
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tomasz Sadkowski | -- | 3587 | 2023-08-31 15:53:16 | | | |
| 2 | Lindsay Dong | Meta information modification | 3587 | 2023-09-01 02:47:30 | | |
Video Upload Options
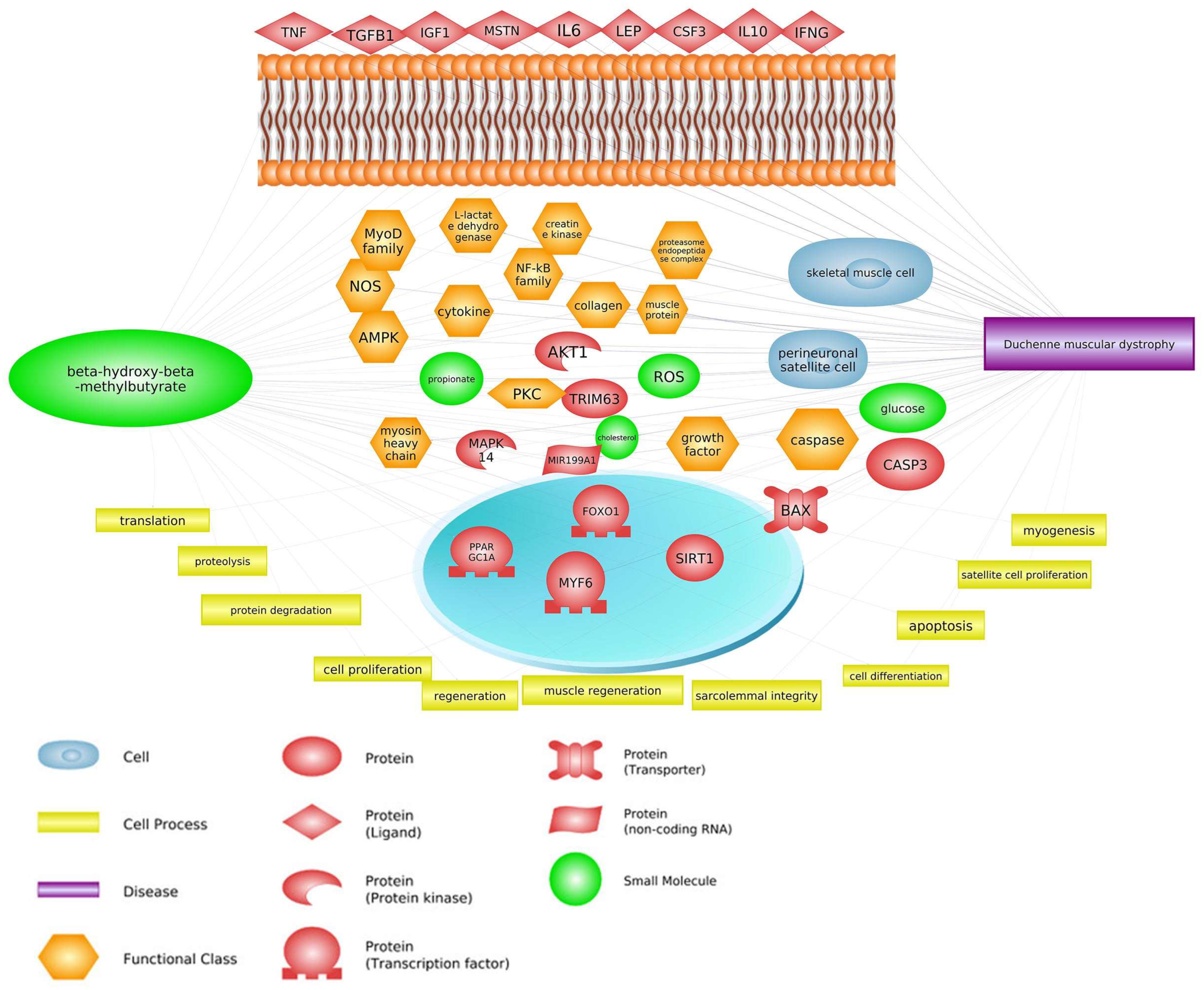
Skeletal muscle is the protein reservoir of our body and an important regulator of glucose and lipid homeostasis. The dystrophin gene is the largest gene and has a key role in skeletal muscle construction and function. Mutations in the dystrophin gene cause Duchenne and Becker muscular dystrophy in humans, mice, dogs, and cats. Duchenne muscular dystrophy (DMD) is an X-linked neuromuscular condition causing progressive muscle weakness and premature death. β-hydroxy β-methylbutyrate (HMB) prevents deleterious muscle responses under pathological conditions, including tumor and chronic steroid therapy-related muscle losses. The use of HMB as a dietary supplement allows for increasing lean weight gain; has a positive immunostimulatory effect; is associated with decreased mortality; and attenuates sarcopenia in elderly animals and individuals.
1. Introduction
2. Common Pathways
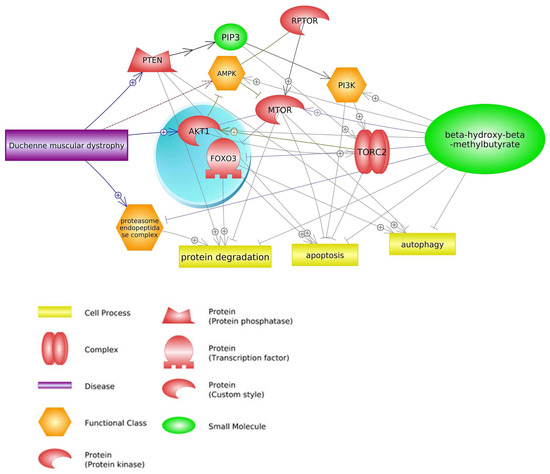
2.1. mTOR Signaling Pathway
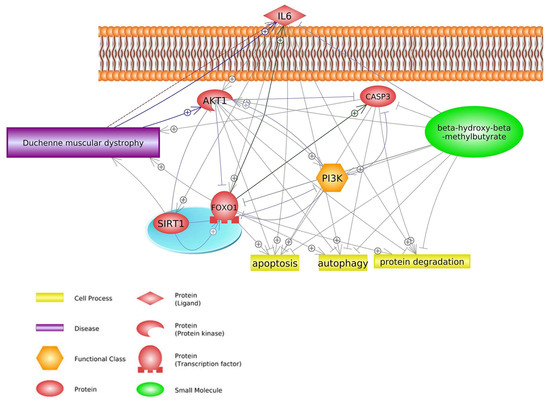
2.2. FOXO1 Signaling Pathway
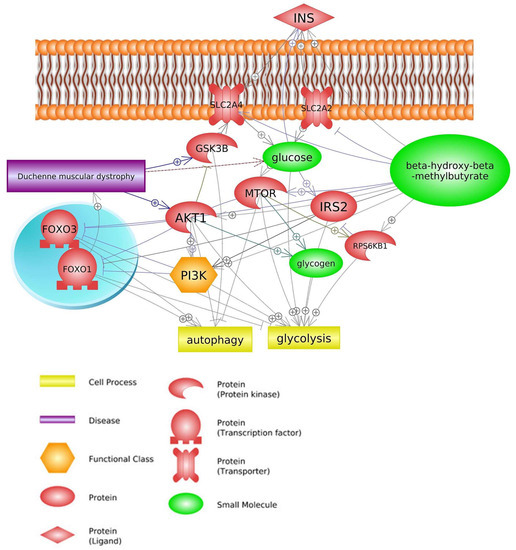
2.3. Insulin Signaling Pathway
3. Common Genes
| Gene | DMD Effect | References | HMB Effect | References |
|---|---|---|---|---|
| MSTN | inhibitor | Chiu, W. et al. (2020) [76] | stimulator | Shirvani, H. et al. (2020) [77] |
| AKT1 | stimulator | Peter, A.K.; Crosbie, R.H. (2006) [44], Kornegay, J.N. et al. (2012) [78] |
stimulator | Salto, R. et al. (2015) [46] Schnuck, J.K. et al. (2016) [74] |
| LEP | stimulator | Söderpalm, A.C. et al. (2007) [79] | stimulator | Świetlicka, I. et al. (2021) [80] |
| IL6 | stimulator | Rodríguez-Cruz, M. et al. (2018) [14] Gallardo, F.S. et al. (2021) [56] |
inhibitor | Miyake, S. et al. (2019) [54] |
| CASP3 | positive effect | Parrotta, E.I. et al. (2020) [60] | inhibitor | Eley, H.L. et al. (2008) [59] Hao, Y. et al. (2011) [64] |
| MAPK (MAPK14) | biomarker | Cotán, D. et al. (2016) [81] | inhibitor | Eley, H.L. et al. (2008) [59] |
| MyoD family | unknown | Gonçalves, M.A.F.V. et al. (2008 and 2011) [82][83] | stimulator | Kim, J.-S. et al. (2013) [43] |
| nNOS | unknown | Tian, L.J. et al. (2014) [84] | stimulator | Peterson, A.L. et al. (1999) [85] |
| NF-κB | stimulator | Wattin, M. et al. (2018) [50] Finkel, R.S. et al. (2021) [86] |
inhibitor | Smith, H.J. et al. (2005) [4] Miyake, S. et al. (2019) [54] |
| LDH | stimulator | Luce, L.N. et al. (2016) [87] Sadek, A.A. et al. (2017) [88] |
unknown | Tsuchiya, Y. et al. (2019) [89] |
4. Therapeutic Agents Used for DMD Treatment
4.1. Statins
4.2. Glucocorticoids
References
- Nissen, S.L.; Abumrad, N.N. Nutritional Role of the Leucine Metabolite β-Hydroxy β-Methylbutyrate (HMB). J. Nutr. Biochem. 1997, 8, 300–311.
- Engelen, M.P.K.J.; Deutz, N.E.P. Is HMB an Effective Anabolic Agent to Improve Outcome in Older Diseased Populations? Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 207–213.
- Wilkinson, D.J.; Hossain, T.; Limb, M.C.; Phillips, B.E.; Lund, J.; Williams, J.P.; Brook, M.S.; Cegielski, J.; Philp, A.; Ashcroft, S.; et al. Impact of the Calcium Form of β-Hydroxy-β-Methylbutyrate upon Human Skeletal Muscle Protein Metabolism. Clin. Nutr. Edinb. Scotl. 2018, 37, 2068–2075.
- Smith, H.J.; Khal, J.; Tisdale, M.J. Downregulation of Ubiquitin-Dependent Protein Degradation in Murine Myotubes during Hyperthermia by Eicosapentaenoic Acid. Biochem. Biophys. Res. Commun. 2005, 332, 83–88.
- Holecek, M.; Muthny, T.; Kovarik, M.; Sispera, L. Effect of Beta-Hydroxy-Beta-Methylbutyrate (HMB) on Protein Metabolism in Whole Body and in Selected Tissues. Food Chem. Toxicol. 2009, 47, 255–259.
- Clark, R.H.; Feleke, G.; Din, M.; Yasmin, T.; Singh, G.; Khan, F.A.; Rathmacher, J.A. Nutritional Treatment for Acquired Immunodeficiency Virus-Associated Wasting Using β-Hydroxy β-Methylbutyrate, Glutamine, and Arginine: A Randomized, Double-Blind, Placebo-Controlled Study. J. Parenter. Enter. Nutr. 2000, 24, 133–139.
- Kuhls, D.A.; Rathmacher, J.A.; Musngi, M.D.; Frisch, D.A.; Nielson, J.; Barber, A.; MacIntyre, A.D.; Coates, J.E.; Fildes, J.J. Beta-Hydroxy-Beta-Methylbutyrate Supplementation in Critically Ill Trauma Patients. J. Trauma 2007, 62, 125–131; discussion 131–132.
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.; Hart, N.; Connolly, B.; Whelan, K. β-Hydroxy-β-Methylbutyrate and Its Impact on Skeletal Muscle Mass and Physical Function in Clinical Practice: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2019, 109, 1119–1132.
- Theadom, A.; Rodrigues, M.; Roxburgh, R.; Balalla, S.; Higgins, C.; Bhattacharjee, R.; Jones, K.; Krishnamurthi, R.; Feigin, V. Prevalence of Muscular Dystrophies: A Systematic Literature Review. Neuroepidemiology 2014, 43, 259–268.
- Straub, V.; Campbell, K.P. Muscular Dystrophies and the Dystrophin-Glycoprotein Complex. Curr. Opin. Neurol. 1997, 10, 168–175.
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Trifirò, G. Global Epidemiology of Duchenne Muscular Dystrophy: An Updated Systematic Review and Meta-Analysis. Orphanet J. Rare Dis. 2020, 15, 141.
- Del Rocío Cruz-Guzmán, O.; Rodríguez-Cruz, M.; Escobar Cedillo, R.E. Systemic Inflammation in Duchenne Muscular Dystrophy: Association with Muscle Function and Nutritional Status. BioMed Res. Int. 2015, 2015.
- Tidball, J.G. Inflammatory Processes in Muscle Injury and Repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R345–R353.
- Rodríguez-Cruz, M.; Cruz-Guzmán, O.D.R.; Almeida-Becerril, T.; Solís-Serna, A.D.; Atilano-Miguel, S.; Sánchez-González, J.R.; Barbosa-Cortés, L.; Ruíz-Cruz, E.D.; Huicochea, J.C.; Cárdenas-Conejo, A.; et al. Potential Therapeutic Impact of Omega-3 Long Chain-Polyunsaturated Fatty Acids on Inflammation Markers in Duchenne Muscular Dystrophy: A Double-Blind, Controlled Randomized Trial. Clin. Nutr. Edinb. Scotl. 2018, 37, 1840–1851.
- De Paepe, B.; De Bleecker, J.L. Cytokines and Chemokines as Regulators of Skeletal Muscle Inflammation: Presenting the Case of Duchenne Muscular Dystrophy. Mediat. Inflamm. 2013, 2013, 540370.
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol. Rev. 2016, 96, 253–305.
- Guiraud, S.; Davies, K.E. Pharmacological Advances for Treatment in Duchenne Muscular Dystrophy. Curr. Opin. Pharmacol. 2017, 34, 36–48.
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 1: Diagnosis, and Neuromuscular, Rehabilitation, Endocrine, and Gastrointestinal and Nutritional Management. Lancet Neurol. 2018, 17, 251–267.
- Emery, A.E. Population Frequencies of Inherited Neuromuscular Diseases—A World Survey. Neuromuscul. Disord. 1991, 1, 19–29.
- Wehling-Henricks, M.; Lee, J.J.; Tidball, J.G. Prednisolone Decreases Cellular Adhesion Molecules Required for Inflammatory Cell Infiltration in Dystrophin-Deficient Skeletal Muscle. Neuromuscul. Disord. NMD 2004, 14, 483–490.
- Payne, E.T.; Yasuda, N.; Bourgeois, J.M.; Devries, M.C.; Rodriguez, M.C.; Yousuf, J.; Tarnopolsky, M.A. Nutritional Therapy Improves Function and Complements Corticosteroid Intervention in Mdx Mice. Muscle Nerve 2006, 33, 66–77.
- Howell, J.J.; Manning, B.D. MTOR Couples Cellular Nutrient Sensing to Organismal Metabolic Homeostasis. Trends Endocrinol. Metab. 2011, 22, 94–102.
- Yang, H.; Rudge, D.G.; Koos, J.D.; Vaidialingam, B.; Yang, H.J.; Pavletich, N.P. MTOR Kinase Structure, Mechanism and Regulation. Nature 2013, 497, 217–223.
- Yamamoto, M.; Teramoto, K.; Katoh, H. Epidermal Growth Factor Promotes Glioblastoma Cell Death under Glucose Deprivation via Upregulation of XCT (SLC7A11). Cell. Signal. 2021, 78, 109874.
- Qian, B.; Yao, Z.; Yang, Y.; Li, N.; Wang, Q. Downregulation of SDCBP Inhibits Cell Proliferation and Induces Apoptosis by Regulating PI3K/AKT/MTOR Pathway in Gastric Carcinoma. Biotechnol. Appl. Biochem. 2022, 69, 240–247.
- Pimentel, G.D.; Rosa, J.C.; Lira, F.S.; Zanchi, N.E.; Ropelle, E.R.; Oyama, L.M.; Oller do Nascimento, C.M.; de Mello, M.T.; Tufik, S.; Santos, R.V. β-Hydroxy-β-Methylbutyrate (HMβ) Supplementation Stimulates Skeletal Muscle Hypertrophy in Rats via the MTOR Pathway. Nutr. Metab. 2011, 8, 11.
- Wan, H.; Zhu, J.; Su, G.; Liu, Y.; Hua, L.; Hu, L.; Wu, C.; Zhang, R.; Zhou, P.; Shen, Y.; et al. Dietary Supplementation with β-Hydroxy-β-Methylbutyrate Calcium during the Early Postnatal Period Accelerates Skeletal Muscle Fibre Growth and Maturity in Intra-Uterine Growth-Retarded and Normal-Birth-Weight Piglets. Br. J. Nutr. 2016, 115, 1360–1369.
- de, A. Costa Riela, N.; Alvim Guimarães, M.M.; Oliveira de Almeida, D.; Araujo, E.M.Q. Effects of Beta-Hydroxy-Beta-Methylbutyrate Supplementation on Elderly Body Composition and Muscle Strength: A Review of Clinical Trials. Ann. Nutr. Metab. 2021, 77, 16–22.
- Baier, S.; Johannsen, D.; Abumrad, N.; Rathmacher, J.A.; Nissen, S.; Flakoll, P. Year-Long Changes in Protein Metabolism in Elderly Men and Women Supplemented with a Nutrition Cocktail of β-Hydroxy-β-Methylbutyrate (HMB), L-Arginine, and L-Lysine. J. Parenter. Enter. Nutr. 2009, 33, 71–82.
- Stout, J.R.; Fukuda, D.H.; Kendall, K.L.; Smith-Ryan, A.E.; Moon, J.R.; Hoffman, J.R. β-Hydroxy-β-Methylbutyrate (HMB) Supplementation and Resistance Exercise Significantly Reduce Abdominal Adiposity in Healthy Elderly Men. Exp. Gerontol. 2015, 64, 33–34.
- Ellis, A.C.; Hunter, G.R.; Goss, A.M.; Gower, B.A. Oral Supplementation with Beta-Hydroxy-Beta-Methylbutyrate, Arginine, and Glutamine Improves Lean Body Mass in Healthy Older Adults. J. Diet. Suppl. 2019, 16, 281–293.
- Ortiz, A. β-Hydroxy-β-Methylbutyrate Supplementation in Special Populations. Strength Cond. J. 2013, 35, 73–77.
- Kornasio, R.; Riederer, I.; Butler-Browne, G.; Mouly, V.; Uni, Z.; Halevy, O. β-Hydroxy-β-Methylbutyrate (HMB) Stimulates Myogenic Cell Proliferation, Differentiation and Survival via the MAPK/ERK and PI3K/Akt Pathways. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2009, 1793, 755–763.
- Portal, S.; Zadik, Z.; Rabinowitz, J.; Pilz-Burstein, R.; Adler-Portal, D.; Meckel, Y.; Cooper, D.M.; Eliakim, A.; Nemet, D. The Effect of HMB Supplementation on Body Composition, Fitness, Hormonal and Inflammatory Mediators in Elite Adolescent Volleyball Players: A Prospective Randomized, Double-Blind, Placebo-Controlled Study. Eur. J. Appl. Physiol. 2011, 111, 2261–2269.
- Amini-Khoei, H.; Saghaei, E.; Mobini, G.-R.; Sabzevary-Ghahfarokhi, M.; Ahmadi, R.; Bagheri, N.; Mokhtari, T. Possible Involvement of PI3K/AKT/MTOR Signaling Pathway in the Protective Effect of Selegiline (Deprenyl) against Memory Impairment Following Ischemia Reperfusion in Rat. Neuropeptides 2019, 77, 101942.
- Brooks, D.L.; Garza, A.E.; Katayama, I.A.; Romero, J.R.; Adler, G.K.; Pojoga, L.H.; Williams, G.H. Aldosterone Modulates the Mechanistic Target of Rapamycin Signaling in Male Mice. Endocrinology 2019, 160, 716–728.
- Jhanwar-Uniyal, M.; Wainwright, J.V.; Mohan, A.L.; Tobias, M.E.; Murali, R.; Gandhi, C.D.; Schmidt, M.H. Diverse Signaling Mechanisms of MTOR Complexes: MTORC1 and MTORC2 in Forming a Formidable Relationship. Adv. Biol. Regul. 2019, 72, 51–62.
- Kumari, S.; Khan, S.; Sekhri, R.; Mandil, H.; Behrman, S.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. Protein Kinase D1 Regulates Metabolic Switch in Pancreatic Cancer via Modulation of MTORC1. Br. J. Cancer 2020, 122, 121–131.
- Koundouros, N.; Poulogiannis, G. Reprogramming of Fatty Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 4–22.
- Horman, S.; Vertommen, D.; Heath, R.; Neumann, D.; Mouton, V.; Woods, A.; Schlattner, U.; Wallimann, T.; Carling, D.; Hue, L. Insulin Antagonizes Ischemia-Induced Thr172 Phosphorylation of AMP-Activated Protein Kinase α-Subunits in Heart via Hierarchical Phosphorylation of Ser485/491. J. Biol. Chem. 2006, 281, 5335–5340.
- Boukouris, A.E.; Zervopoulos, S.D.; Michelakis, E.D. Metabolic Enzymes Moonlighting in the Nucleus: Metabolic Regulation of Gene Transcription. Trends Biochem. Sci. 2016, 41, 712–730.
- Fitschen, P.J.; Wilson, G.J.; Wilson, J.M.; Wilund, K.R. Efficacy of β-Hydroxy-β-Methylbutyrate Supplementation in Elderly and Clinical Populations. Nutrition 2013, 29, 29–36.
- Kim, J.-S.; V Khamoui, A.; Jo, E.; Park, B.-S.; Jun Lee, W. β-Hydroxy-β-Methylbutyrate as a Countermeasure for Cancer Cachexia: A Cellular and Molecular Rationale. Anti-Cancer Agents Med. Chem. 2013, 13, 1188–1196.
- Peter, A.K.; Crosbie, R.H. Hypertrophic Response of Duchenne and Limb-Girdle Muscular Dystrophies Is Associated with Activation of Akt Pathway. Exp. Cell Res. 2006, 312, 2580–2591.
- Alway, S.E.; Pereira, S.L.; Edens, N.K.; Hao, Y.; Bennett, B.T. β-Hydroxy-β-Methylbutyrate (HMB) Enhances the Proliferation of Satellite Cells in Fast Muscles of Aged Rats during Recovery from Disuse Atrophy. Exp. Gerontol. 2013, 48, 973–984.
- Salto, R.; Vílchez, J.D.; Girón, M.D.; Cabrera, E.; Campos, N.; Manzano, M.; Rueda, R.; López-Pedrosa, J.M. β-Hydroxy-β-Methylbutyrate (HMB) Promotes Neurite Outgrowth in Neuro2a Cells. PLoS ONE 2015, 10, e0135614.
- Zhong, Y.; Zeng, L.; Deng, J.; Duan, Y.; Li, F. β-Hydroxy-β-Methylbutyrate (HMB) Improves Mitochondrial Function in Myocytes through Pathways Involving PPARβ/δ and CDK4. Nutrition 2019, 60, 217–226.
- Parveen, A.; Wen, Y.; Roy, A.; Kumar, A. Therapeutic Targeting of PTEN in Duchenne Muscular Dystrophy. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 8–9.
- Zheng, J.; Zheng, C.; Song, B.; Guo, Q.; Zhong, Y.; Zhang, S.; Zhang, L.; Duan, G.; Li, F.; Duan, Y. HMB Improves Lipid Metabolism of Bama Xiang Mini-Pigs via Modulating the Bacteroidetes-Acetic Acid-AMPKα Axis. Front. Microbiol. 2021, 12, 736997.
- Wattin, M.; Gaweda, L.; Muller, P.; Baritaud, M.; Scholtes, C.; Lozano, C.; Gieseler, K.; Kretz-Remy, C. Modulation of Protein Quality Control and Proteasome to Autophagy Switch in Immortalized Myoblasts from Duchenne Muscular Dystrophy Patients. Int. J. Mol. Sci. 2018, 19, 178.
- Mirza, K.A.; Pereira, S.L.; Voss, A.C.; Tisdale, M.J. Comparison of the Anticatabolic Effects of Leucine and Ca-β-Hydroxy-β-Methylbutyrate in Experimental Models of Cancer Cachexia. Nutrition 2014, 30, 807–813.
- Gross, D.N.; Van Den Heuvel, A.P.J.; Birnbaum, M.J. The Role of FoxO in the Regulation of Metabolism. Oncogene 2008, 27, 2320–2336.
- Zubair, A.; Frieri, M. Role of Nuclear Factor-ĸB in Breast and Colorectal Cancer. Curr. Allergy Asthma Rep. 2013, 13, 44–49.
- Miyake, S.; Ogo, A.; Kubota, H.; Teramoto, F.; Hirai, T. β-Hydroxy-β-Methylbutyrate Suppresses NF-\sckB Activation and IL6 Production in TE-1 Cancer Cells. In Vivo 2019, 33, 353–358.
- Messina, S.; Vita, G.L.; Aguennouz, M.; Sframeli, M.; Romeo, S.; Rodolico, C.; Vita, G. Activation of NF-KappaB Pathway in Duchenne Muscular Dystrophy: Relation to Age. Acta Myol. 2011, 30, 16–23.
- Gallardo, F.S.; Córdova-Casanova, A.; Brandan, E. The Linkage between Inflammation and Fibrosis in Muscular Dystrophies: The Axis Autotaxin–Lysophosphatidic Acid as a New Therapeutic Target? J. Cell Commun. Signal. 2021, 15, 317–334.
- Dudek, M.; Lohr, K.; Donakonda, S.; Baumann, T.; Lüdemann, M.; Hegenbarth, S.; Duebbel, L.; Eberhagen, C.; Michailidou, S.; Yassin, A. IL6-Induced FOXO1 Activity Determines the Dynamics of Metabolism in CD8 T Cells Cross-Primed by Liver Sinusoidal Endothelial Cells. Cell Rep. 2022, 38, 110389.
- Ait Ahmed, Y.; Fu, Y.; Rodrigues, R.M.; He, Y.; Guan, Y.; Guillot, A.; Ren, R.; Feng, D.; Hidalgo, J.; Ju, C. Kupffer Cell Restoration after Partial Hepatectomy Is Mainly Driven by Local Cell Proliferation in IL6-Dependent Autocrine and Paracrine Manners. Cell. Mol. Immunol. 2021, 18, 2165–2176.
- Eley, H.L.; Russell, S.T.; Tisdale, M.J. Mechanism of Attenuation of Muscle Protein Degradation Induced by Tumor Necrosis Factor-α and Angiotensin II by β-Hydroxy-β-Methylbutyrate. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E1417–E1426.
- Parrotta, E.I.; Lucchino, V.; Scaramuzzino, L.; Scalise, S.; Cuda, G. Modeling Cardiac Disease Mechanisms Using Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Progress, Promises and Challenges. Int. J. Mol. Sci. 2020, 21, 4354.
- Sandri, M.; El Meslemani, A.H.; Sandri, C.; Schjerling, P.; Vissing, K.; Andersen, J.L.; Rossini, K.; Carraro, U.; Angelini, C. Caspase 3 Expression Correlates with Skeletal Muscle Apoptosis in Duchenne and Facioscapulo Human Muscular Dystrophy. A Potential Target for Pharmacological Treatment? J. Neuropathol. Exp. Neurol. 2001, 60, 302–312.
- Almushayt, S.J.; Hussain, S.; Wilkinson, D.J.; Selby, N.M. A Systematic Review of the Acute Effects of Hemodialysis on Skeletal Muscle Perfusion, Metabolism, and Function. Kidney Int. Rep. 2020, 5, 307–317.
- Noh, K.K.; Chung, K.W.; Choi, Y.J.; Park, M.H.; Jang, E.J.; Park, C.H.; Yoon, C.; Kim, N.D.; Kim, M.K.; Chung, H.Y. β-Hydroxy β-Methylbutyrate Improves Dexamethasone-Induced Muscle Atrophy by Modulating the Muscle Degradation Pathway in SD Rat. PLoS ONE 2014, 9, e102947.
- Hao, Y.; Jackson, J.R.; Wang, Y.; Edens, N.; Pereira, S.L.; Alway, S.E. β-Hydroxy-β-Methylbutyrate Reduces Myonuclear Apoptosis during Recovery from Hind Limb Suspension-Induced Muscle Fiber Atrophy in Aged Rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R701–R715.
- Alexander, M.S.; Casar, J.C.; Motohashi, N.; Vieira, N.M.; Eisenberg, I.; Marshall, J.L.; Gasperini, M.J.; Lek, A.; Myers, J.A.; Estrella, E.A. MicroRNA-486–Dependent Modulation of DOCK3/PTEN/AKT Signaling Pathways Improves Muscular Dystrophy–Associated Symptoms. J. Clin. Investig. 2014, 124, 2651–2667.
- Yao, C.; Guo, G.; Huang, R.; Tang, C.; Zhu, Q.; Cheng, Y.; Kong, L.; Ren, J.; Fang, M. Manual Therapy Regulates Oxidative Stress in Aging Rat Lumbar Intervertebral Discs through the SIRT1/FOXO1 Pathway. Aging 2022, 14, 2400.
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of Glycogen Synthase Kinase-3 by Insulin Mediated by Protein Kinase B. Nature 1995, 378, 785–789.
- Kulkarni, R.N.; Brüning, J.C.; Winnay, J.N.; Postic, C.; Magnuson, M.A.; Kahn, C.R. Tissue-Specific Knockout of the Insulin Receptor in Pancreatic Beta Cells Creates an Insulin Secretory Defect Similar to That in Type 2 Diabetes. Cell 1999, 96, 329–339.
- Saltiel, A.R.; Kahn, C.R. Insulin Signalling and the Regulation of Glucose and Lipid Metabolism. Nature 2001, 414, 799–806.
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715.
- Shaham, O.; Wei, R.; Wang, T.J.; Ricciardi, C.; Lewis, G.D.; Vasan, R.S.; Carr, S.A.; Thadhani, R.; Gerszten, R.E.; Mootha, V.K. Metabolic Profiling of the Human Response to a Glucose Challenge Reveals Distinct Axes of Insulin Sensitivity. Mol. Syst. Biol. 2008, 4, 214.
- Sharma, U.; Atri, S.; Sharma, M.C.; Sarkar, C.; Jagannathan, N.R. Skeletal Muscle Metabolism in Duchenne Muscular Dystrophy (DMD): An in-Vitro Proton NMR Spectroscopy Study. Magn. Reson. Imaging 2003, 21, 145–153.
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of Leucine and Its Metabolite β-Hydroxy-β-Methylbutyrate on Human Skeletal Muscle Protein Metabolism. J. Physiol. 2013, 591, 2911–2923.
- Schnuck, J.K.; Johnson, M.A.; Gould, L.M.; Gannon, N.P.; Vaughan, R.A. Acute β-Hydroxy-β-Methyl Butyrate Suppresses Regulators of Mitochondrial Biogenesis and Lipid Oxidation While Increasing Lipid Content in Myotubes. Lipids 2016, 51, 1127–1136.
- Sharawy, M.H.; El-Awady, M.S.; Megahed, N.; Gameil, N.M. The Ergogenic Supplement β-Hydroxy-β-Methylbutyrate (HMB) Attenuates Insulin Resistance through Suppressing GLUT-2 in Rat Liver. Can. J. Physiol. Pharmacol. 2016, 94, 488–497.
- Chiu, W.; Hsun, Y.-H.; Chang, K.-J.; Yarmishyn, A.A.; Hsiao, Y.-J.; Chien, Y.; Chien, C.-S.; Ma, C.; Yang, Y.-P.; Tsai, P.-H. Current Genetic Survey and Potential Gene-Targeting Therapeutics for Neuromuscular Diseases. Int. J. Mol. Sci. 2020, 21, 9589.
- Shirvani, H.; Rahmati-Ahmadabad, S.; Kowsari, E.; Fry, H.; Kazemi, M.; Kaviani, M. Effects of 2-Week HMB-FA Supplementation with or without Eccentric Resistance Exercise on Expression of Some Genes Related to Muscle Protein Turnover and Serum Irisin and IGF1 IGF1 Concentrations. Gene 2020, 760, 145018.
- Kornegay, J.N.; Childers, M.K.; Bogan, D.J.; Bogan, J.R.; Nghiem, P.; Wang, J.; Fan, Z.; Howard, J.F.; Schatzberg, S.J.; Dow, J.L.; et al. The Paradox of Muscle Hypertrophy in Muscular Dystrophy. Phys. Med. Rehabil. Clin. N. Am. 2012, 23, 149–172.
- Söderpalm, A.-C.; Magnusson, P.; Åhlander, A.-C.; Karlsson, J.; Kroksmark, A.-K.; Tulinius, M.; Swolin-Eide, D. Low Bone Mineral Density and Decreased Bone Turnover in Duchenne Muscular Dystrophy. Neuromuscul. Disord. 2007, 17, 919–928.
- Świetlicka, I.; Muszyński, S.; Prein, C.; Clausen-Schaumann, H.; Aszodi, A.; Arciszewski, M.B.; Blicharski, T.; Gagoś, M.; Świetlicki, M.; Dobrowolski, P.; et al. Fourier Transform Infrared Microspectroscopy Combined with Principal Component Analysis and Artificial Neural Networks for the Study of the Effect of β-Hydroxy-β-Methylbutyrate (HMB) Supplementation on Articular Cartilage. Int. J. Mol. Sci. 2021, 22, 9189.
- Cotán, D.; Villanueva Paz, M.; Alcocer-Gómez, E.; Garrido-Maraver, J.; de la Mata, M.; Delgado Pavón, A.; de Lavera, I.; Galán, F.; Ybot-González, P.; A Sánchez-Alcázar, J. AMPK as a Target in Rare Diseases. Curr. Drug Targets 2016, 17, 921–931.
- Gonçalves, M.A.; Janssen, J.M.; Nguyen, Q.G.; Athanasopoulos, T.; Hauschka, S.D.; Dickson, G.; De Vries, A.A. Transcription Factor Rational Design Improves Directed Differentiation of Human Mesenchymal Stem Cells Into Skeletal Myocytes. Mol. Ther. 2011, 19, 1331–1341.
- Gonçalves, M.A.; Swildens, J.; Holkers, M.; Narain, A.; Van Nierop, G.P.; Van De Watering, M.J.; Knaän-Shanzer, S.; De Vries, A.A. Genetic Complementation of Human Muscle Cells via Directed Stem Cell Fusion. Mol. Ther. 2008, 16, 741–748.
- Tian, L.J.; Cao, J.H.; Deng, X.Q.; Zhang, C.L.; Qian, T.; Song, X.X.; Huang, B.S. Gene Expression Profiling of Duchenne Muscular Dystrophy Reveals Characteristics along Disease Progression. Genet. Mol. Res. 2014, 13, 1402–1411.
- Peterson, A.L.; Qureshi, M.A.; Ferket, P.R.; Jr, J.C.F. In Vitro Exposure with β-Hydroxy-β-Methylbutyrate Enhances Chicken Macrophage Growth and Function. Vet. Immunol. Immunopathol. 1999, 67, 67–78.
- Finkel, R.S.; Finanger, E.; Vandenborne, K.; Sweeney, H.L.; Tennekoon, G.; Shieh, P.B.; Willcocks, R.; Walter, G.; Rooney, W.D.; Forbes, S.C. Disease-Modifying Effects of Edasalonexent, an NF-ΚB Inhibitor, in Young Boys with Duchenne Muscular Dystrophy: Results of the MoveDMD Phase 2 and Open Label Extension Trial. Neuromuscul. Disord. 2021, 31, 385–396.
- Luce, L.N.; Dalamon, V.; Ferrer, M.; Parma, D.; Szijan, I.; Giliberto, F. MLPA Analysis of an Argentine Cohort of Patients with Dystrophinopathy: Association of Intron Breakpoints Hot Spots with STR Abundance in DMD Gene. J. Neurol. Sci. 2016, 365, 22–30.
- Sadek, A.A.; Mahmoud, S.M.; El-Aal, M.A.; Allam, A.A.; El-Halim, W.I.A. Evaluation of Cardiac Functions in Children with Duchenne Muscular Dystrophy: A Prospective Case-Control Study. Electron. Phys. 2017, 9, 5732–5739.
- Tsuchiya, Y.; Hirayama, K.; Ueda, H.; Ochi, E. Two and Four Weeks of β-Hydroxy-β-Methylbutyrate (HMB) Supplementations Reduce Muscle Damage Following Eccentric Contractions. J. Am. Coll. Nutr. 2019, 38, 373–379.
- Gazzerro, P.; Proto, M.C.; Gangemi, G.; Malfitano, A.M.; Ciaglia, E.; Pisanti, S.; Santoro, A.; Laezza, C.; Bifulco, M. Pharmacological Actions of Statins: A Critical Appraisal in the Management of Cancer. Pharmacol. Rev. 2012, 64, 102–146.
- Zhou, Q.; Liao, J.K. Pleiotropic Effects of Statins: Basic Research and Clinical Perspectives. Circ. J. 2010, 74, 818–826.
- Yamada, A.K.; Ferretti, R.; Matsumura, C.Y.; Antunes, L.; da Silva, C.A.; Pertille, A. Beta-Hydroxy-Beta-Methylbutyrate Associated with Low-Intensity Exercise Training Improves Skeletal Muscle Regeneration through the IGF-Akt Pathway. Braz. J. Med. Biol. Res. 2022, 55, e11597.
- Laoutaris, I.D.; Adamopoulos, S.; Manginas, A.; Panagiotakos, D.B.; Cokkinos, D.V.; Dritsas, A. Inspiratory Work Capacity Is More Severely Depressed than Inspiratory Muscle Strength in Patients with Heart Failure: Novel Applications for Inspiratory Muscle Training. Int. J. Cardiol. 2016, 221, 622–626.
- Rahman, F.A.; Quadrilatero, J. Mitochondrial Apoptotic Signaling Involvement in Remodeling During Myogenesis and Skeletal Muscle Atrophy. Semin. Cell Dev. Biol. 2023, 143, 66–74.
- Essid, S.M.; Bevington, A.; Brunskill, N.J. Proinsulin C-Peptide Enhances Cell Survival and Protects against Simvastatin-Induced Myotoxicity in L6 Rat Myoblasts. Int. J. Mol. Sci. 2019, 20, 1654.
- Bongiovanni, T.; Genovesi, F.; Nemmer, M.; Carling, C.; Alberti, G.; Howatson, G. Nutritional Interventions for Reducing the Signs and Symptoms of Exercise-Induced Muscle Damage and Accelerate Recovery in Athletes: Current Knowledge, Practical Application and Future Perspectives. Eur. J. Appl. Physiol. 2020, 120, 1965–1996.
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A Practical Guide to the Monitoring and Management of the Complications of Systemic Corticosteroid Therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30.
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31.
- Gloss, D.; Moxley, R.T.; Ashwal, S.; Oskoui, M. Practice Guideline Update Summary: Corticosteroid Treatment of Duchenne Muscular Dystrophy: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 465–472.
- Pasquini, F.; Guerin, C.; Blake, D.; Davies, K.; Karpati, G.; Holland, P. The Effect of Glucocorticoids on the Accumulation of Utrophin by Cultured Normal and Dystrophic Human Skeletal Muscle Satellite Cells. Neuromuscul. Disord. 1995, 5, 105–114.




