer 2011, 10, 141.
- Marques, A.C.; Ponting, C.P. Intergenic lncRNAs and the Evolution of Gene Expression. Curr. Opin. Genet. Dev. 2014, 27, 48–53.
- Hori, Y.; Tanimoto, Y.; Takahashi, S.; Furukawa, T.; Koshiba-Takeuchi, K.; Takeuchi, J.K. Important Cardiac Transcription Factor Genes Are Accompanied by Bidirectional Long Non-Coding RNAs. BMC Genom. 2018, 19, 967.
- Hou, Y.; Zhang, R.; Sun, X. Enhancer LncRNAs Influence Chromatin Interactions in Different Ways. Front. Genet. 2019, 10, 936.
- Long, Y.; Wang, X.; Youmans, D.T.; Cech, T.R. How do lncRNAs regulate transcription? Sci. Adv. 2017, 3, eaao2110.
- Böhmdorfer, G.; Wierzbicki, A.T. Control of Chromatin Structure by Long Noncoding RNA. Trends Cell Biol. 2015, 25, 623–632.
- Huang, W.; Li, H.; Yu, Q.; Xiao, W.; Wang, D.O. LncRNA-mediated DNA methylation: An emerging mechanism in cancer and beyond. J. Exp. Clin. Cancer Res. 2022, 41, 100.
- Pisignano, G.; Ladomery, M. Epigenetic Regulation of Alternative Splicing: How LncRNAs Tailor the Message. Non-Coding RNA 2021, 7, 21.
- Yoon, J.H.; Abdelmohsen, K.; Gorospe, M. Posttranscriptional gene regulation by long noncoding RNA. J. Mol. Biol. 2013, 425, 3723–3730.
- Lou, W.; Ding, B.; Fu, P. Pseudogene-Derived lncRNAs and Their miRNA Sponging Mechanism in Human Cancer. Front. Cell Dev. Biol. 2020, 8, 85.
- Szcześniak, M.W.; Makałowska, I. lncRNA-RNA Interactions across the Human Transcriptome. PLoS ONE 2016, 11, e0150353.
- Luo, J.; Qu, L.; Gao, F.; Lin, J.; Liu, J.; Lin, A. LncRNAs: Architectural Scaffolds or More Potential Roles in Phase Separation. Front. Genet. 2021, 12, 626234.
- Blythe, A.J.; Fox, A.H.; Bond, C.S. The ins and outs of lncRNA structure: How, why and what comes next? Biochim. Biophys. Acta 2016, 1859, 46–58.
- Guh, C.Y.; Hsieh, Y.H.; Chu, H.P. Functions and properties of nuclear lncRNAs-from systematically mapping the interactomes of lncRNAs. J. Biomed. Sci. 2020, 27, 44.
- Hu, X.Y.; Hou, P.F.; Li, T.T.; Quan, H.Y.; Li, M.L.; Lin, T.; Liu, J.J.; Bai, J.; Zheng, J.N. The roles of Wnt/β-catenin signaling pathway related lncRNAs in cancer. Int. J. Biol. Sci. 2018, 14, 2003–2011.
- Fu, P.F.; Zheng, X.; Fan, X.; Lin, A.F. Role of cytoplasmic lncRNAs in regulating cancer signaling pathways. J. Zhejiang Univ. Sci. B 2019, 20, 1–8.
- Gong, Z.; Zhang, Y.; Yang, Y.; Yang, Y.; Zhang, J.; Wang, Y.; Zhao, L.; Yu, N.; Wu, Z.; Guo, W. LncRNA LINC01094 Promotes Cells Proliferation and Metastasis through the PTEN/AKT Pathway by Targeting AZGP1 in Gastric Cancer. Cancers 2023, 15, 1261.
- Jiang, N.; Zhang, X.; Gu, X.; Li, X.; Shang, L. Progress in understanding themolecular mechanisms of the antitumour effects of the long non-coding RNA MEG3. Cancers 2023, 15, 1230.
- Lingadahalli, S.; Jadhao, S.; Sung, Y.Y.; Chen, M.; Hu, L.; Chen, X.; Cheung, E. Novel lncRNA LINC00844 Regulates Prostate Cancer Cell Migration and Invasion through AR Signaling. Mol. Cancer Res. 2018, 16, 1865–1878.
- Zhang, W.; Yan, Y.; Peng, J.; Thakur, A.; Bai, N.; Yang, K.; Xu, Z. Decoding Roles of Exosomal lncRNAs in Tumor-Immune Regulation and Therapeutic Potential. Cancers 2022, 15, 286.
- Kuo, F.C.; Neville, M.J.; Sabaratnam, R.; Wesolowska-Andersen, A.; Phillips, D.; Wittemans, L.B.L.; van Dam, A.D.; Loh, N.Y.; Todorčević, M.; Denton, N.; et al. HOTAIR interacts with PRC2 complex regulating the regional preadipocyte transcriptome and human fat distribution. Cell Rep. 2022, 40, 111136.
- Xiao, Y.; Pan, J.; Geng, Q.; Wang, G. LncRNA MALAT1 Increases the Stemness of Gastric Cancer Cells via Enhancing SOX2 mRNA Stability. FEBS Open Bio 2019, 9, 1212–1222.
- Ji, Q.; Cai, G.; Liu, X.; Zhang, Y.; Wang, Y.; Zhou, L.; Sui, H.; Li, Q. MALAT1 Regulates the Transcriptional and Translational Levels of Proto-Oncogene RUNX2 in Colorectal Cancer Metastasis. Cell Death Dis. 2019, 10, 378.
- Gordon, M.A.; Babbs, B.; Cochrane, D.R.; Bitler, B.G.; Richer, J.K. The long non-coding RNA MALAT1 promotes ovarian cancer progression by regulating RBFOX2-mediated alternative splicing. Mol. Carcinog. 2019, 58, 196–205.
- Taiana, E.; Ronchetti, D.; Todoerti, K.; Nobili, L.; Tassone, P.; Amodio, N.; Neri, A. LncRNA NEAT1 in Paraspeckles: A Structural Scaffold for Cellular DNA Damage Response Systems? Noncoding RNA 2020, 6, 26.
- Pardini, B.; Calin, G.A. MicroRNAs and Long Non-Coding RNAs and Their Hormone-Like Activities in Cancer. Cancers 2019, 11, 378.
- Abramowicz, A.; Story, M.D. The Long and Short of It: The Emerging Roles of Non-Coding RNA in Small Extracellular Vesicles. Cancers 2020, 12, 1445.
- Bao, Z.; Yang, Z.; Huang, Z.; Zhou, Y.; Cui, Q.; Dong, D. LncRNADisease 2.0: An Updated Database of Long Non-Coding RNA-Associated Diseases. Nucleic Acids Res. 2019, 47, D1034–D1037.
- Gao, Y.; Shang, S.; Guo, S.; Li, X.; Zhou, H.; Liu, H.; Sun, Y.; Wang, J.; Wang, P.; Zhi, H.; et al. Lnc2Cancer 3.0: An Updated Resource for Experimentally Supported lncRNA/circRNA Cancer Associations and Web Tools Based on RNA-Seq and scRNA-Seq Data. Nucleic Acids Res. 2021, 49, D1251–D1258.
- Ning, L.; Cui, T.; Zheng, B.; Wang, N.; Luo, J.; Yang, B.; Du, M.; Cheng, J.; Dou, Y.; Wang, D. MNDR v3.0: Mammal ncRNA-Disease Repository with Increased Coverage and Annotation. Nucleic Acids Res. 2021, 49, D160–D164.
- Volders, P.J.; Anckaert, J.; Verheggen, K.; Nuytens, J.; Martens, L.; Mestdagh, P.; Vandesompele, J. LNCipedia 5: Towards a Reference Set of Human Long Non-Coding RNAs. Nucleic Acids Res. 2019, 47, D135–D139.
- Liu, L.; Li, Z.; Liu, C.; Zou, D.; Li, Q.; Feng, C.; Jing, W.; Luo, S.; Zhang, Z.; Ma, L. LncRNAWiki 2.0: A Knowledgebase of Human Long Non-Coding RNAs with Enhanced Curation Model and Database System. Nucleic Acids Res. 2022, 50, D190–D195.
- Bhartiya, D.; Pal, K.; Ghosh, S.; Kapoor, S.; Jalali, S.; Panwar, B.; Jain, S.; Sati, S.; Sengupta, S.; Sachidanandan, C.; et al. lncRNome: A Comprehensive Knowledgebase of Human Long Noncoding RNAs. Database 2013, 2013, bat034.
- Zhao, H.; Yin, X.; Xu, H.; Liu, K.; Liu, W.; Wang, L.; Zhang, C.; Bo, L.; Lan, X.; Lin, S.; et al. LncTarD 2.0: An Updated Comprehensive Database for Experimentally-Supported Functional lncRNA-Target Regulations in Human Diseases. Nucleic Acids Res. 2023, 51, D199–D207.
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356.
- Ten Hoorn, S.; de Back, T.R.; Sommeijer, D.W.; Vermeulen, L. Clinical Value of Consensus Molecular Subtypes in Colorectal Cancer: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2022, 114, 503–516.
- Chowdhury, S.; Hofree, M.; Lin, K.; Maru, D.; Kopetz, S.; Shen, J.P. Implications of Intratumor Heterogeneity on Consensus Molecular Subtype (CMS) in Colorectal Cancer. Cancers 2021, 13, 4923.
- Zhou, X.; Chen, J.; Tang, W. The Molecular Mechanism of HOTAIR in Tumorigenesis, Metastasis, and Drug Resistance. Acta Biochim. Biophys. Sin. 2014, 46, 1011–1015.
- Yu, X.; Li, Z. Long Non-Coding RNA HOTAIR: A Novel Oncogene. Mol. Med. Rep. 2015, 12, 5611–5618.
- Qiu, W.; Yang, J.; Wang, B.; Yang, M.; Tian, G.; Wang, P.; Yang, J. Evaluating the Microsatellite Instability of Colorectal Cancer Based on Multimodal Deep Learning Integrating Histopathological and Molecular Data. Front. Oncol. 2022, 12, 925079.
- Pashapour, S.; Shanehbandi, D.; Bornehdeli, S.; Zafari, V.; Khani, M.R.; Hashemzadeh, S.; Asvadi Kermani, T. Overexpression of HOTAIR in Tumor Tissues of Patients with Colon Cancer Correlates with Tumor Metastasis and Differentiation. Middle East. J. Cancer 2020, 11, 410–414.
- Hu, Q.; Ye, Y.; Chan, L.C.; Li, Y.; Liang, K.; Lin, A.; Egranov, S.D.; Zhang, Y.; Xia, W.; Gong, J.; et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat. Immunol. 2019, 20, 835–851.
- Lin, A.; Hu, Q.; Li, C.; Xing, Z.; Ma, G.; Wang, C.; Li, J.; Ye, Y.; Yao, J.; Liang, K.; et al. The LINK-A LncRNA Interacts with PtdIns(3,4,5)P3 to Hyperactivate AKT and Confer Resistance to AKT Inhibitors. Nat. Cell Biol. 2017, 19, 238–251.
- Zhao, C.; Gan, C.; Xiao, Y.; Liu, R.; Zhang, L.; Lan, T.; Ye, Y.; Tong, H.; Huang, Z.; Tang, C.; et al. High expression of long non-coding RNA Linc-A associates with poor survival in patients with colorectal cancer. Mol. Biol. Rep. 2020, 47, 7497–7504.
- Chen, B.; Dragomir, M.P.; Yang, C.; Li, Q.; Horst, D.; Calin, G.A. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct. Target. Ther. 2022, 7, 121.
- Javed, Z.; Khan, K.; Sadia, H.; Raza, S.; Salehi, B.; Sharifi-Rad, J.; Cho, W.C. LncRNA & Wnt signaling in colorectal cancer. Cancer Cell Int. 2020, 20, 326.
- Liu, Z.; Chen, Q.; Hann, S.S. The functions and oncogenic roles of CCAT1 in human cancer. Biomed. Pharmacother. 2019, 115, 108943.
- Lu, Y.; Sha, H.; Sun, X.; Zhang, Y.; Wu, Y.; Zhang, J.; Zhang, H.; Wu, J.; Feng, J. CRNDE: An Oncogenic Long Non-Coding RNA in Cancers. Cancer Cell Int. 2020, 20, 162.
- Han, P.; Li, J.W.; Zhang, B.M.; Lv, J.C.; Li, Y.M.; Gu, X.Y.; Yu, Z.W.; Jia, Y.H.; Bai, X.F.; Li, L.; et al. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol. Cancer 2017, 16, 9.
- Neve, B.; Jonckheere, N.; Vincent, A.; Van Seuningen, I. Single-Cell Analysis May Shed New Lights on the Role of LncRNAs in Chemoresistance in Gastrointestinal Cancers. RNA Technol. 2020, 11, 229–253.
- Tang, F.; Xu, Y.; Wang, H.; Bian, E.; Zhao, B. LncRNA-ATB in Cancers: What Do We Know So Far? Mol. Biol. Rep. 2020, 47, 4077–4086.
- Liu, X.; Wang, C. Long Non-Coding RNA ATB Is Associated with Metastases and Promotes Cell Invasion in Colorectal Cancer via Sponging miR-141-3p. Exp. Ther. Med. 2020, 20, 261, Erratum in Exp. Ther. Med. 2022, 23, 238.
- Chi, Y.; Wang, D.; Wang, J.; Yu, W.; Yang, J. Long Non-Coding RNA in the Pathogenesis of Cancers. Cells 2019, 8, 1015.
- Yuan, J.-H.; Yang, F.; Wang, F.; Ma, J.-Z.; Guo, Y.-J.; Tao, Q.-F.; Liu, F.; Pan, W.; Wang, T.-T.; Zhou, C.-C.; et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell 2014, 25, 666–681.
- Shi, D.; Zheng, H.; Zhuo, C.; Peng, J.; Li, D.; Xu, Y.; Li, X.; Cai, G.; Cai, S. Low expression of novel lncRNA RP11-462C24.1 suggests a biomarker of poor prognosis in colorectal cancer. Med. Oncol. 2014, 31, 31.
- Zhang, H.; Zhang, G.; Liu, H.; Shan, Y.; Zhang, X. RP11-462C24.1 suppresses proliferation and invasion of colorectal carcinoma cells by regulating HSP70 through PI3K/AKT signaling pathway. Hum. Cell. 2021, 34, 132–151.
- Yang, J.; Qi, M.; Fei, X.; Wang, X.; Wang, K. LncRNA H19: A Novel Oncogene in Multiple Cancers. Int. J. Biol. Sci. 2021, 17, 3188–3208.
- Xie, J.; Hu, Y.; Sun, D.; Liu, C.; Li, Z.; Zhu, J. Targeting non-coding RNA H19: A potential therapeutic approach in pulmonary diseases. Front. Pharmacol. 2022, 13, 978151.
- Xiong, H.; Shen, J.; Chen, Z.; Yang, J.; Xie, B.; Jia, Y.; Jayasinghe, U.; Wang, J.; Zhao, W.; Xie, S.; et al. H19/let 7/Lin28 ceRNA network mediates autophagy inhibiting epithelial mesenchymal transition in breast cancer. Int. J. Oncol. 2020, 56, 794–806.
- Kou, N.; Liu, S.; Li, X.; Li, W.; Zhong, W.; Gui, L.; Chai, S.; Ren, X.; Na, R.; Zeng, T.; et al. H19 Facilitates Tongue Squamous Cell Carcinoma Migration and Invasion via Sponging miR-let-7. Oncol. Res. 2019, 27, 173–182.
- Lu, W.; Huang, Z.; Wang, J.; Liu, H. Long non-coding RNA DANCR accelerates colorectal cancer progression via regulating the miR-185-5p/HMGA2 axis. J. Biochem. 2022, 171, 389–398.
- Huarte, M. The Emerging Role of LncRNAs in Cancer. Nat. Med. 2015, 21, 1253–1261.
- Huang, T.; Wang, M.; Huang, B.; Chang, A.; Liu, F.; Zhang, Y.; Jiang, B. Long Noncoding RNAs in the mTOR Signaling Network: Biomarkers and Therapeutic Targets. Apoptosis 2018, 23, 255–264.
- Tu, X.; Zhang, Y.; Zheng, X.; Deng, J.; Li, H.; Kang, Z.; Cao, Z.; Huang, Z.; Ding, Z.; Dong, L.; et al. TGF-β-induced hepatocyte lincRNA-p21 contributes to liver fibrosis in mice. Sci. Rep. 2017, 7, 2957.
- Amirinejad, R.; Rezaei, M.; Shirvani-Farsani, Z. An update on long intergenic noncoding RNA p21: A regulatory molecule with various significant functions in cancer. Cell Biosci. 2020, 10, 82.
- Ma, Y.; Shen, N.; Wicha, M.S.; Luo, M. The Roles of the Let-7 Family of MicroRNAs in the Regulation of Cancer Stemness. Cells 2021, 10, 2415.
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602.
- Ma, Z.J.; Wang, Y.H.; Li, Z.G.; Wang, Y.; Li, B.Y.; Kang, H.Y.; Wu, X.Y. Immunosuppressive Effect of Exosomes from Mesenchymal Stromal Cells in Defined Medium on Experimental Colitis. Int. J. Stem Cells 2019, 12, 440–448.
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131.
- Kos, K.; Salvagno, C.; Wellenstein, M.D.; Aslam, M.A.; Meijer, D.A.; Hau, C.-S.; Vrijland, K.; Kaldenbach, D.; Raeven, E.A.; Schmittnaegel, M.; et al. Tumor-associated macrophages promote intratumoral conversion of conventional CD4+ T cells into regulatory T cells via PD-1 signalling. Oncoimmunology 2022, 11, 2063225.
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022, 10, 20503121211069012.
- Horzum, U.; Yanik, H.; Taskiran, E.Z.; Esendagli, G. Effector Th1 cells under PD-1 and CTLA-4 checkpoint blockade abrogate the upregulation of multiple inhibitory receptors and by-pass exhaustion. Immunology 2022, 167, 640–650.
- Iglesias-Escudero, M.; Arias-González, N.; Martínez-Cáceres, E. Regulatory cells and the effect of cancer immunotherapy. Mol. Cancer 2023, 22, 26.
- Tay, C.; Tanaka, A.; Sakaguchi, S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell 2023, 41, 450–465.
- Ando, M.; Ito, M.; Srirat, T.; Kondo, T.; Yoshimura, A. Memory T cell, exhaustion, and tumor immunity. Immunol. Med. 2020, 43, 1–9.
- Vanhaver, C.; van der Bruggen, P.; Bruger, A.M. MDSC in Mice and Men: Mechanisms of Immunosuppression in Cancer. J. Clin. Med. 2021, 10, 2872.
- Grzywa, T.M.; Sosnowska, A.; Matryba, P.; Rydzynska, Z.; Jasinski, M.; Nowis, D.; Golab, J. Myeloid Cell-Derived Arginase in Cancer Immune Response. Front. Immunol. 2020, 11, 938.
- Mortezaee, K.; Majidpoor, J. Roles for macrophage-polarizing interleukins in cancer immunity and immunotherapy. Cell Oncol. 2022, 45, 333–353.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
485
Revisions:
2 times
(View History)
Update Date:
08 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




