Globally, vitamin D deficiency is a significant public health problem—a pandemic—that has overtaken iron deficiency as the most common nutritional deficiency in the world. Vitamin D deficiency is associated with many chronic diseases and increases the risk of acute and worsened chronic infections. Both vitamin D and [25(OH]D: calcifediol) and its active form, 1,25-dihydroxyvitamin D [1,25(OH)2D: calcitriol], play critical roles in protecting humans from invasive pathogens, reducing risks of autoimmunity, and maintaining better health. Conversely, low 25(OH)D status increases susceptibility to infections and developing autoimmunity. Individuals obtain optimal results by maintaining serum 25(OH)D concentrations above 50 ng/mL (125 nmol/L) (above 40 ng/mL in the population): this also minimizes community outbreaks and autoimmune disorders. In over 97.5% of people, this can be achieved through daily sun exposure (except in countries far from the equator during winter) or taking between 5,000 and 8,000 IU vitamin D supplements daily (average, ~70 to 90 IU/kg body weight). Only those with gastrointestinal malabsorption, obesity, or on medications that increase catabolism of vitamin D, and a few specific disorders require higher intake.
1. Introduction
1,25-dihydroxycholecalciferol (calciferol) is the most active vitamin D metabolite and a potent immune modulator essential for combating pathogens
[1][2][32,33]. As described below, the circulating hormonal form of calcitriol does not affect immune cell functions. The functionality of these cells depends on adequate generation of calcitriol within them. Calcitriol (i) activates cytosol’s vitamin D (calcitriol)receptors (VDRs), then the complex, translocates into the nucleus to modulate genomic functions, and (ii) acts as signaling molecules for its non-genomic actions, membrane effects, and autocrine and paracrine signaling.
Calcitriol concentrations in the circulation are controlled mostly by parathyroid hormone (PTH) partly via circulatory ionized calcium. In contrast, in target tissues, the production of calcitriol is mostly regulated by circulatory 25(OH)D concentration and the feedback catabolic activity of tissue 24-hydroxylase enzyme, not by PTH. Except for providing as a vital substrate, vitamin D has no demonstrable action: it is measured only in research. Consequently, vitamin D physiologic correlations are focused on the serum 25(OH)D concentration.
2. Vitamin D Activates Immune Cells
The calcitriol—receptor complex translocates to the nucleus, where it binds to the upstream DNA, and modulates over 1200 genes
[3][34]. Calcitriol down-regulates inflammation and oxidative stress, primarily by suppressing inflammatory cytokines. The immunomodulatory effects of vitamin D include activation of immune cells such as T and B cells and macrophage and dendritic cells, as well as increased production of antimicrobial peptides and neutralizing antibodies
[4][5][6][35,36,37].
As with COVID-19 multiple infections, vaccines, and repeated bivalent COVID-19 booster doses, cause immune paresis, increasing the vulnerability to infections, especially to intracellular bacteria such as tuberculosis and
[7][38] and respiratory viruses
[8][9][39,40], and SARS-CoV-2
[10][11][12][41,42][43], especially in those with hypovitaminosis D, leading to severe complications. Those who developed complications and died from it had a high prevalence of vitamin D deficiency—serum 25(OH)D concentrations less than 12 ng/mL (
p = 0.004), compared with those with negative results
[13][44].
In addition, vulnerable people—older people with comorbidities—low vitamin D strongly correlate with cytokine storm—a hyper-inflammatory condition caused by an uncontrolled, overactive immune status, with high mortality
[14][45]. Symptomatic disease, complications, and deaths from viral infections, including SARS-CoV-2, are due to weak immune systems and high viral load. Vitamin D does not totally prevent viral infections (i.e., contracting COVID-19), it significantly reduces symptomatic disease, complications, and deaths
[12][15][16][17][18][19][20][43,46,47,48,49,50,51].
Unsurprisingly, having pre-pandemic hypovitaminosis D increases these risks and vulnerability
[21][22][23][24][52,53,54,55]. Similarly, vitamin D deficiency at diagnosis of SARS-CoV-2 markedly increases the severity and mortality
[12][17][25][26][43,48,56,57]. Whereas vitamin D sufficiency is protective against severe COVID-19 disease and deaths (
p<0.01)
[15][17][27][28][46,48,58,59]. Overall data confirmed Bradford Hill criteria
[29][60]—vitamin D deficiency as a cause for infection, severity, and mortality from SARS-CoV-2 virus
[12][15][16][17][18][19][20][43,46,47,48,49,50,51].
Vitamin D controls autoimmunity by suppressing adaptive immunity via T- and B-lymphocyte activity
[30][61]. Therefore, hypovitaminosis D causes immune dysfunction; a prime reason for autoimmune responses
[31][32][33][34][35][62,63,64,65,66], worsens existing autoimmune diseases
[36][37][67,68], such as multiple sclerosis (MS)
[38][69], and increases risks for autoimmune diseases
[34][35][65,66]. Persons with several autoimmune disorders, such as type 1 diabetes, autoimmune adrenal disease, MS, Hashimoto’s thyroiditis, etc., consistently have lower concentrations of serum 25(OH)D (calcifediol)
[32][33][39][63,64,70]. Data also supports a strong inverse relationship between vitamin D status and autoimmunity—the lower the serum 25(OH)D concentrations, the higher the risks for autoimmunity—incidence and severity
[33][34][35][64,65,66].
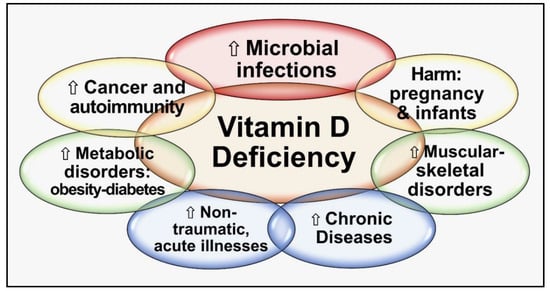
Figure 1 summarizes the critical negative outcomes of chronic vitamin D deficiency.
Figure 1.
Major negative consequences are categorized into groups of chronic vitamin D deficiency.
In contrast, vitamin D sufficiency reduces not only acute infections like SARS-CoV-2 but also the risks of chronic infections, such as tuberculosis. Sufficient generation of calcitriol within the immune cells regulates innate and adaptive immunity, potentiating the innate response (monocytes/macrophages with anti-microbial activity)
[40][41][42][43][44][10,11,12,13,71]. Calcitriol also modulates B lymphocytes and plasma cells for immunoglobulin production and stabilizes B-cells
[30][45][61,72], increasing anti-microbial peptide synthesis
(Section 3.5 and Section 3.6).
3. Modes of Stimulating the Immune System by Vitamin D
Following interactions of vitamin D and VDR (genomic interactions)
[31][62], and activating membrane and signaling systems (non-genomic actions) in immune cells leads to control of inflammation and oxidative stress, increased anti-inflammatory responses
[46][73], and decreased autoimmune tendencies
[47][74]. However, those with sustained hypovitaminosis D have less effective innate and adaptive immune systems. Impaired genomic activity results in hypo-responsivity of autoreactive T cells
[48][49][75,76]. In contrast, when 25(OH)D concentrations are adequate, T-cell responsiveness is restored, and autoimmunity risks are reduced
[45][72].
The active form of vitamin D calcitriol is essential for modulating immune cells, such as monocytes, macrophages, dendritic cells, and T and B lymphocytes
[49][50][76,77]. These cells express the enzyme CYP27B1 that hydroxylase calcifediol [25(OH)D] to calcitriol and synthesize VDR)
[49][50][76,77]. These interactions produce anti-microbial peptides, such as cathelicidin and β-defensin 2
(see Section 3.6). In infected cells, calcitriol increases the expression of nucleotide-binding oligomerization domain-containing protein 2, which damages the cell membranes of bacteria and viruses by activating signaling cascades
[45][72].
In addition to anti-microbial peptides, calcitriol induces autophagy and gap protein
[51][78] which strengthens gap junctions
[52][79] and the integrity of epithelial and endothelial cells, preventing viral penetration and fluid leaks
[53][80]. Vitamin D also enhances the expression of angiotensin-converting enzyme-2 (ACE-2)
[54][81], suppressing the renin–angiotensin system and dampening inflammation
[55][82]. Increased expression of soluble ACE-2 neutralizes circulatory viruses, preventing SARS-CoV-2 from binding to cell membrane-bound ACE-2 receptors and cellular entry
[56][57][83,84].
4. Vitamin D and Immune System
Evaluation of epidemiological studies illustrates that vitamin D deficiency significantly increases the susceptibility to infections and autoimmunity
[31][33][35][58][62,64,66,85], and acquired autoimmunity
[32][63]. 1α-hydroxylase (CYP27B1) and VDR are expressed in all immune cells, including by neutrophils, lymphocytes, dendritic cells, macrophages (antigen-presenting cells), and B lymphocytes, CD4+, and CD8+: these are stimulated when pathogens and foreign antigens are detected by Toll-like receptors-4 (TLR-4)
[58][59][85,86].
With intracellular signaling initiated by sufficient calcitriol, accelerate 1α-hydroxylation of 25(OH)D to 1,25-(OH)
2D and synthesis of VDR. Calcitriol then suppresses the transcription of inflammatory cytokines and blocks IgE-mediated mast cell degranulation. The latter is one of the mechanisms to alleviate hives, allergic reactions, and disorders that exacerbate inflammation
[45][72]. In contrast, vitamin D adequacy stabilizes mast cells, suppressing the release of histamine and TNF-α
[31][62].
Vitamin D modulates several types of immune cells, including monocytes/macrophages, dendritic cells, and B and T cells
[44][71]. Hypovitaminosis D increases vulnerability to inflammatory diseases and disorders with an autoimmune element, such as lupus, metabolic syndrome, and T1D
[60][61][62][63][64][87,88,89,90,91] (see below). Following supplementing with vitamin D, clinically meaningful disease risk reductions have been reported in persons with MS, chronic fatigue syndrome, Behcet’s disease, inflammatory bowel diseases
[50][65][66][67][77,92,93,94], and rheumatoid arthritis
[68][69][95,96]. However, not all study results agree
[70][71][97,98].
In addition to the anti-inflammatory effects of vitamin D on T-helper cells, B cells, macrophages, and dendritic cells, vitamin D has broader immunomodulatory actions on innate and adaptive immune responses
[72][73][99,100]. Regulation of immune responses by calcitriol partially inhibits B-cell expressing IgE and increases expression of IL-10, via dendritic cells and T cells
[48][74][75][76][75,101,102,103]. The following section discusses some non-genomic effects of calcitriol on the immune cells.
5. Vitamin D Is Fundamental to the Defense against Microbes and Preventing Autoimmunity
Vitamin D deficiency leads to a dysfunctional immune system, creating increased susceptibility to bacterial infections, mainly intracellular bacterial infections
[77][78][104,105], such as mycobacteria tuberculosis
[79][80][106,107], and a variety of viral infections, including influenza A
[9][78][81][40,105,108] (
Table 1). In contrast, adequate circulating 25(OH)D concentrations are associated with decreased incidences of infections
[82][109], enhanced immunity, and improved ability to overcome bacterial and viral infections
[78][83][105,110].
Table 1 illustrates some examples of infections and autoimmune disorders improved with adequate serum 25(OH)D concentrations, demonstrating multiple mechanisms; calcitriol combats pathogens
[84][85][24,111] and prevents autoimmunity
[1][2][32,33] (
Table 1).
Table 1.
Infections and autoimmune diseases significantly improved by vitamin D *.