
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ankur Vaidya | -- | 1529 | 2023-09-07 12:57:05 | | | |
| 2 | Wendy Huang | Meta information modification | 1529 | 2023-09-08 13:24:57 | | |
Video Upload Options
Lipid nanoparticles (LNPs) are spherical vesicles composed of ionizable lipids that are neutral at physiological pH. Despite their benefits, unmodified LNP drug delivery systems have substantial drawbacks, including a lack of targeted selectivity, a short blood circulation period, and in vivo instability. lipid–polymer hybrid nanoparticles (LPHNPs) are the next generation of nanoparticles, having the combined benefits of polymeric nanoparticles and liposomes. LPHNPs are being prepared from both natural and synthetic polymers with various techniques, including one- or two-step methods, emulsification solvent evaporation (ESE) method, and the nanoprecipitation method. Varieties of LPHNPs, including monolithic hybrid nanoparticles, core–shell nanoparticles, hollow core–shell nanoparticles, biomimetic lipid–polymer hybrid nanoparticles, and polymer-caged liposomes, have been investigated for various drug delivery applications.
1. Introduction
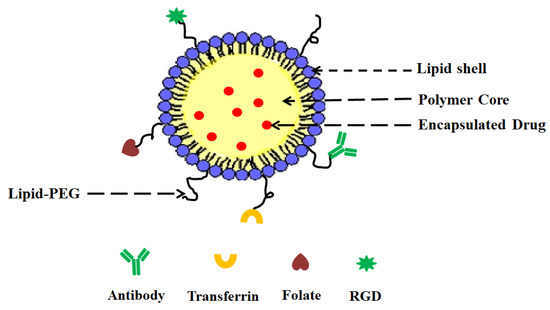
- (i)
-
A drug encapsulating polymer core;
- (ii)
-
A lipid layer surrounding the polymer core;
- (iii)
-
An outer lipid–PEG layer.
2. LPHNPs Composition and Its Influence
| Polymer Used | Lipid Used | Reference |
|---|---|---|
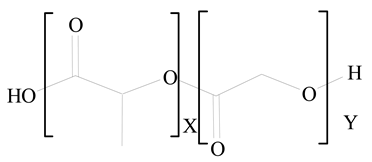 Polylactic-co-glycolic acid (PLGA) |
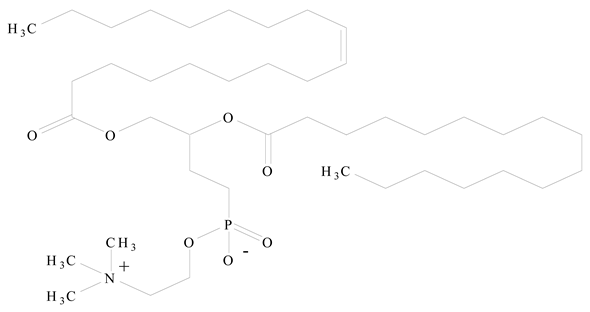 Phosphatidyl choline (lecithin) |
[16] |
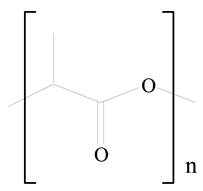 Polylactic acid (PLA) |
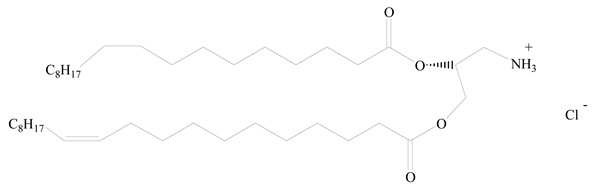 1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP) |
[17] |
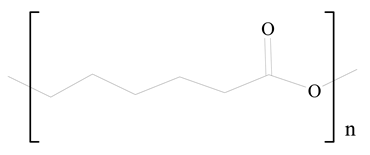 Polycaprolactone (PCL) |
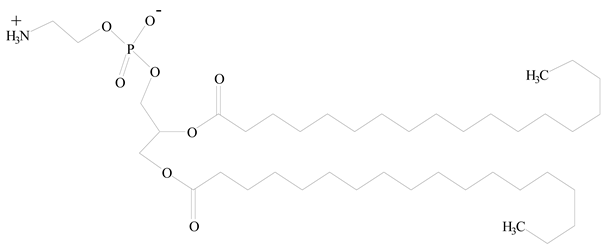 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE) |
[18] |
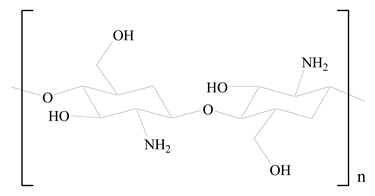 D-Glucosamine and N-acetyl-D-glucosamine (chitosan) |
 Glyceryl monooleate (GMO) |
[19] |
 Poly(vinyl alcohol) (PVA) |
 Stearic acid |
[20] |
 Hyaluronic acid (HA) |
 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE) |
[21] |
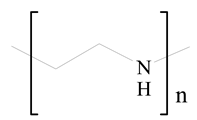 Polyethylenimine (PEI) |
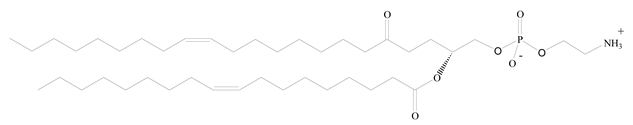 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) |
[22] |
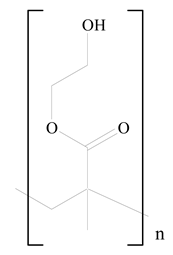 Poly(2-hydroxyethyl methacrylate) (PHEMA) |
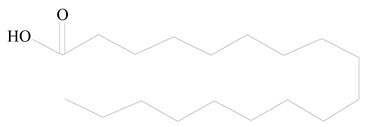 Stearic acid |
[23] |
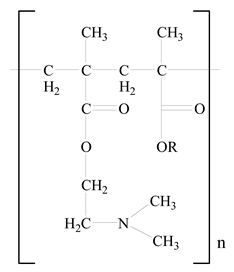 Eudragit |
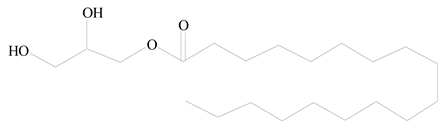 Glycerol monostearate |
[24] |
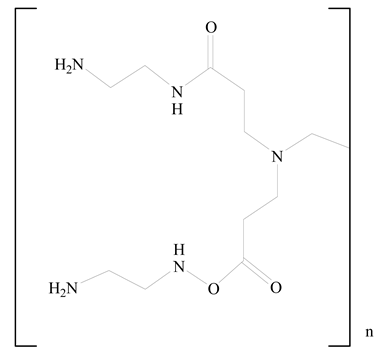 Polyamidoamine |
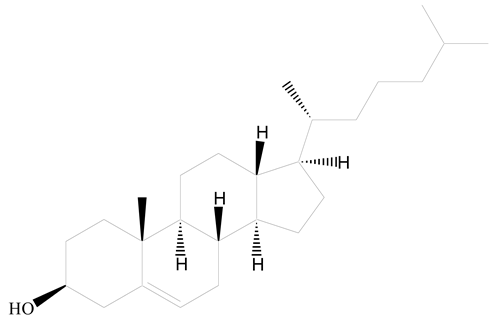 (3β)-Cholest-5-en-3-ol (cholesterol) |
[25] |
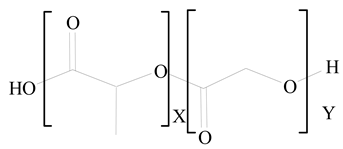 Polylactic-co-glycolic acid (PLGA) |
 1,2-Dimyristoyl-sn-glycero-3-phosphoethanolaminediethylene (DMPE) |
[26] |
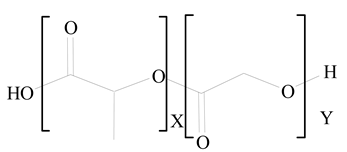 Polylactic-co-glycolic acid (PLGA) |
 Diethylenetriaminepentaacetate (DTPA) |
[26] |
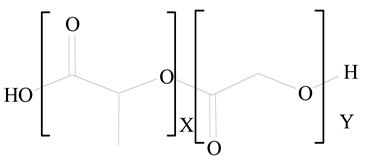 Polylactic-co-glycolic acid (PLGA) |
 1,2-Dilauroyl-sn-glycero-3-phosphocholine structure (DLPC) |
[27] |
References
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191.
- Nakmode, D.; Bhavana, V.; Thakor, P.; Madan, J.; Singh, P.K.; Singh, S.B.; Rosenholm, J.M.; Bansal, K.K.; Mehra, N.K. Fundamental Aspects of Lipid-Based Excipients in Lipid-Based Product Development. Pharmaceutics 2022, 14, 831.
- Marzi, M.; Rostami Chijan, M.; Zarenezhad, E. Hydrogels as Promising Therapeutic Strategy for the Treatment of Skin Cancer. J. Mol. Struct. 2022, 1262, 133014.
- Bansal, K.K.; Rosenholm, J.M. Synthetic Polymers from Renewable Feedstocks: An Alternative to Fossil-Based Materials in Biomedical Applications. Ther. Deliv. 2020, 11, 297–300.
- Bansal, K.K.; Kakde, D.; Purdie, L.; Irvine, D.J.; Howdle, S.M.; Mantovani, G.; Alexander, C. New Biomaterials from Renewable Resources—Amphiphilic Block Copolymers from δ-Decalactone. Polym. Chem. 2015, 6, 7196–7210.
- Bansal, K.; Sasso, L.; Makwana, H.; Awwad, S.; Brocchini, S.; Alexander, C. Nanopharmacy: Exploratory Methods for Polymeric Materials; John Wiley & Sons: Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2017; Volume 1, ISBN 978-3-527-34054-5.
- Jain, P.; Vaidya, A.; Jain, R.; Shrivastava, S.; Khan, T.; Jain, A. Ethyl Cellulose Coated Chitosan Microspheres of Metronidazole as Potential Anti-Amoebic Agent. J. Bionanosci. 2017, 11, 599–607.
- Vaidya, A.; Jain, S.; Agrawal, R.K.; Jain, S.K. Pectin–Metronidazole Prodrug Bearing Microspheres for Colon Targeting. J. Saudi Chem. Soc. 2015, 19, 257–264.
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109.
- Attama, A.A.; Momoh, M.A.; Builders, P.F.; Attama, A.A.; Momoh, M.A.; Builders, P.F. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage Form Design and Development. In Recent Advances in Novel Drug Carrier Systems; IntechOpen: London, UK, 2012; ISBN 978-953-51-0810-8.
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015.
- Teixeira, M.C.; Carbone, C.; Souto, E.B. Beyond Liposomes: Recent Advances on Lipid Based Nanostructures for Poorly Soluble/Poorly Permeable Drug Delivery. Prog. Lipid Res. 2017, 68, 1–11.
- Jampílek, J.; Kráľová, K. Chapter 8—Recent Advances in Lipid Nanocarriers Applicable in the Fight against Cancer**This Chapter Is Sincerely Dedicated to the Memory of Prof. Ervin Wolfram (1923–1985), the Long-Time Head of the Department of Colloid Chemistry and Colloid Technology, the Faculty of Science, Eötvös Loránd University, Budapest, Hungary. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 219–294. ISBN 978-0-12-816200-2.
- Mendozza, M.; Caselli, L.; Salvatore, A.; Montis, C.; Berti, D. Nanoparticles and Organized Lipid Assemblies: From Interaction to Design of Hybrid Soft Devices. Soft Matter 2019, 15, 8951–8970.
- Wang, Q.; Alshaker, H.; Böhler, T.; Srivats, S.; Chao, Y.; Cooper, C.; Pchejetski, D. Core Shell Lipid-Polymer Hybrid Nanoparticles with Combined Docetaxel and Molecular Targeted Therapy for the Treatment of Metastatic Prostate Cancer. Sci. Rep. 2017, 7, 5901.
- Mieszawska, A.J.; Gianella, A.; Cormode, D.P.; Zhao, Y.; Meijerink, A.; Langer, R.; Farokhzad, O.C.; Fayad, Z.A.; Mulder, W.J.M. Engineering of Lipid-Coated PLGA Nanoparticles with a Tunable Payload of Diagnostically Active Nanocrystals for Medical Imaging. Chem. Commun. 2012, 48, 5835–5837.
- Jain, A.K.; Bataille, C.J.R.; Milhas, S.; Miller, A.; Zhang, J.; Rabbitts, T.H. Immunopolymer Lipid Nanoparticles for Delivery of Macromolecules to Antigen-Expressing Cells. ACS Appl. Bio Mater. 2020, 3, 8481–8495.
- Shao, Y.; Luo, W.; Guo, Q.; Li, X.; Zhang, Q.; Li, J. In Vitro and in Vivo Effect of Hyaluronic Acid Modified, Doxorubicin and Gallic Acid Co-Delivered Lipid-Polymeric Hybrid Nano-System for Leukemia Therapy. Drug Des. Dev. Ther. 2019, 13, 2043–2055.
- Dong, W.; Wang, X.; Liu, C.; Zhang, X.; Zhang, X.; Chen, X.; Kou, Y.; Mao, S. Chitosan Based Polymer-Lipid Hybrid Nanoparticles for Oral Delivery of Enoxaparin. Int. J. Pharm. 2018, 547, 499–505.
- Sailor, G.U.; Ramani, V.D.; Shah, N.; Parmar, G.R.; Gohil, D.; Balaraman, R.; Seth, A. Design of Experiment Approach Based Formulation Optimization of Berberine Loaded Solid Lipid Nanoparticle for Antihyperlipidemic Activity. Indian J. Pharm. Sci. 2021, 83, 204–218.
- Lee, J.-Y.; Yang, H.; Yoon, I.-S.; Kim, S.-B.; Ko, S.-H.; Shim, J.-S.; Sung, S.H.; Cho, H.-J.; Kim, D.-D. Nanocomplexes Based on Amphiphilic Hyaluronic Acid Derivative and Polyethylene Glycol–Lipid for Ginsenoside Rg3 Delivery. J. Pharm. Sci. 2014, 103, 3254–3262.
- Wei, W.; Sun, J.; Guo, X.-Y.; Chen, X.; Wang, R.; Qiu, C.; Zhang, H.-T.; Pang, W.-H.; Wang, J.-C.; Zhang, Q. Microfluidic-Based Holonomic Constraints of SiRNA in the Kernel of Lipid/Polymer Hybrid Nanoassemblies for Improving Stable and Safe In Vivo Delivery. ACS Appl. Mater. Interfaces 2020, 12, 14839–14854.
- Kumar, S.S.D.; Mahesh, A.; Mahadevan, S.; Mandal, A.B. Synthesis and Characterization of Curcumin Loaded Polymer/Lipid Based Nanoparticles and Evaluation of Their Antitumor Effects on MCF-7 Cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 1913–1922.
- Kumar, V.; Chaudhary, H.; Kamboj, A. Development and Evaluation of Isradipine via Rutin-Loaded Coated Solid-Lipid Nanoparticles. Interv. Med. Appl. Sci. 2018, 10, 236–246.
- Gao, L.-Y.; Liu, X.-Y.; Chen, C.-J.; Wang, J.-C.; Feng, Q.; Yu, M.-Z.; Ma, X.-F.; Pei, X.-W.; Niu, Y.-J.; Qiu, C.; et al. Core-Shell Type Lipid/RPAA-Chol Polymer Hybrid Nanoparticles for in Vivo SiRNA Delivery. Biomaterials 2014, 35, 2066–2078.
- Wang, A.Z.; Yuet, K.; Zhang, L.; Gu, F.X.; Huynh-Le, M.; Radovic-Moreno, A.F.; Kantoff, P.W.; Bander, N.H.; Langer, R.; Farokhzad, O.C. ChemoRad Nanoparticles: A Novel Multifunctional Nanoparticle Platform for Targeted Delivery of Concurrent Chemoradiation. Nanomedicine 2010, 5, 361–368.
- Liu, Y.; Li, K.; Pan, J.; Liu, B.; Feng, S.-S. Folic Acid Conjugated Nanoparticles of Mixed Lipid Monolayer Shell and Biodegradable Polymer Core for Targeted Delivery of Docetaxel. Biomaterials 2010, 31, 330–338.
- Salvador-Morales, C.; Zhang, L.; Langer, R.; Farokhzad, O.C. Immunocompatibility Properties of Lipid–Polymer Hybrid Nanoparticles with Heterogeneous Surface Functional Groups. Biomaterials 2009, 30, 2231–2240.
- Dave, V.; Yadav, R.B.; Kushwaha, K.; Yadav, S.; Sharma, S.; Agrawal, U. Lipid-Polymer Hybrid Nanoparticles: Development & Statistical Optimization of Norfloxacin for Topical Drug Delivery System. Bioact. Mater. 2017, 2, 269–280.
- Valencia, P.M.; Basto, P.A.; Zhang, L.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Single-Step Assembly of Homogenous Lipid-Polymeric and Lipid-Quantum Dot Nanoparticles Enabled by Microfluidic Rapid Mixing. ACS Nano 2010, 4, 1671–1679.
- Troutier, A.-L.; Delair, T.; Pichot, C.; Ladavière, C. Physicochemical and Interfacial Investigation of Lipid/Polymer Particle Assemblies. Langmuir 2005, 21, 1305–1313.
- Thevenot, J.; Troutier, A.-L.; David, L.; Delair, T.; Ladavière, C. Steric Stabilization of Lipid/Polymer Particle Assemblies by Poly(Ethylene Glycol)-Lipids. Biomacromolecules 2007, 8, 3651–3660.
- Yeh, M.-K.; Chang, W.-K.; Tai, Y.-J.; Chiang, C.-H.; Hu, C.; Hong, P.-D. The Comparison of Protein-Entrapped Liposomes and Lipoparticles: Preparation, Characterization, and Efficacy of Cellular Uptake. Int. J. Nanomed. 2011, 6, 2403–2417.
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core–Shell-Type Lipid–Polymer Hybrid Nanoparticles as a Drug Delivery Platform. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 474–491.
- Wakaskar, R.R. General Overview of Lipid-Polymer Hybrid Nanoparticles, Dendrimers, Micelles, Liposomes, Spongosomes and Cubosomes. J. Drug Target. 2018, 26, 311–318.
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid Polymer Hybrid Nanocarriers: Insights into Synthesis Aspects, Characterization, Release Mechanisms, Surface Functionalization and Potential Implications. Colloid Interface Sci. Commun. 2022, 46, 100570.




