Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eldar Kopishev | -- | 1271 | 2023-09-07 11:30:29 | | | |
| 2 | Peter Tang | Meta information modification | 1271 | 2023-09-08 04:25:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Keldibekova, R.; Suleimenova, S.; Nurgozhina, G.; Kopishev, E. Interpolymer Complexes Based on Cellulose Ethers. Encyclopedia. Available online: https://encyclopedia.pub/entry/48913 (accessed on 07 February 2026).
Keldibekova R, Suleimenova S, Nurgozhina G, Kopishev E. Interpolymer Complexes Based on Cellulose Ethers. Encyclopedia. Available at: https://encyclopedia.pub/entry/48913. Accessed February 07, 2026.
Keldibekova, Raushan, Symbat Suleimenova, Gulden Nurgozhina, Eldar Kopishev. "Interpolymer Complexes Based on Cellulose Ethers" Encyclopedia, https://encyclopedia.pub/entry/48913 (accessed February 07, 2026).
Keldibekova, R., Suleimenova, S., Nurgozhina, G., & Kopishev, E. (2023, September 07). Interpolymer Complexes Based on Cellulose Ethers. In Encyclopedia. https://encyclopedia.pub/entry/48913
Keldibekova, Raushan, et al. "Interpolymer Complexes Based on Cellulose Ethers." Encyclopedia. Web. 07 September, 2023.
Copy Citation
Interpolymer complexes based on cellulose ethers are formed by combining different polymers through non-covalent interactions, resulting in stable structures.
interpolymer complexes
cellulose ethers
mucoadhesion
hydrogels
drug delivery
1. Introduction
The initial reference to interpolymer complexes (IPCs) (also referred to as polymer-polymer complexes in some sources) can be traced back to the research documented in [1]. Subsequently, Bungenberg de Jong extended these investigations, as indicated in references [2][3][4][5]. The systematic studies in this field were first undertaken by Liquori, as evidenced by works [6][7]. During the period of 1970–1980, extensive research was conducted, which established the fundamental basis for the subsequent rapid progress in the study and application of interpolymer complexes. Notable contributions in this era were made by prominent scientists, such as Bekturov [8], Zezin [9], Papisov [10], Izumrudov, Kabanov [11], and Tsuchida [12], who played pivotal roles in leading these scientific endeavours.
IPCs exhibit a complex structural arrangement, which arises from the interaction between macromolecules of polymers possessing complementary chemical structures when in a solution. The characteristics of macromolecules are determined not only by the chemical structure of the polymer chain but also by the formation of macromolecular aggregates. Thus, the macromolecules exhibit higher-order structures, including the configuration and conformation of the polymer chains, in addition to their primary structures. The effects of aggregation are typically observed as phase separation phenomena, such as precipitation, gelation, coacervation, emulsification, as well as the crystallization and liquid crystallization of polymers, or the self-assembly of biopolymer subunits. At present, IPCs are recognized as highly promising materials for their ability to act as structure-forming agents in various types of dispersions [13][14][15][16].
The investigation into the growth of interpolymer complex (IPC) films is of significant interest both from a scientific perspective and for practical applications. The cooperative interaction of complementary structures found in IPCs plays a crucial role in chemistry, polymer physics, and molecular biology. Despite the extensive research conducted on interpolymer complexes, there remain unanswered questions concerning their experimental and theoretical data, highlighting the need for further scientific advancements in this field.
A comprehensive analysis of the available data leads to the following conclusions: the primary focus of IPC research lies in understanding the material’s structural construction, the physicochemical parameters that influence polycomplex growth, and the diverse range of applications for IPCs. Essential factors influencing the structural growth of IPCs include solution pH, the temperature at which functional group binding occurs, solution concentration, the presence of low molecular weight compounds, and other relevant parameters.
Based on the forces of interaction involved, intermacromolecular complexes can be categorized into the following four distinct classes (Figure 1):
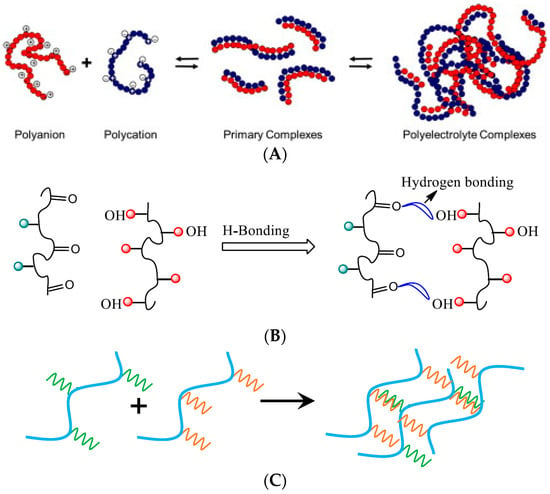
2. Methods of Obtaining
Several methods have been established for obtaining interpolymer complexes, including the following:
Among these approaches, one of the feasible and reliable methods for creating interpolymer complexes involves employing joint assembly processes within multicomponent polymer systems that contain complementary macromolecular components. Specifically, electrostatically controlled joint assembly is utilized, where water solutions of oppositely charged polyelectrolytes are mixed, leading to the formation of IPCs (Figure 2). These complexes represent macromolecular co-assemblies stabilized by an interconnected network of interpolymer salt bonds [28].
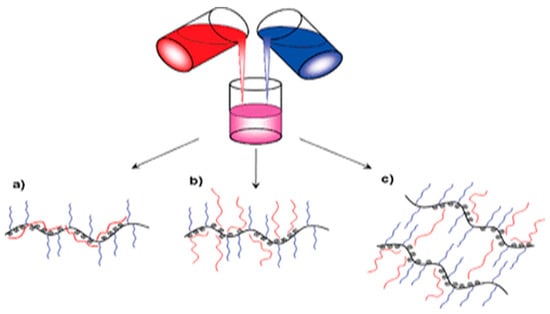
Figure 2. Mixing complementary macromolecules in solutions [32]. (a) Poly(sodium styrenesulfonate (PSS) is buried among the poly(ethylene oxide) brushes, (b) extended PSS chains from complex core, and (c) PSS chains bridging between complexes.
Matrix polymerization is an intriguing approach based on the conventional matrix synthesis model, which offers a means to synthesize composite materials that may be challenging or unattainable using alternative methods. Originally, the method was denoted as rreplica polymerization [46]; subsequently, it was assigned two additional designations, namely, “Template polymerization” and “Matrix polymerization”, which ultimately superseded the former term. The interpolymer complexes obtained through this method exhibit sufficient stability, and their polymer components can only be separated by disrupting the cooperative systems of intermolecular bonds [35]. Połowiński [34] differentiates between two distinct mechanisms of polymerization (Figure 3).
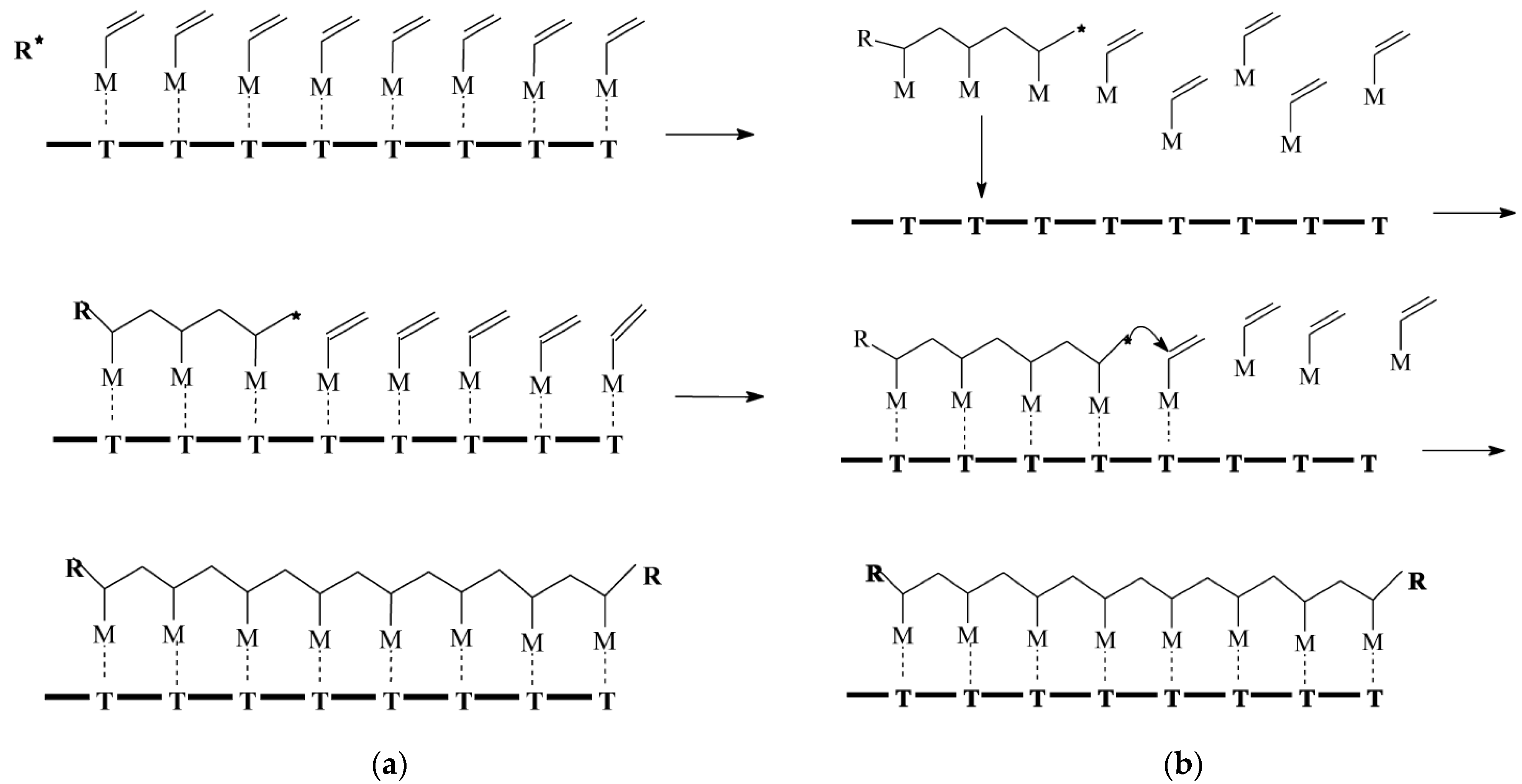
Figure 3. Example of matrix polymerization [34]. (a) The ‘zip’ mechanism, *—electrostatic, hydrogen bridges and (b) ‘pick-up’ mechanism, *—oligomer with critical length.
Another method for obtaining IPCs involves conducting a reaction at the interface of two immiscible liquid compounds. This technique relies on two solutions containing complementary polymers in separate solvents, resulting in the formation of a thin film of IPC at the phase boundary (Figure 4) [38].
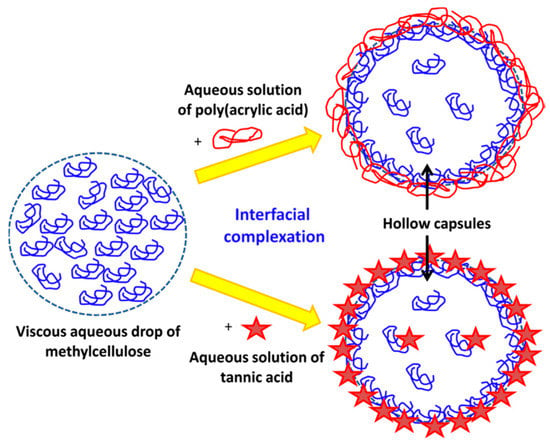
Figure 4. Example of interfacial complexation [41].
The application method on both solid and flexible surfaces entails the sequential deposition of polymer solutions capable of forming soluble and insoluble interpolymer complexes. This approach offers notable advantages, including precise control over coating thickness and the ability to develop materials with tailored physicochemical properties. Multilayer coatings can be applied to various surfaces, holding potential applications in the medical field and other scientific disciplines (Figure 5) [43][45].
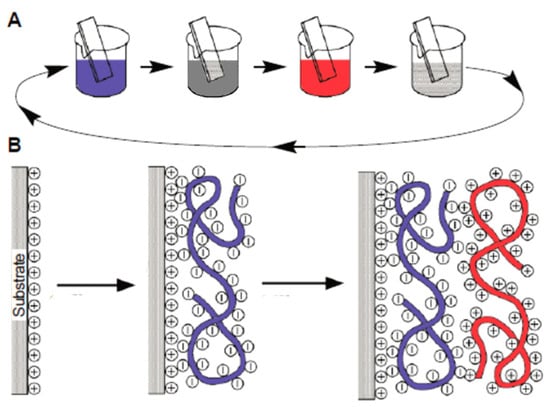
Figure 5. Application methods on solid and flexible surfaces [45]. (A) Schematic of the film deposition process using slides and beakers. (B) Simplified molecular picture of the first two adsorption steps, depicting film deposition starting with a positively charged substrate. The molecules and solutions of the polyanion and polycation are distinguished by the utilization of red and blue colors, respectively.
3. Methods of Studying IPCs
Throughout this research, a substantial body of scientific literature on interpolymer complexes and their preparation and analysis methods has been thoroughly examined. Notably, standardized approaches exist for investigating the partially formed complexes, including the following:
4. IPCs Based on Cellulose Ethers
Cellulose ethers are derivatives of cellulose with a general chemical formula [C6H7O2(OH)3−x(OR)x]n, where n represents the degree of polymerization. In this formula, x denotes the number of hydroxyl (OH) groups substituted in a single unit of the cellulose macromolecule, while R represents an alkyl, acyl, or mineral acid residue. Each unit of the macromolecule contains three hydroxyl groups, which have the capability to undergo reactions to form ethers and esters. In the case of mixed cellulose esters, different substitutive radicals are present.
The most commonly encountered cellulose ethers and esters include ethers, such as carboxymethylcellulose, methylcellulose, ethyl cellulose, as well as methylhydroxypropyl cellulose, oxypropyl cellulose, and cyanethyl cellulose. Esters include cellulose acetates, cellulose nitrates, as well as acetylphthalyl cellulose, acetopropionates, acetobutyrates, and cellulose sulfates (Figure 6).
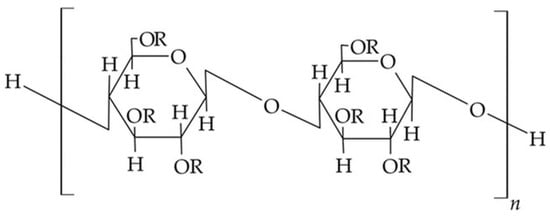
Figure 6. Chemical structure of cellulose esters/ether derivatives [55].
Cellulosic materials encompass a variety of cellulose derivatives, including cellulose esters, such as acetate, acetate trimellitate, acetate phthalate (CAP), hydroxypropyl methyl (HPM, also known as hypromellose) phthalate, and HPM acetate succinate. These esters are formed through the esterification of hydroxyl groups in cellulose using acetic, trimellitic, dicarboxylic phthalic, and succinic acids, or a combination of them.
Additionally, cellulosic materials also comprise cellulose ethers, such as methyl, ethyl, hydroxyethyl, hydroxyethyl methyl, hydroxypropyl (HP), HPM, and carboxymethyl ethers of cellulose. These ethers are formed by etherification of hydroxyl groups in cellulose using the appropriate alkyl halide (R-Cl) after the alkalization of cellulose, typically derived from wood pulp (Table 1).
Table 1. Cellulose and its derivatives (Figure 6).
|
Cellulose Esters |
R Groups |
Cellulose Ethers |
R Groups |
|---|---|---|---|
|
Acetate |
H, I |
Methylcellulose |
H, CH3 |
|
Acetate trimellitate |
H, I, II |
Ethyl cellulose |
H, CH2CH3 |
|
Acetate phthalate |
I, III |
Hydroxyethylmethyl cellulose |
H, CH3, [CH2CH2O]nH |
|
Hydroxypropyl methyl phthalate |
H, CH3, CH2CH(OH)CH3, III, IV |
Hydroxypropyl cellulose |
H, [CH2CH(CH3)O]nH |
|
Hydroxypropyl methyl phthalate acetate succinate |
H, CH3, CH2CH(OH)CH3, I, V |
Carboxymethylcellulose |
H, CH2COONa |
References
- Kossel, A. Ueber Die Peptone Und Ihr Verhältniss Zu Den Eiweisskörpern. Pflüger Arch. Für Die Gesammte Physiol. Des Menschen Und Der Thiere 1880, 21, 179–184.
- de Jong, H.G.B.; Winkler, K.C. Entmischung in Kristalloiden Neutralsalzlösungen, Analog Der Komplexkoazervation von Biokolloidsolen. Z. Für Anorg. Und Allg. Chem. 1937, 232, 119–132.
- Holleman, L.W.J.; de Jong, H.G.B.; Modderman, R.S.T. Zur Kenntnis Der Lyophilen Kolloide-XXI. Mitteilung. Über Koazervation I: Einfache Koazervation von Gelatinesolen. Kolloid-Beihefte 1934, 39, 334–420.
- Bungenberg de Jong, H.G.; Dekker, W.A.L. Zur Kenntnis Der Lyophilen Kolloide-XXVI. Mitteilung. Über Koazervation III: Komplexkoazervation Des Systems Gummiarabikum-Gelatine. Zweiter Teil. Kolloid-Beihefte 1936, 43, 213–271.
- de Jong, H.G.B.; Dekker, W.A.L. Zur Kenntnis Der Lyophilen Kolloide-XXV. Mitteilung. Über Koazervation II: Komplexkoazervation Des Systems Gummiarabikum-Gelatine. Kolloid-Beihefte 1935, 43, 143–212.
- Liquori, A.M.; Anzuino, G.; Coiro, V.M.; D’Alagni, M.; De Santis, P.; Savino, M. Complementary Stereospecific Interaction between Isotactic and Syndiotactic Polymer Molecules. Nature 1965, 206, 358–362.
- Barone, G.; Crescenzi, V.; Liquori, A.M.; Quadrifoglio, F. Solubilization of Polycyclic Aromatic Hydrocarbons in Poly(Methacrylic Acid) Aqueous Solutions. Phys. Chem. 1967, 71, 2341–2345.
- Bekturov, E.A.; Bimendina, L.A. Interpolymer Complexes. In Speciality Polymers; Springer: Berlin/Heidelberg, Germany, 1981; pp. 99–147. ISBN 978-3-540-38525-7.
- Kabanov, V.A.; Zezin, A.B. Soluble Interpolymeric Complexes as a New Class of Synthetic Polyelectrolytes. Pure Appl. Chem. 1984, 56, 343–354.
- Baranovsky, V.Y.; Litmanovich, A.A.; Papisov, I.M.; Kabanov, V.A. Quantitative Studies of Interaction between Complementary Polymers and Oligomers in Solutions. Eur. Polym. J. 1981, 17, 969–979.
- Kabanov, A.V.; Bronich, T.K.; Kabanov, V.A.; Yu, K.; Eisenberg, A. Soluble Stoichiometric Complexes from Poly(N-Ethyl-4-Vinylpyridinium) Cations and Poly(Ethylene Oxide)-Block-Polymethacrylate Anions. Macromolecules 1996, 29, 6797–6802.
- Tsuchida, E.; Abe, K. Interactions Between Macromolecules in Solution and Intermacromolecular Complexes; Tsuchida, E., Abe, K., Eds.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1982; Volume 45, ISBN 3-540-11624-9.
- Khutoryanskiy, V.V.; Smyslov, R.Y.; Yakimansky, A.V. Modern Methods for Studying Polymer Complexes in Aqueous and Organic Solutions. Polym. Sci. Ser. A 2018, 60, 553–576.
- Pavlyuchenko, V.N.; Ivanchev, S.S. Composite Polymer Hydrogels. Polym. Sci. Ser. A 2009, 51, 743–760.
- Bizley, S.C.; Williams, A.C.; Khutoryanskiy, V.V. Thermodynamic and Kinetic Properties of Interpolymer Complexes Assessed by Isothermal Titration Calorimetry and Surface Plasmon Resonance. Soft Matter 2014, 10, 8254–8260.
- Izumrudov, V.A. Self-Assembly and Molecular “Recognition” Phenomena in Solutions of (Bio)Polyelectrolyte Complexes. Russ. Chem. Rev. 2008, 77, 401–415.
- Du, J.; Dai, J.; Liu, J.-L.; Dankovich, T. Novel PH-Sensitive Polyelectrolyte Carboxymethyl Konjac Glucomannan-Chitosan Beads as Drug Carriers. React. Funct. Polym. 2006, 66, 1055–1061.
- Kabanov, V.A. Polyelectrolyte Complexes in Solution and in Bulk. Russ. Chem. Rev. 2005, 74, 3–20.
- Putro, J.N.; Lunardi, V.B.; Soetaredjo, F.E.; Yuliana, M.; Santoso, S.P.; Wenten, I.G.; Ismadji, S. A Review of Gum Hydrocolloid Polyelectrolyte Complexes (PEC) for Biomedical Applications: Their Properties and Drug Delivery Studies. Processes 2021, 9, 1796.
- Bourganis, V.; Karamanidou, T.; Kammona, O.; Kiparissides, C. Polyelectrolyte Complexes as Prospective Carriers for the Oral Delivery of Protein Therapeutics. Eur. J. Pharm. Biopharm. 2017, 111, 44–60.
- Khutoryanskiy, V.V. Hydrogen-Bonded Interpolymer Complexes as Materials for Pharmaceutical Applications. Int. J. Pharm. 2007, 334, 15–26.
- Yan, S.; Yao, L.; Kang, B.; Lee, J.Y. Solvent Effect on Hydrogen Bonded Tyr⋯Asp⋯Arg Triads: Enzymatic Catalyzed Model System. Comput. Biol. Chem. 2016, 65, 140–147.
- Gadwal, I. A Brief Overview on Preparation of Self-Healing Polymers and Coatings via Hydrogen Bonding Interactions. Macromol 2020, 1, 18–36.
- Anufrieva, E.V.; Krakovyak, M.G.; Nekrasova, T.N.; Smyslov, R.Y. Effect of Solvent on the Formation of Stereocomplexes in Poly(Methyl Methacrylate) Solutions. Polym. Sci. Ser. A 1996, 38, 190–193.
- Munim, S.A.; Raza, Z.A. Poly(Lactic Acid) Based Hydrogels: Formation, Characteristics and Biomedical Applications. J. Porous Mater. 2019, 26, 881–901.
- De Geest, B.G.; Sukhorukov, G.B.; Möhwald, H. The Pros and Cons of Polyelectrolyte Capsules in Drug Delivery. Expert Opin. Drug Deliv. 2009, 6, 613–624.
- Mazzoldi, P.; Carturan, S.; Quaranta, A.; Sada, C.; Sglavo, V.M. Ion Exchange Process: History, Evolution and Applications. Riv. del Nuovo Cim. 2013, 36, 397–460.
- Pergushov, D.V.; Borisov, O.V.; Zezin, A.B.; Müller, A.H.E. Interpolyelectrolyte Complexes Based on Polyionic Species of Branched Topology. Adv. Polym. Sci. 2011, 241, 131–161.
- Yashin, V.V.; Ermakov, I.V.; Litmanovich, A.D.; Plate, N.A. Theory of Macromolecular Reaction in the Polymer Mixture Complicated by Diffusion. Vysokomol. Soedin. Ser. A Ser. B Ser. C Kratk. Soobshcheniya 1994, 36, 955–963.
- Izumrudov, V.A.; Zezin, A.B.; Kabanov, V.A. Equilibria in Interpolyelectrolyte Reactions and the Phenomenon of Molecular “Recognition” in Solutions of Interpolyelectrolyte Complexes. Russ. Chem. Rev. 1991, 60, 792–806.
- Kabanov, V.A.; Papisov, I.M. Formation of Complexes between Complementary Synthetic Polymers and Oligomers in Dilute Solution Review. Polym. Sci. USSR 1979, 21, 261–307.
- Shovsky, A.; Varga, I.; Makuška, R.; Claesson, P.M. Formation and Stability of Water-Soluble, Molecular Polyelectrolyte Complexes: Effects of Charge Density, Mixing Ratio, and Polyelectrolyte Concentration. Langmuir 2009, 25, 6113–6121.
- Zhang, Y.; Jin, Q.; Zhao, J.; Wu, C.; Fan, Q.; Wu, Q. Facile Fabrication of PH-Sensitive Core-Shell Nanoparticles Based on HEC and PMAA via Template Polymerization. Eur. Polym. J. 2010, 46, 1425–1435.
- Połowiński, S. Template Polymerisation and Co-Polymerisation. Prog. Polym. Sci. 2002, 27, 537–577.
- Papisov, I.M. Matrix Polymerization and Other Matrix and Pseudomatrix Processes as a Method to Obtain Composite Materials. Polym. Sci. Ser. B 1997, 39, 122–133.
- Novakov, I.A.; Navrotskij, A.V.; Navrotskii, A.V. Polymerization of 1,2-Dimethyl-5-Vinylpyridinium Methyl Sulfate and the Properties of the Resultant Polyelectrolytes. Polym. Sci. Ser. C 2002, 44, 49–62.
- Salimi, H.; Aryanasab, F.; Banazadeh, A.R.; Shabanian, M.; Seidi, F. Designing Syntheses of Cellulose and Starch Derivatives with Basic or Cationic N -Functions: Part I-Cellulose Derivatives. Polym. Adv. Technol. 2016, 27, 5–32.
- Bekturov, E.A.; Bimendina, L.A. Complexes of Water-Soluble Polymers. J. Macromol. Sci. Part C 1997, 37, 501–518.
- Panarin, E.F.; Kopejkin, V.V. Biological Activity of Synthetic Polyelectrolyte Complexes of Ionogenic Surfactants. Vysokomol. Soedin. Ser. A Ser. B Ser. C Kratk. Soobshcheniya 2002, 44, 2340–2351.
- Gazizov, A.; Zhumadilova, G.; Bimendina, L.; Kudaibergenov, S. Interpolymer Complexes of Some Vinyl Copolymers in a Solution and on the Boundary of Two Liquid Phases. Polymer 2000, 41, 5793–5797.
- Driver, K.; Baco, S.; Khutoryanskiy, V.V. Hollow Capsules Formed in a Single Stage via Interfacial Hydrogen-Bonded Complexation of Methylcellulose with Poly(Acrylic Acid) and Tannic Acid. Eur. Polym. J. 2013, 49, 4249–4256.
- Piculell, L.; Thuresson, K.; Lindman, B. Mixed Solutions of Surfactant and Hydrophobically Modified Polymer. Polym. Adv. Technol. 2001, 12, 44–69.
- De Geest, B.G.; De Koker, S.; Sukhorukov, G.B.; Kreft, O.; Parak, W.J.; Skirtach, A.G.; Demeester, J.; De Smedt, S.C.; Hennink, W.E. Polyelectrolyte Microcapsules for Biomedical Applications. Soft Matter 2009, 5, 282–291.
- Khutoryanskaya, O.V.; Williams, A.C.; Khutoryanskiy, V.V. PH-Mediated Interactions between Poly(Acrylic Acid) and Methylcellulose in the Formation of Ultrathin Multilayered Hydrogels and Spherical Nanoparticles. Macromolecules 2007, 40, 7707–7713.
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237.
- Szwarc, M. Replica Polymerization. J. Polym. Sci. 1954, 13, 317–318.
- Petrov, A.I.; Antipov, A.A.; Sukhorukov, G.B. Base−Acid Equilibria in Polyelectrolyte Systems: From Weak Polyelectrolytes to Interpolyelectrolyte Complexes and Multilayered Polyelectrolyte Shells. Macromolecules 2003, 36, 10079–10086.
- Mun, G.A.; Khutoryanskiy, V.V.; Akhmetkalieva, G.T.; Shmakov, S.N.; Dubolazov, A.V.; Nurkeeva, Z.S.; Park, K. Interpolymer Complexes of Poly(Acrylic Acid) with Poly(2-Hydroxyethyl Acrylate) in Aqueous Solutions. Colloid Polym. Sci. 2004, 283, 174–181.
- Bumbu, G.G.; Munteanu, B.S.; Eckelt, J.; Vasile, C. Influence of the Composition of Hydroxypropyl Cellulose/Maleic Acid-Alt-Styrene Copolymer Blends on Their Properties as Matrix for Drug Release. Express Polym. Lett. 2009, 3, 286–294.
- Castro-Guerrero, C.F.; Morales-Cepeda, A.; Rivera-Armenta, J.; Mendoza-Martfnez, A.; Álvarez-Castillo, A. Gels from Acrylic Acid and Hydroxypropyl Cellulose via Free Radical Polymerization. E-Polymers 2008, 147, 1697–1704.
- Li, H.; Shen, X.-Y.; Gong, G.-L.; Wang, D. Compatibility Studies with Blends Based on Hydroxypropylcellulose and Polyacrylonitrile. Carbohydr. Polym. 2008, 73, 191–200.
- Liu, W.; Liu, Y.; Hao, X.; Zeng, G.; Wang, W.; Liu, R.; Huang, Y. Backbone-Collapsed Intra- and Inter-Molecular Self-Assembly of Cellulose-Based Dense Graft Copolymer. Carbohydr. Polym. 2012, 88, 290–298.
- Zhang, R.; Chu, G.; Vasilyev, G.; Martin, P.; Camposeo, A.; Persano, L.; Pisignano, D.; Zussman, E. Hybrid Nanocomposites for 3D Optics: Using Interpolymer Complexes with Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2019, 11, 19324–19330.
- Gärdlund, L.; Wågberg, L.; Norgren, M. New Insights into the Structure of Polyelectrolyte Complexes. J. Colloid Interface Sci. 2007, 312, 237–246.
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater. 2018, 7, 153–174.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
493
Revisions:
2 times
(View History)
Update Date:
08 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




