
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sunil J. Wimalawansa | -- | 1885 | 2023-09-06 00:54:55 | | | |
| 2 | Sirius Huang | -5 word(s) | 1880 | 2023-09-06 03:45:51 | | |
Video Upload Options
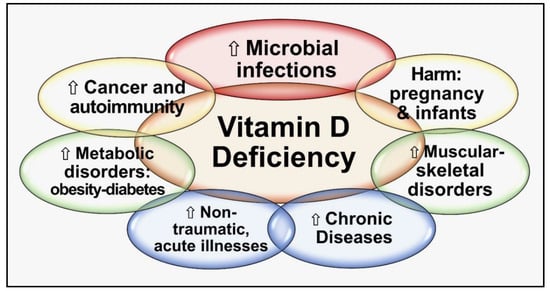
Globally, vitamin D deficiency is a significant public health problem—a pandemic—that has overtaken iron deficiency as the most common nutritional deficiency in the world. Vitamin D deficiency is associated with many chronic diseases and increases the risk of acute and worsened chronic infections. Both vitamin D and [25(OH]D: calcifediol) and its active form, 1,25-dihydroxyvitamin D [1,25(OH)2D: calcitriol], play critical roles in protecting humans from invasive pathogens, reducing risks of autoimmunity, and maintaining better health. Conversely, low 25(OH)D status increases susceptibility to infections and developing autoimmunity. Individuals obtain optimal results by maintaining serum 25(OH)D concentrations above 50 ng/mL (125 nmol/L) (above 40 ng/mL in the population): this also minimizes community outbreaks and autoimmune disorders. In over 97.5% of people, this can be achieved through daily sun exposure (except in countries far from the equator during winter) or taking between 5,000 and 8,000 IU vitamin D supplements daily (average, ~70 to 90 IU/kg body weight). Only those with gastrointestinal malabsorption, obesity, or on medications that increase catabolism of vitamin D, and a few specific disorders require higher intake.
1. Introduction
2. Vitamin D Activates Immune Cells
3. Modes of Stimulating the Immune System by Vitamin D
4. Vitamin D and Immune System
5. Vitamin D Is Fundamental to the Defense against Microbes and Preventing Autoimmunity
| Examples of Infections | Autoimmune Diseases and Others |
|---|---|
| Tuberculosis, leprosy, common cold (intracellular microorganisms) | Allergy/eczema |
| Influenza type A $ | Asthma |
| Coryza (common cold) | Chronic hives |
| Upper respiratory tract infections | Fibromyalgia |
| Lower urinary tract infections | Inflammatory bowel disease |
| Bacterial vaginosis in pregnant women | Multiple sclerosis |
| Periodontal gum disease and infections | Myositis and periostitis |
| Osteonecrosis of the jaw | Primary biliary cirrhosis |
| Miscellaneous fungal infections | Psoriasis |
| Yeast infection | Polyautoimmunity |
| Coxsackie A and B | Rheumatoid arthritis/ Behcet’s disease |
| SARS-CoV-2 | Type 1 diabetes mellitus |
6. The Importance of Intracellular Generation of Calcitriol for Immune Cell Signaling
Approximately 75% of the innate [1] and over 50% of the adaptive immune systems [90] are driven by intracellularly generated calcitriol [91]. However, present hormonal calcitriol has little or no effect on intracellular signal transduction or genomic functions in immune cells. Therefore, administration of pharmacological doses of calcitriol should be avoided in infections. The correct approach is to provide appropriate higher doses of vitamin D (including an upfront loading dose if indicated) [92], except for oral administration of calcifediol in emergencies [84][91].
Intracellular calcitriol is critical for modulating both genomic [93] and non-genomic activities like signal transduction [94][95]. The latter includes the tightening of gap-junctions [96] and autocrine (intracrine) and paracrine signaling [5][6][84]. Intracrine functions are initiated following the detection of foreign proteins, microbes, etc., by a series of cell surface receptors: the most important is the membrane-bound (sensing/detecting) Toll-like receptor-4 TLR-4 [97], which is also involved in the production of antimicrobial peptides [59]. Intermittent signals derived from TLR-4 - lead to over-drive peak production of calcitriol and VDR in microsomes [98] (see below for details). However, calcitriol is indicated in calcitriol/VDR-resistant syndromes, hypoparathyroidism, and chronic renal failure, where exogenous calcitriol is lifesaving [99].
When circulating D3 and/or 25(OH)D is adequate (e.g., over 50 ng/mL), they diffuse into immune cells, facilitating the generation of calcitriol intracellualrly. Calcitriol suppresses the pathological process and hyper-immune reactions with its genomic actions and autocrine signaling mechanisms [5][6]. These actions reduce the risks of cytokine storms and ARDS and are associated with severe pulmonary and cardiovascular complications in persons with severe infections like COVID-19 [100][101].
7. Conclusion
Maintenance of sufficient circulating 25(OH)D has a profound helpful effect on the musculoskeletal system, decreasing many common disorders. In addition to its endocrine effects, vitamin D exerts genomic and crucial autocrine and paracrine effects in peripheral target tissues subject to epigenesis modulation [102]. Maintaining mean population vitamin D status—serum 25(OH)D concentrations— above 40 ng/mL leads to broader benefits and reduced healthcare costs. This will significantly impact achieving full physiological benefits, including reducing the risks of chronic diseases, infections, and all-cause mortality [103]. This is the most cost-effective approach, instead of treating people after developing complications from chronic hypovitaminosis D and many related disease conditions. Therefore, adopting this to clinical practice guidelines and healthcare insurance protocols is warranted.
References
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, e1909.
- Gotelli, E.; Soldano, S.; Hysa, E.; Paolino, S.; Campitiello, R.; Pizzorni, C.; Sulli, A.; Smith, V.; Cutolo, M. Vitamin D and COVID-19: Narrative Review after 3 Years of Pandemic. Nutrients 2022, 14, 4907.
- Hanel, A.; Bendik, I.; Carlberg, C. Transcriptome-Wide Profile of 25-Hydroxyvitamin D3 in Primary Immune Cells from Human Peripheral Blood. Nutrients 2021, 13, 4100.
- Aygun, H. Vitamin D can prevent COVID-19 infection-induced multiple organ damage. Naunyn-Schmiedeberg's Arch. Pharmacol. 2020, 393, 1157–1160.
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat. Immunol. 2022, 23, 62–74.
- McGregor, E.; Kazemian, M.; Afzali, B.; Freiwald, T.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Zhang, Z.; Teague, H.; West, E.E.; et al. An autocrine Vitamin D-driven Th1 shutdown program can be exploited for COVID-19. bioRxiv 2020.
- Wimalawansa, S.J. Biology of vitamin D. J. Steroids Horm. Sci. 2019, 10, 198.
- Grant, W.B. Variations in Vitamin D Production Could Possibly Explain the Seasonality of Childhood Respiratory Infections in Hawaii. Pediatr. Infect. Dis. J. 2008, 27, 853.
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583.
- Wimalawansa, S.J. Fighting against COVID-19: Boosting the immunity with micronutrients, stress reduction, physical activity, and vitamin D. Nutr. Food Sci. J. 2020, 3, 126.
- Chetty, V.V.; Chetty, M. Potential benefit of vitamin D supplementation in people with respiratory illnesses, during the COVID-19 pandemic. Clin. Transl. Sci. 2021, 14, 2111–2116.
- Wimalawansa, S.J.; Polonowita, A. Boosting immunity with vitamin D for preventing complications and deaths from COVID-19. In Proceedings of the COVID 19: Impact, Mitigation, Opportunities and Building Resilience “From Adversity to Serendipity,” Perspectives of Global Relevance Based on Research, Experience and Successes in Combating COVID-19 in Sri Lanka, Colombo, Sri Lanka, 27 January 2021; pp. 171–198.
- D’avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolò, A.; Lucchini, R.; Keller, F.; Cantù, M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359.
- DiNicolantonio, J.J.; O’keefe, J.H. Magnesium and Vitamin D Deficiency as a Potential Cause of Immune Dysfunction, Cytokine Storm and Disseminated Intravascular Coagulation in COVID-19 patients. Mo. Med. 2021, 118, 68–73.
- Cicero, A.F.G.; Fogacci, F.; Borghi, C. Vitamin D Supplementation and COVID-19 Outcomes: Mounting Evidence and Fewer Doubts. Nutrients 2022, 14, 3584.
- Xu, Y.; Baylink, D.J.; Chen, C.-S.; Reeves, M.E.; Xiao, J.; Lacy, C.; Lau, E.; Cao, H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. 2020, 18, 322.
- Argano, C.; Bocchio, R.M.; Natoli, G.; Scibetta, S.; Monaco, M.L.; Corrao, S. Protective Effect of Vitamin D Supplementation on COVID-19-Related Intensive Care Hospitalization and Mortality: Definitive Evidence from Meta-Analysis and Trial Sequential Analysis. Pharmaceuticals 2023, 16, 130.
- Davies, G.; Mazess, R.B.; Benskin, L.L. Letter to the editor in response to the article: “Vitamin D concentrations and COVID-19 infection in UK biobank” (Hastie et al.). Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 643–644.
- Hastie, C.E.; Pell, J.P.; Sattar, N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 2020, 60, 545–548.
- Raisi-Estabragh, Z.; McCracken, C.; Bethell, M.S.; Cooper, J.; Cooper, C.; Caulfield, M.J.; Munroe, P.B.; Harvey, N.C.; E Petersen, S. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: Study of 1326 cases from the UK Biobank. J. Public Health 2020, 42, 451–460.
- Annweiler, C.; Hanotte, B.; de L’eprevier, C.G.; Sabatier, J.-M.; Lafaie, L.; Célarier, T. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J. Steroid Biochem. Mol. Biol. 2020, 204, 105771.
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377.
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Deficiency and Treatment with COVID-19 Incidence. medRxiv 2020.
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics with COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722.
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757.
- Baktash, V.; Hosack, T.; Patel, N.; Shah, S.; Kandiah, P.; Abbeele, K.V.D.; Mandal, A.K.J.; Missouris, C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2020, 97, 442–447.
- Greiller, C.L.; Martineau, A.R. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients 2015, 7, 4240–4270.
- Gönen, M.S.; Alaylıoğlu, M.; Durcan, E.; Özdemir, Y.; Şahin, S.; Konukoğlu, D.; Nohut, O.K.; Ürkmez, S.; Küçükece, B.; Balkan, I.I.; et al. Rapid and Effective Vitamin D Supplementation May Present Better Clinical Outcomes in COVID-19 (SARS-CoV-2) Patients by Altering Serum INOS1, IL1B, IFNg, Cathelicidin-LL37, and ICAM1. Nutrients 2021, 13, 4047.
- Hill, A.B. The Environment and Disease: Association or Causation? Proc. R. Soc. Med. 1965, 58, 295–300.
- Cutolo, M.; Pizzorni, C.; Sulli, A. Vitamin D endocrine system involvement in autoimmune rheumatic diseases. Autoimmun. Rev. 2011, 11, 84–87.
- Caccamo, D.; Ricca, S.; Currò, M.; Ientile, R. Health Risks of Hypovitaminosis D: A Review of New Molecular Insights. Int. J. Mol. Sci. 2018, 19, 892.
- Delvin, E.; Souberbielle, J.-C.; Viard, J.-P.; Salle, B. Role of vitamin D in acquired immune and autoimmune diseases. Crit. Rev. Clin. Lab. Sci. 2014, 51, 232–247.
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 17, 9784.
- Gallo, D.; Baci, D.; Kustrimovic, N.; Lanzo, N.; Patera, B.; Tanda, M.L.; Piantanida, E.; Mortara, L. How Does Vitamin D Affect Immune Cells Crosstalk in Autoimmune Diseases? Int. J. Mol. Sci. 2023, 24, 4689.
- Székely, J.I.; Pataki, Á. Effects of vitamin D on immune disorders with special regard to asthma, COPD and autoimmune diseases: A short review. Expert Rev. Respir. Med. 2012, 6, 683–704.
- Hassan, V.; Hassan, S.; Seyed-Javad, P.; Ahmad, K.; Asieh, H.; Maryam, S.; Farid, F.; Siavash, A. Association between Serum 25 (OH) Vitamin D Concentrations and Inflammatory Bowel Diseases (IBDs) Activity. Med. J. Malays. 2013, 68, 34–38.
- Sainaghi, P.P.; Bellan, M.; Nerviani, A.; Sola, D.; Molinari, R.; Cerutti, C.; Pirisi, M. Superiority of a High Loading Dose of Cholecalciferol to Correct Hypovitaminosis D in Patients with Inflammatory/Autoimmune Rheumatic Diseases. J. Rheumatol. 2013, 40, 166–172.
- Tuohimaa, P.; Keisala, T.; Minasyan, A.; Cachat, J.; Kalueff, A. Vitamin D, nervous system and aging. Psychoneuroendocrinology 2009, 34, S278–S286.
- Pani, M.; Regulla, K.; Segni, M.; Krause, M.; Hofmann, S.; Hufner, M.; Herwig, J.; Pasquino, A.; Usadel, K.; Badenhoop, K. Vitamin D 1alpha-hydroxylase (CYP1alpha) polymorphism in Graves' disease, Hashimoto's thyroiditis and type 1 diabetes mellitus. Eur. J. Endocrinol. 2002, 146, 777–781.
- Ganmaa, D.; Enkhmaa, D.; Nasantogtokh, E.; Sukhbaatar, S.; Tumur-Ochir, K.; Manson, J. Vitamin D, respiratory infections, and chronic disease: Review of meta-analyses and randomized clinical trials. J. Intern. Med. 2021, 291, 141–164.
- Vintilescu, B.; E Niculescu, C.; Stepan, M.D.; Ioniță, E. Involvement of Vitamin D in Chronic Infections of the Waldeyer`s Ring in the School Aged Child. Curr. Health Sci. J. 2019, 45, 291–295.
- Juszczak, A.B.; Kupczak, M.; Konecki, T. Does Vitamin Supplementation Play a Role in Chronic Kidney Disease? Nutrients 2023, 15, 2847.
- Özdemir, B.; Köksal, B.T.; Karakaş, N.M.; Tekindal, M.A.; Özbek, Y. Serum Vitamin D Levels in Children with Recurrent Respiratory Infections and Chronic Cough. Indian. J. Pediatr. 2016, 83, 777–782.
- Song, L.; Papaioannou, G.; Zhao, H.; Luderer, H.F.; Miller, C.; Dall’osso, C.; Nazarian, R.M.; Wagers, A.J.; Demay, M.B. The Vitamin D Receptor Regulates Tissue Resident Macrophage Response to Injury. Endocrinology 2016, 157, 4066–4075.
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624.
- Bikle, D.D. Vitamin D and the immune system: Role in protection against bacterial infection. Curr. Opin. Nephrol. Hypertens. 2008, 17, 348–352.
- Lin, R.; White, J.H. The pleiotropic actions of vitamin D. BioEssays 2004, 26, 21–28.
- Zhou, Q.; Qin, S.; Zhang, J.; Zhon, L.; Pen, Z.; Xing, T. 1,25(OH) 2 D 3 induces regulatory T cell differentiation by influencing the VDR/PLC-γ1/TGF-β1/pathway. Mol. Immunol. 2017, 91, 156–164.
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496.
- Shirvani, S.S.; Nouri, M.; Sakhinia, E.; Babaloo, Z.; Mohammadzaeh, A.; Alipour, S.; Jadideslam, G.; Khabbazi, A. The molecular and clinical evidence of vitamin D signaling as a modulator of the immune system: Role in Behçet’s disease. Immunol. Lett. 2019, 210, 10–19.
- Du, Y.; Tong, Y.; Quan, Y.; Wang, G.; Cheng, H.; Gu, S.; Jiang, J.X. Protein kinase A activation alleviates cataract formation via increased gap junction intercellular communication. iScience 2023, 26, 106114.
- Miller, S.C.; De Saint-Georges, L.; Bowman, B.M.; Jee, W.S. Bone lining cells: Structure and Function. Scanning Microsc. 1989, 3, 953–960. Available online: https://europepmc.org/article/med/2694361 (accessed on 16 January 2021).
- Valiunas, V. Biophysical Properties of Connexin-45 Gap Junction Hemichannels Studied in Vertebrate Cells. J. Gen. Physiol. 2002, 119, 147–164.
- Tikellis, C.; Thomas, M.C. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int. J. Pept. 2012, 2012, 256294.
- Wimalawansa, S.J. ACE inhibitors and angiotensin receptor blockers reduce the complications associated with COVID-19 infection. World J. Pharma Res. 2021, 10, 2579–2600.
- Yalcin, H.C.; Sukumaran, V.; Al-Ruweidi, M.K.A.A.; Shurbaji, S. Do Changes in ACE-2 Expression Affect SARS-CoV-2 Virulence and Related Complications: A Closer Look into Membrane-Bound and Soluble Forms. Int. J. Mol. Sci. 2021, 22, 6703.
- Bradding, P.; Richardson, M.; Hinks, T.S.; Howarth, P.H.; Choy, D.F.; Arron, J.R.; Wenzel, S.E.; Siddiqui, S. ACE2, TMPRSS2, and furin gene expression in the airways of people with asthma—Implications for COVID-19. J. Allergy Clin. Immunol. 2020, 146, 208–211.
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97.
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773.
- Khan, H.; Kunutsor, S.; Franco, O.H.; Chowdhury, R. Vitamin D, type 2 diabetes and other metabolic outcomes: A systematic review and meta-analysis of prospective studies. Proc. Nutr. Soc. 2013, 72, 89–97.
- Takiishi, T.; Gysemans, C.; Bouillon, R.; Mathieu, C. Vitamin D and Diabetes. Rheum. Dis. Clin. N. Am. 2012, 38, 179–206.
- Garg, M.; Rosella, O.; Rosella, G.; Wu, Y.; Lubel, J.S.; Gibson, P.R. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin. Nutr. 2017, 37, 1375–1382.
- Wasse, H.; Cardarelli, F.; De Staercke, C.; Hooper, C.; Veledar, E.; Guessous, I. 25-hydroxyvitamin D concentration is inversely associated with serum MMP-9 in a cross-sectional study of African American ESRD patients. BMC Nephrol. 2011, 12, 24.
- WöBke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244.
- Mascitelli, L.; Pezzetta, F.; Goldstein, M.R. Inflammatory bowel disease and the vitamin D endocrine system. Intern. Med. J. 2011, 41, 369–370.
- Raman, M.; Milestone, A.N.; Walters, J.R.; Hart, A.L.; Ghosh, S. Vitamin D and gastrointestinal diseases: Inflammatory bowel disease and colorectal cancer. Ther. Adv. Gastroenterol. 2011, 4, 49–62.
- Ardizzone, S.; Cassinotti, A.; Bevilacqua, M.; Clerici, M.; Porro, G.B. Vitamin D and Inflammatory Bowel Disease. Vitam. Horm. 2011, 86, 367–377.
- Attar, S.M. Vitamin D deficiency in rheumatoid arthritis. Prevalence and association with disease activity in Western Saudi Arabia. Saudi Med. J. 2012, 33, 520–525.
- Furuya, T.; Hosoi, T.; Tanaka, E.; Nakajima, A.; Taniguchi, A.; Momohara, S.; Yamanaka, H. Prevalence of and factors associated with vitamin D deficiency in 4,793 Japanese patients with rheumatoid arthritis. Clin. Rheumatol. 2013, 32, 1081–1087.
- Rossini, M.; Bongi, S.M.; La Montagna, G.; Minisola, G.; Malavolta, N.; Bernini, L.; Cacace, E.; Sinigaglia, L.; Di Munno, O.; Adami, S. Vitamin D deficiency in rheumatoid arthritis: Prevalence, determinants and associations with disease activity and disability. Arthritis Res. Ther. 2010, 12, R216.
- Ishikawa, L.L.W.; Colavite, P.M.; Fraga-Silva, T.F.d.C.; Mimura, L.A.N.; França, T.G.D.; Zorzella-Pezavento, S.F.G.; Chiuso-Minicucci, F.; Marcolino, L.D.; Penitenti, M.; Ikoma, M.R.V.; et al. Vitamin D Deficiency and Rheumatoid Arthritis. Clin. Rev. Allergy Immunol. 2017, 52, 373–388.
- Lin, Z.; Li, W. The Roles of Vitamin D and Its Analogs in Inflammatory Diseases. Curr. Top. Med. Chem. 2016, 16, 1242–1261.
- Wang, T.; Wu, M.-B.; Zhang, R.-H.; Chen, Z.-J.; Hua, C.; Lin, J.-P.; Yang, L.-R. Advances in Computational Structure-Based Drug Design and Application in Drug Discovery. Curr. Top. Med. Chem. 2016, 16, 901–916.
- Heine, G.; Niesner, U.; Chang, H.-D.; Steinmeyer, A.; Zügel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D3promotes IL-10 production in human B cells. Eur. J. Immunol. 2008, 38, 2210–2218.
- Bikle, D.D. Vitamin D regulation of immune function during covid-19. Rev. Endocr. Metab. Disord. 2022, 23, 279–285.
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1061–1094.
- E Nnoaham, K.; Clarke, A. Low serum vitamin D levels and tuberculosis: A systematic review and meta-analysis. Leuk. Res. 2008, 37, 113–119.
- Grant, W.B.; Goldstein, M.; Mascitelli, L. Ample evidence exists from human studies that vitamin D reduces the risk of selected bacterial and viral infections. Exp. Biol. Med. 2010, 235, 1395–1396.
- Liu, N.; Kaplan, A.; Low, J.; Nguyen, L.; Liu, G.; Equils, O.; Hewison, M. Vitamin D Induces Innate Antibacterial Responses in Human Trophoblasts via an Intracrine Pathway1. Biol. Reprod. 2009, 80, 398–406.
- Vasilyevna Belyaeva, I.; Pavlovitch Churilov, L.; Robertovnsmalla, C.M.L.; Vladimirovitch Nikolaev, A.; Andreevna Starshinova, A.; Kazimirovitch Yablonsky, P. Vitamin D, Cathelicidin, Prolactin, Autoantibodies, and Cytokines in Different Forms of Pulmonary Tuberculosis versus Sarcoidosis. Isr. Med. Assoc. J. 2017, 19, 499–505.
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A., Jr. Association Between Serum 25-Hydroxyvitamin D Level and Upper Respiratory Tract Infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384–390.
- Berry, D.J.; Hesketh, K.; Power, C.; Hyppönen, E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 2011, 106, 1433–1440.
- Anty, R.; Anstee, Q.M.; Gual, P.; Tran, A. Prophylaxis of bacterial infections in cirrhosis: Is an optimal 25-OH vitamin D level required? J. Hepatol. 2014, 61, 965–966.
- Wimalawansa, S.J. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections—Sepsis and COVID-19. Nutrients 2022, 14, 2997.
- Grant, W.B.; Boucher, B.J.; Pludowski, P.; Wimalawansa, S.J. The emerging evidence for non-skeletal health benefits of vitamin D supplementation in adults. Nat. Rev. Endocrinol. 2022, 18, 323.
- Wimalawansa, S.J. Vitamin D: Everything You Need to Know; Karunaratne & Sons: Homagama, Sri Lanka, 2012; Volume 1.0, ISBN 978-955-9098-94-2.
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 175, 60–81.
- Armas, L.A.G.; Hollis, B.W.; Heaney, R.P. Vitamin D2 Is Much Less Effective than Vitamin D3 in Humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391.
- Urashima, M.; Mezawa, H.; Noya, M.; Camargo, C.A., Jr. Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: A randomized controlled trial. Food Funct. 2014, 5, 2365–2370.
- White, J.H. Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Arch. Biochem. Biophys. 2012, 523, 58–63.
- Wimalawansa, S. Overcoming Infections Including COVID-19, by Maintaining Circulating 25(OH)D Concentrations Above 50 ng/mL. Pathol. Lab. Med. Int. 2022, ume 14, 37–60.
- Wimalawansa, S.J.; Whittle, R. Vitamin D: A single initial dose is not bogus if followed by an appropriate maintenance intake. JBMR Plus 2022, 6, e10606.
- Trochoutsou, A.I.; Kloukina, V.; Samitas, K.; Xanthou, G. Vitamin-D in the Immune System: Genomic and Non-Genomic Actions. Mini-Rev. Med. Chem. 2015, 15, 953–963.
- Bravo, S.; Paredes, R.; Izaurieta, P.; Lian, J.B.; Stein, J.L.; Stein, G.S.; Hinrichs, M.V.; Olate, J.; Aguayo, L.G.; Montecino, M. The classic receptor for 1α,25-dihydroxy vitamin D3 is required for non-genomic actions of 1α,25-dihydroxy vitamin D3 in osteosarcoma cells. J. Cell. Biochem. 2006, 99, 995–1000.
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135.
- Stio, M.; Retico, L.; Annese, V.; Bonanomi, A.G. Vitamin D regulates the tight-junction protein expression in active ulcerative colitis. Scand. J. Gastroenterol. 2016, 51, 1193–1199.
- Valdés-López, J.F.; Velilla, P.; Urcuqui-Inchima, S. Vitamin D modulates the expression of Toll-like receptors and pro-inflammatory cytokines without affecting Chikungunya virus replication, in monocytes and macrophages. Acta Trop. 2022, 232, 106497.
- Ellfolk, M.; Norlin, M.; Wikvall, K. Isolation and properties of the CYP2D25 promoter: Transcriptional regulation by vitamin D3 metabolites. Biochem. Biophys. Res. Commun. 2006, 345, 568–572.
- Oristrell, J.; Oliva, J.C.; Subirana, I.; Casado, E.; Domínguez, D.; Toloba, A.; Aguilera, P.; Esplugues, J.; Fafián, P.; Grau, M. Association of Calcitriol Supplementation with Reduced COVID-19 Mortality in Patients with Chronic Kidney Disease: A Population-Based Study. Biomedicines 2021, 9, 509.
- Xu, J.; Sriramula, S.; Xia, H.; Moreno-Walton, L.; Culicchia, F.; Domenig, O.; Poglitsch, M.; Lazartigues, E. Clinical Relevance and Role of Neuronal AT 1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ. Res. 2017, 121, 43–55.
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438.
- Kato, S.; Nishimura, K.-I.; Mori, J.-I. Update on recent progress in vitamin D research. Molecular basis of epigenetic regulation by vitamin D via its nuclear receptor. Clin. Calcium 2017, 27, 1543–1550.
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755.




