Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Satoru Matsuda | -- | 1534 | 2023-09-07 15:45:26 | | | |
| 2 | Peter Tang | Meta information modification | 1534 | 2023-09-08 04:32:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Matsuda, S. ROS and Autophagy in Cisplatin-Induced Acute Kidney Injury. Encyclopedia. Available online: https://encyclopedia.pub/entry/48928 (accessed on 08 February 2026).
Yoshikawa S, Taniguchi K, Sawamura H, Ikeda Y, Tsuji A, Matsuda S. ROS and Autophagy in Cisplatin-Induced Acute Kidney Injury. Encyclopedia. Available at: https://encyclopedia.pub/entry/48928. Accessed February 08, 2026.
Yoshikawa, Sayuri, Kurumi Taniguchi, Haruka Sawamura, Yuka Ikeda, Ai Tsuji, Satoru Matsuda. "ROS and Autophagy in Cisplatin-Induced Acute Kidney Injury" Encyclopedia, https://encyclopedia.pub/entry/48928 (accessed February 08, 2026).
Yoshikawa, S., Taniguchi, K., Sawamura, H., Ikeda, Y., Tsuji, A., & Matsuda, S. (2023, September 07). ROS and Autophagy in Cisplatin-Induced Acute Kidney Injury. In Encyclopedia. https://encyclopedia.pub/entry/48928
Yoshikawa, Sayuri, et al. "ROS and Autophagy in Cisplatin-Induced Acute Kidney Injury." Encyclopedia. Web. 07 September, 2023.
Copy Citation
Cisplatin-induced acute kidney injury (AKI) is the main factor restraining the clinical application of cisplatin. As increased levels of reactive oxygen species (ROS) may promote the progression of the injury, the elimination of ROS has been considered as an effective method to prevent the cisplatin-induced AKI. In addition, it has been revealed that an inducer of autophagy could protect kidney cells in the autophagy dependent manner. Induction of autophagy could also modulate the production of ROS in cases of renal injury.
cisplatin
reactive oxygen species
autophagy
histone deacetylases
cancer
acute kidney injury
1. Introduction
Cisplatin is one of the most widely used broad-spectrum anticancer agents, and is used for the treatment of various solid tumors such as ovarian cancer, prostate cancer, bladder cancer, head or neck cancer, and lung cancer [1][2]. The antitumor mechanisms of cisplatin are mainly DNA damage via the enhanced generation of reactive oxygen species (ROS) [3]. The excess ROS such as superoxide anion, hydrogen peroxide, and hydroxyl radical would cause oxidative damage to various important molecules including proteins, lipids, and/or DNAs, leading to the critical damage of cancer cells [4]. It had been suggested that hydrogen peroxide is involved in the cisplatin-induced necrosis, whereas hydroxyl radical is responsible for the cisplatin-induced apoptosis [5]. Accordingly, the protective effects of hydroxyl radical scavengers are associated with an inhibition of cytochrome c release and caspase activation [5]. In general, cancer cells have higher levels of ROS than normal cells as a result of hyper-metabolism [6]. In addition to their extreme cytotoxicity, cisplatin could also have a variety of non-specific adverse reactions in cancer patients [7]. Studies have suggested that the accumulation of intracellular ROS is a hallmark of cisplatin-induced acute kidney injury (AKI) [8]. Cisplatin may show high activity in the fast proliferating cells, thereby causing cellular damage. In particular, about 30% of cisplatin-administered patients suffer from renal dysfunction and/or injury [9]. Increased formation of ROS in renal proximal convoluted tubule cells may be associated with the cisplatin-induced AKI [10], which is a substantial complication of cisplatin chemotherapy related to the ROS-dependent death of renal cells [11]. The AKI may be associated with high morbidity and mortality. To date, there are few strategies for preventing cisplatin-induced AKI [12], and it is urgent to search for novel therapeutic procedures to protect the kidney against the nephrotoxicity of cisplatin. In recent years, remarkable advances have been made in effective protective regimens for the nephrotoxicity of cisplatin [13]. Basically, protection of kidney from cisplatin-induced AKI might be attainable with antioxidant-based therapeutic interventions that increase antioxidant levels and thus improve the damage from ROS.
2. ROS and Autophagy in Cisplatin-Induced Acute Kidney Injury
The excessive generation of ROS has been regarded as the critical role during the pathogenetic process induced by cisplatin, by which DNA damage and/or cell death could occur. Increased ROS production is also known to change the mitochondrial electron transport chain, and eventually lead to apoptosis [14]. In particular, the dysfunction of the mitochondrial respiratory chain results in the further excess production of ROS that contributes to severe kidney injury [15]. Consequently, the mitochondrial dysfunction induced by the treatment with cisplatin could be considered as due to increased levels of ROS resulting in various cellular apoptosis including kidney cells [2][16]. Increased levels of ROS can also contribute to the tubular cell apoptosis in kidney, thereby causing more severe kidney injury during AKI [17]. Further generation of ROS in the tubular cells mitochondria might thus possibly contribute to the exacerbation of cisplatin-induced AKI. Therefore, the elimination of ROS has long been considered as an effective procedure to prevent the cisplatin-induced AKI [18]. In addition to increased ROS levels, treatment with cisplatin also impairs the activity of antioxidants such as superoxide dismutases (SODs), catalase, and glutathione peroxidase, which could function to reduce ROS levels [19]. The SODs are ROS-eliminating super enzymes with several subcellular localizations [20]. Targeting the cisplatin-induced oxidative stress via manipulation of the cellular antioxidant system including the expression of SODs could be beneficial for protecting against cisplatin nephrotoxicity. In fact, some agents with antioxidant and/or anti-inflammation activities could alleviate the cisplatin-induced cell damage by reduced production of ROS [21]. These therapeutic approaches may enhance the tolerance to cisplatin and hence might enable greater dose intensity associated with better outcomes. It has been shown that sirt1 expression on proximal tubules in the kidney may save cisplatin-induced AKI by preserving the function of peroxisomes and the elimination of ROS, which could be a potential therapeutic target for the treatment of cisplatin-induced AKI [22] (Figure 1).
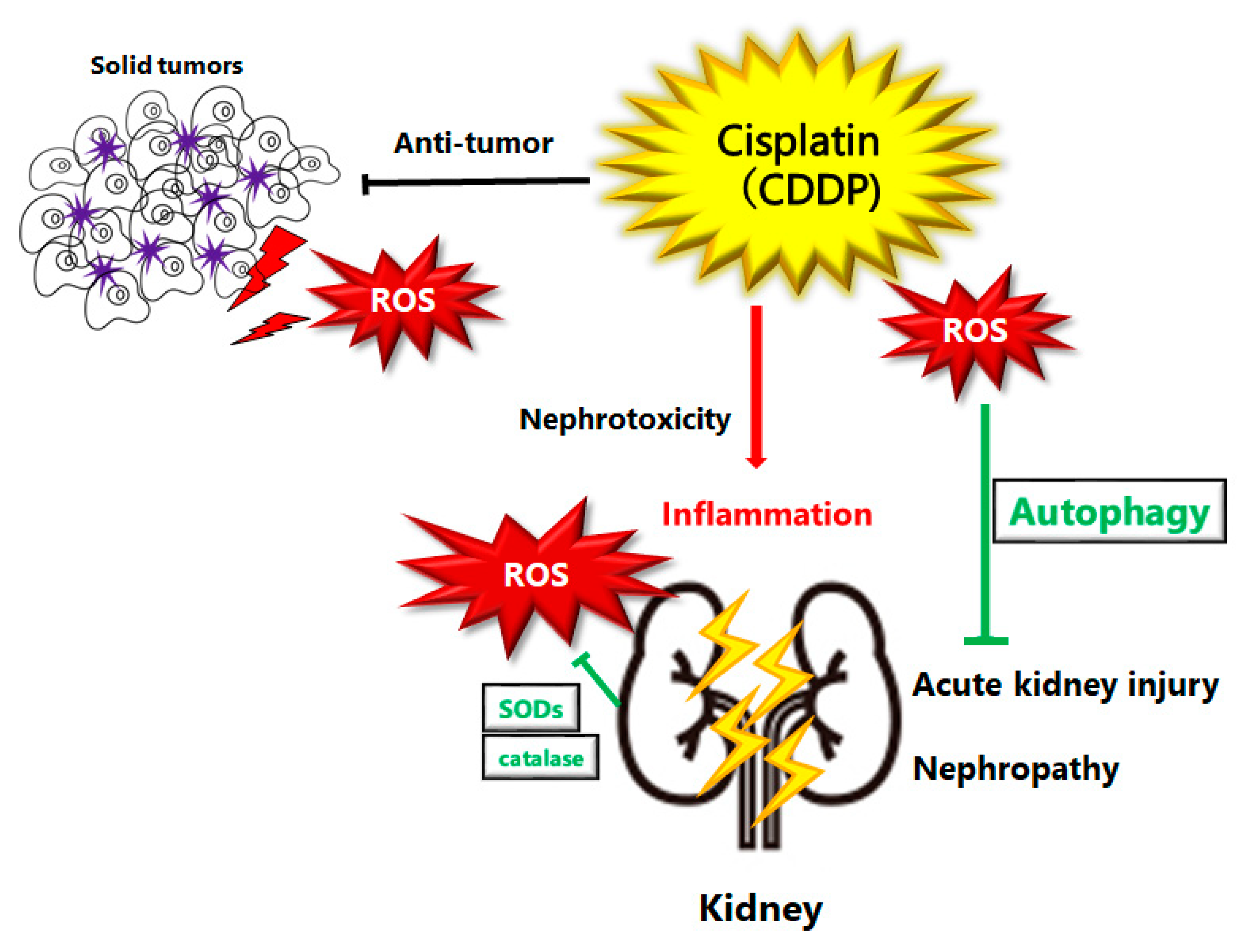
Figure 1. Schematic illustration of pathogenesis of cisplatin induced acute kidney injury or nephropathy. Reactive oxygen species (ROS), inflammation, and autophagy are all involved in the pathogenesis of cisplatin induced acute kidney injury. ROS may damage DNA or organelles within a cell. The damage could be treated with autophagy to enhance the survival of kidney cells. If the damage is too severe to be repaired, cells might undergo cell-death leading to kidney injury or nephropathy. Note that several significant features have been omitted for clarity.
ROS-induced autophagy may also lead to a different outcome of cell fate that may result in cell survival or cell death, depending on the severity of ROS exposure [23]. ROS impact on autophagy is mediated by specific signaling pathways, which might reduce the oxidative damage by degrading and/or recycling intracellular oxidized macromolecules and dysfunctional organelles [24].
3. Autophagy as a Target for the Treatment of Cisplatin-Induced Acute Kidney Injury
Autophagy, a highly conserved multistep catabolic pathway in eukaryotic cells, degrades and/or recycles macromolecules and/or dysfunctional organelles, which also contributes to the maintenance of the homeostasis of the kidney. For example, an inducer of autophagy could protect cells in an autophagy dependent manner [25]. In addition, the activity of autophagy regulator beclin 1 brings kidney protection via the reduction of kidney damage, subsequently refining kidney recovery post-AKI [26]. The beneficial effect of autophagy has a potential clinical significance in minimizing or preventing cisplatin nephrotoxicity [27]. In fact, autophagy occurs in AKI, and this might be an imperative mechanism for protection of cell survival [28]. Autophagy can protect kidney proximal tubules against AKI, possibly by alleviating DNA damage and/or ROS production [29][30]. In general, adenosine-monophosphate activated-protein kinase (AMPK) and mammalian target of rapamycin (mTOR) are major positive and negative regulators of autophagy, respectively. Therefore, inhibition of AMPK could lead to autophagy in cisplatin-induced AKI, resulting in more cellular or tubular kidney damage [31]. It has been indicated that penicilliumin-B denotes a different AMPK activator that might provide considerable protection against the apoptosis of renal tubular cells through the activated AMPK-induced autophagy and/or mitochondrial regeneration [32]. Likewise, metformin might protect against the cisplatin-induced apoptosis of tubular cells and/or AKI through stimulating AMPK-activation and/or inducing autophagy [33]. In addition, it has been shown that the pre-activation of autophagy could improve the survival and differentiation of kidney cells by inhibiting the mTOR signaling pathway, which in turn could mitigate the cisplatin-induced AKI [34].
In this regard, various compounds have an impact on the autophagy within kidney diseases. For example, chlorogenic acids may decrease cisplatin-induced AKI through alterations of inflammation, oxidative stress, apoptosis and/or autophagy, with the improvement in kidney restoration [35]. Also, ginsenoside effectively protects against cisplatin-induced AKI by activating the autophagy-mediated pathway [36]. The ginsenoside mediated improvement has been found due to the regulation of AMPK and/or mTOR-mediated autophagy and the inhibition of apoptosis [37]. Autophagy-mediated inhibition of apoptosis might also play a crucial role in astragaloside-mediated protection against cisplatin-induced renotoxicity [38]. In addition, berberine could play a protecting role in cisplatin-induced AKI by up-regulating mitophagy that is a kind of autophagy [39]. Similarly, Pink1 or Parkin dependent mitophagy has also identified potential targets for the treatment of cisplatin-induced AKI [40]. Honokiol treatment may cause noticeable kidney protection and attenuation of the cisplatin-induced kidney changes via preventing mitochondrial dysfunction [41]. Morin hydrate, a natural flavonoid, could also improve autophagy and/or inflammatory responses and decrease the cellular death in kidney, suggesting morin hydrate as a potential therapeutic agent against cisplatin-induced nephrotoxicity [42]. Trehalose treatment similarly conserves mitochondrial function via the activation of autophagy, and then attenuates cisplatin-induced AKI [43]. Cordyceps cicadae, a traditional Chinese medicine, may have a potential kidney protective effectfor prevention of cisplatin-induced AKI through the inhibition of various oxidative stresses by activating AMPK [44]. It is reported that treatment with 3-dehydroxyceanothetric acid 2-methyl ester isolated from the root of Ziziphus jujuba has decreased the autophagic vesicles via the altered protein expressions of AMPK and/or mTOR dependent pathway against cisplatin-induced AKI [45]. Retinoic acids could also improve cisplatin-induced AKI through the activation of autophagy, and the retinoic acids might have some protective effects for cisplatin-based chemotherapy [46]. AMPK activation is probably essential for the protection of kidney via the lithium-induced tubular cell autophagy in cases of cisplatin-induced AKI [47]. In addition, IFN-γ could accelerate autophagic change in kidney and increase the viability of kidney tubular cells, thereby attenuating cisplatin-induced AKI [48]. Deficiency of neutral ceramidase, an enzyme responsible for converting ceramide into sphingosine, could protect against cisplatin-induced AKI by the mechanism of increased autophagy [49]. Amniotic fluid stem cells may lead to amelioration of cisplatin-induced AKI, which is mediated by inhibition of apoptosis and/or activation of autophagy [50]. However, persistent autophagy after AKI induces pro-fibrotic cytokines in renal tubular cells, promoting renal fibrosis and chronic kidney disease (CKD) [51]. As described above, autophagy is deeply involved in the cisplatin-induced AKI, and autophagy could be a target for kidney protection.
References
- Martinho, N.; Santos, T.C.B.; Florindo, H.F.; Silva, L.C. Cisplatin-Membrane Interactions and Their Influence on Platinum Complexes Activity and Toxicity. Front. Physiol. 2019, 9, 1898.
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518.
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50.
- Fasnacht, M.; Polacek, N. Oxidative Stress in Bacteria and the Central Dogma of Molecular Biology. Front. Mol. Biosci. 2021, 8, 671037.
- Baek, S.M.; Kwon, C.H.; Kim, J.H.; Woo, J.S.; Jung, J.S.; Kim, Y.K. Differential roles of hydrogen peroxide and hydroxyl radical in cisplatin-induced cell death in renal proximal tubular epithelial cells. J. Lab. Clin. Med. 2003, 142, 178–186.
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid Med Cell Longev. 2019, 2019, 5381692.
- Jordan, P.; Carmo-Fonseca, M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol. Life Sci. 2000, 57, 1229–1235.
- Guan, J.; Tong, X.; Zhang, Y.; Xu, F.; Zhang, Y.; Liang, X.; Jin, J.; Jing, H.; Guo, L.; Ni, X.; et al. Nephrotoxicity induced by cisplatin is primarily due to the activation of the 5-hydroxytryptamine degradation system in proximal renal tubules. Chem. Biol. Interact. 2021, 349, 109662.
- Tan, Z.; Guo, F.; Huang, Z.; Xia, Z.; Liu, J.; Tao, S.; Li, L.; Feng, Y.; Du, X.; Ma, L.; et al. P harmacological and genetic inhibition of fatty acid-binding protein 4 alleviated cisplatin-induced acute kidney injury. Cell Mol. Med. 2019, 23, 6260–6270.
- Mapuskar, K.A.; Steinbach, E.J.; Zaher, A.; Riley, D.P.; Beardsley, R.A.; Keene, J.L.; Holmlund, J.T.; Anderson, C.M.; Zepeda-Orozco, D.; Buatti, J.M.; et al. Mitochondrial Superoxide Dismutase in Cisplatin-Induced Kidney Injury. Antioxidants (Basel) 2021, 10, 1329.
- Soni, H.; Kaminski, D.; Gangaraju, R.; Adebiyi, A. Cisplatin-induced oxidative stress stimulates renal Fas ligand shedding. Ren. Fail. 2018, 40, 314–322.
- Zhu, H.; Jiang, W.; Zhao, H.; He, C.; Tang, X.; Xu, S.; Xu, C.; Feng, R.; Li, J.; Ma, T.; et al. PSTPIP2 inhibits cisplatin-induced acute kidney injury by suppressing apoptosis of renal tubular epithelial cells. Cell Death Dis. 2020, 11, 1057.
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3011.
- Choi, Y.M.; Kim, H.K.; Shim, W.; Anwar, M.A.; Kwon, J.W.; Kwon, H.K.; Kim, H.J.; Jeong, H.; Kim, H.M.; Hwang, D.; et al. Mechanism of Cisplatin-Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PLoS ONE 2015, 10, e0135083.
- Yang, Y.; Fu, Y.; Wang, P.; Liu, S.; Sha, Y.; Zhang, Y.; Zhang, A.; Jia, Z.; Ding, G.; Huang, S. Intervention of mitochondrial activity attenuates cisplatin-induced acute kidney injury. Int. Urol. Nephrol. 2019, 51, 1207–1218.
- Zhang, J.; Zhao, T.; Wang, C.; Meng, Q.; Huo, X.; Wang, C.; Sun, P.; Sun, H.; Ma, X.; Wu, J.; et al. Catalpol-Induced AMPK Activation Alleviates Cisplatin-Induced Nephrotoxicity through the Mitochondrial-Dependent Pathway without Compromising Its Anticancer Properties. Oxid. Med. Cell Longev. 2021, 2021, 7467156.
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res. 2014, 122, 1–67.
- Li, R.; Hu, L.; Hu, C.; Wang, Q.; Lei, Y.; Zhao, B. Myricitrin protects against cisplatin-induced kidney injury by eliminating excessive reactive oxygen species. Int. Urol. Nephrol. 2020, 52, 187–196.
- Husain, K.; Morris, C.; Whitworth, C.; Trammell, G.L.; Rybak, L.P.; Somani, S.M. Protection by ebselen against cisplatin-induced nephrotoxicity: Antioxidant system. Mo.l Cell Biochem. 1998, 178, 127–133.
- Pechenino, A.S.; Brown, T.R. Superoxide dismutase in the prostate lobes of aging Brown Norway rats. Prostate 2006, 66, 522–535.
- Yan, W.; Xu, Y.; Yuan, Y.; Tian, L.; Wang, Q.; Xie, Y.; Shao, X.; Zhang, M.; Ni, Z.; Mou, S. Renoprotective mechanisms of Astragaloside IV in cisplatin-induced acute kidney injury. Free Radic. Res. 2017, 51, 669–683.
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Sueyasu, K.; Washida, N.; Tokuyama, H.; Tzukerman, M.; et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 2010, 285, 13045–13056.
- Thévenod, F.; Lee, W.K. Cadmium and cellular signaling cascades: Interactions between cell death and survival pathways. Arch. Toxicol. 2013, 87, 1743–1786.
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A. Molecular Interactions Between Reactive Oxygen Species and Autophagy in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3791.
- Park, C.H.; Lee, B.; Han, M.; Rhee, W.J.; Kwak, M.S.; Yoo, T.H.; Shin, J.S. Canagliflozin protects against cisplatin-induced acute kidney injury by AMPK-mediated autophagy in renal proximal tubular cells. Cell Death Discov. 2022, 8, 12.
- Shi, M.; Maique, J.; Shepard, S.; Li, P.; Seli, O.; Moe, O.W.; Hu, M.C. In vivo evidence for therapeutic applications of beclin 1 to promote recovery and inhibit fibrosis after acute kidney injury. Kidney Int. 2022, 101, 63–78.
- Kaushal, G.P.; Kaushal, V.; Herzog, C.; Yang, C. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy 2008, 4, 710–712.
- Periyasamy-Thandavan, S.; Jiang, M.; Wei, Q.; Smith, R.; Yin, X.M.; Dong, Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008, 74, 631–640.
- Takahashi, A.; Kimura, T.; Takabatake, Y.; Namba, T.; Kaimori, J.; Kitamura, H.; Matsui, I.; Niimura, F.; Matsusaka, T.; Fujita, N.; et al. Autophagy guards against cisplatin-induced acute kidney injury. Am. J. Pathol. 2012, 180, 517–525.
- Kaushal, G.P.; Shah, S.V. Autophagy in acute kidney injury. Kidney Int. 2016, 89, 779–791.
- Wei, L.; Chen, W.; Zou, Y.; Huang, H.; Pan, B.; Jin, S.; Huang, R.; Nie, S.; Kong, G. AMP-activated protein kinase regulates autophagic protection against cisplatin-induced tissue injury in the kidney. Genet. Mol. Res. 2015, 14, 12006–12015.
- Shen, W.; Jia, N.; Miao, J.; Chen, S.; Zhou, S.; Meng, P.; Zhou, X.; Tang, L.; Zhou, L. Penicilliumin B Protects against Cisplatin-Induced Renal Tubular Cell Apoptosis through Activation of AMPK-Induced Autophagy and Mitochondrial Biogenesis. Kidney Dis. 2021, 7, 278–292.
- Li, J.; Gui, Y.; Ren, J.; Liu, X.; Feng, Y.; Zeng, Z.; He, W.; Yang, J.; Dai, C. Metformin Protects Against Cisplatin-Induced Tubular Cell Apoptosis and Acute Kidney Injury via AMPKα-regulated Autophagy Induction. Sci. Rep. 2016, 6, 23975.
- Awadalla, A.; Hussein, A.M.; El-Far, Y.M.; El-Senduny, F.F.; Barakat, N.; Hamam, E.T.; Abdeen, H.M.; El-Sherbiny, M.; Serria, M.S.; Sarhan, A.A.; et al. Rapamycin Improves Adipose-Derived Mesenchymal Stem Cells (ADMSCs) Renoprotective Effect against Cisplatin-Induced Acute Nephrotoxicity in Rats by Inhibiting the mTOR/AKT Signaling Pathway. Biomedicines 2022, 10, 1295.
- Domitrović, R.; Cvijanović, O.; Šušnić, V.; Katalinić, N. Renoprotective mechanisms of chlorogenic acid in cisplatin-induced kidney injury. Toxicology 2014, 324, 98–107.
- Zhai, J.; Gao, H.; Wang, S.; Zhang, S.; Qu, X.; Zhang, Y.; Tao, L.; Sun, J.; Song, Y.; Fu, L. Ginsenoside Rg3 attenuates cisplatin-induced kidney injury through inhibition of apoptosis and autophagy-inhibited NLRP3. J. Biochem. Mol. Toxicol. 2021, 35, e22896.
- Xing, J.J.; Hou, J.G.; Ma, Z.N.; Wang, Z.; Ren, S.; Wang, Y.P.; Liu, W.C.; Chen, C.; Li, W. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 2019, 52, e12627.
- Qu, X.; Gao, H.; Tao, L.; Zhang, Y.; Zhai, J.; Sun, J.; Song, Y.; Zhang, S. Astragaloside IV protects against cisplatin-induced liver and kidney injury via autophagy-mediated inhibition of NLRP3 in rats. J. Toxicol. Sci. 2019, 44, 167–175.
- Qi, J.; Xue, Q.; Kuang, L.; Xie, L.; Luo, R.; Nie, X. Berberine alleviates cisplatin-induced acute kidney injury by regulating mitophagy via PINK 1/Parkin pathway. Transl. Androl. Urol. 2020, 9, 1712–1724.
- Zhao, C.; Chen, Z.; Xu, X.; An, X.; Duan, S.; Huang, Z.; Zhang, C.; Wu, L.; Zhang, B.; Zhang, A.; et al. Pink1/Parkin-mediated mitophagy play a protective role in cisplatin induced renal tubular epithelial cells injury. Exp. Cell Res. 2017, 350, 390–397.
- Mao, R.W.; He, S.P.; Lan, J.G.; Zhu, W.Z. Honokiol ameliorates cisplatin-induced acute kidney injury via inhibition of mitochondrial fission. Br. J. Pharmacol. 2022, 179, 3886–3904.
- Singh, M.P.; Chauhan, A.K.; Kang, S.C. Morin hydrate ameliorates cisplatin-induced ER stress, inflammation and autophagy in HEK-293 cells and mice kidney via PARP-1 regulation. Int. Immunopharmacol. 2018, 56, 156–167.
- Zhu, L.; Yuan, Y.; Yuan, L.; Li, L.; Liu, F.; Liu, J.; Chen, Y.; Lu, Y.; Cheng, J. Activation of TFEB-mediated autophagy by trehalose attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Theranostics 2020, 10, 5829–5844.
- Deng, J.S.; Jiang, W.P.; Chen, C.C.; Lee, L.Y.; Li, P.Y.; Huang, W.C.; Liao, J.C.; Chen, H.Y.; Huang, S.S.; Huang, G.J. Cordyceps cicadae Mycelia Ameliorate Cisplatin-Induced Acute Kidney Injury by Suppressing the TLR4/NF-κB/MAPK and Activating the HO-1/Nrf2 and Sirt-1/AMPK Pathways in Mice. Oxid. Med. Cell Longev. 2020, 2020, 7912763.
- Lee, D.; Kang, K.B.; Kim, H.W.; Park, J.S.; Hwang, G.S.; Kang, K.S.; Choi, S.; Yamabe, N.; Kim, K.H. Unique Triterpenoid of Jujube Root Protects Cisplatin-induced Damage in Kidney Epithelial LLC-PK1 Cells via Autophagy Regulation. Nutrients 2020, 12, 677.
- Wu, J.; Zheng, C.; Wan, X.; Shi, M.; McMillan, K.; Maique, J.; Cao, C. Retinoic Acid Alleviates Cisplatin-Induced Acute Kidney Injury Through Activation of Autophagy. Front. Pharmacol. 2020, 11, 987.
- Bao, H.; Zhang, Q.; Liu, X.; Song, Y.; Li, X.; Wang, Z.; Li, C.; Peng, A.; Gong, R. Lithium targeting of AMPK protects against cisplatin-induced acute kidney injury by enhancing autophagy in renal proximal tubular epithelial cells. FASEB J. 2019, 33, 14370–14381.
- Kimura, A.; Ishida, Y.; Inagaki, M.; Nakamura, Y.; Sanke, T.; Mukaida, N.; Kondo, T. Interferon-γ is protective in cisplatin-induced renal injury by enhancing autophagic flux. Kidney Int. 2012, 82, 1093–1104.
- Sears, S.M.; Dupre, T.V.; Shah, P.P.; Davis, D.L.; Doll, M.A.; Sharp, C.N.; Vega, A.A.; Megyesi, J.; Beverly, L.J.; Snider, A.J.; et al. Neutral ceramidase deficiency protects against cisplatin-induced acute kidney injury. J. Lipid. Res. 2022, 63, 100179.
- Minocha, E.; Sinha, R.A.; Jain, M.; Chaturvedi, C.P.; Nityanand, S. Amniotic fluid stem cells ameliorate cisplatin-induced acute renal failure through induction of autophagy and inhibition of apoptosis. Stem Cell Res. Ther. 2019, 10, 370.
- Fu, Y.; Xiang, Y.; Wu, W.; Cai, J.; Tang, C.; Dong, Z. Persistent Activation of Autophagy After Cisplatin Nephrotoxicity Promotes Renal Fibrosis and Chronic Kidney Disease. Front. Pharmacol. 2022, 13, 918732.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
612
Revisions:
2 times
(View History)
Update Date:
08 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




