Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Capaldo | -- | 3309 | 2023-08-31 10:21:07 | | | |
| 2 | Peter Tang | Meta information modification | 3309 | 2023-08-31 10:35:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Capaldo, A. Adrenal Gland of Squamata. Encyclopedia. Available online: https://encyclopedia.pub/entry/48678 (accessed on 25 January 2026).
Capaldo A. Adrenal Gland of Squamata. Encyclopedia. Available at: https://encyclopedia.pub/entry/48678. Accessed January 25, 2026.
Capaldo, Anna. "Adrenal Gland of Squamata" Encyclopedia, https://encyclopedia.pub/entry/48678 (accessed January 25, 2026).
Capaldo, A. (2023, August 31). Adrenal Gland of Squamata. In Encyclopedia. https://encyclopedia.pub/entry/48678
Capaldo, Anna. "Adrenal Gland of Squamata." Encyclopedia. Web. 31 August, 2023.
Copy Citation
The adrenal gland is a complex endocrine organ composed of two components: a steroidogenic tissue, which produces steroid hormones, and a chromaffin tissue, which mainly produces norepinephrine and epinephrine. Through evolution, their relationships with each other changed. They begin as isolated chromaffin and steroidogenic cell aggregates, typical of fish, and end with the advanced compact gland, typical of mammals, which consists of an external steroidogenic cortical zone and an internal chromaffin medullary zone. The adrenal gland of reptiles is unique because, with few exceptions, it is near the gonads and genital ducts, and the chromaffin and steroidogenic tissues are closely associated.
adrenal gland
chromaffin tissue
NE/E cell ratio
Reptilia
Squamata
steroidogenic tissue
1. The Adrenal Gland
All vertebrates have chromaffin tissue, derived from neural crests, which mainly produces the catecholamines norepinephrine (NE) and epinephrine (E), and steroidogenic tissue, of mesodermal origin, which produces steroid hormones. However, the topographical relationships between these two tissues and their own anatomical positions are different in different groups of vertebrates. During evolution, a trend toward developing a close anatomical relationship between these two types of tissue can be observed. The two tissues are separated in hagfish and lampreys, and only occasionally are the chromaffin cells located near the steroidogenic cells. The chromaffin cells can be found within the heart and in the great veins returning blood to the heart [1], and clusters of steroidogenic cells are associated with the posterior cardinal veins and the mesonephric parenchyma [2][3]. Also, in cartilaginous fishes, the steroidogenic and the chromaffin tissues are separated, and their organization is different in different species. In some elasmobranchs and holocephalans, there is one unpaired adrenal gland, made up of adrenocortical cells and placed between the posterior ends of the kidneys. However, in other species, the steroidogenic tissue forms paired strands along the medial border of the posterior kidney and small islets on the anterior surface of the kidneys [3]. The chromaffin cells are associated with the paravertebral autonomic ganglia, called auxiliary bodies [1]. In bony fish, the distribution and localization of the steroidogenic and chromaffin tissues and their relationships with the kidney differ in the various species. In teleosts, depending on the species, steroidogenic cells are present in the head kidney and are frequently associated with the dorsal posterior cardinal veins [3]. The chromaffin cells can be found in the walls of the posterior cardinal veins and the head kidney, where they may be single or may form groups of several cells, separate from steroidogenic cells, or intermingled with them [1][3] (Figure 1).
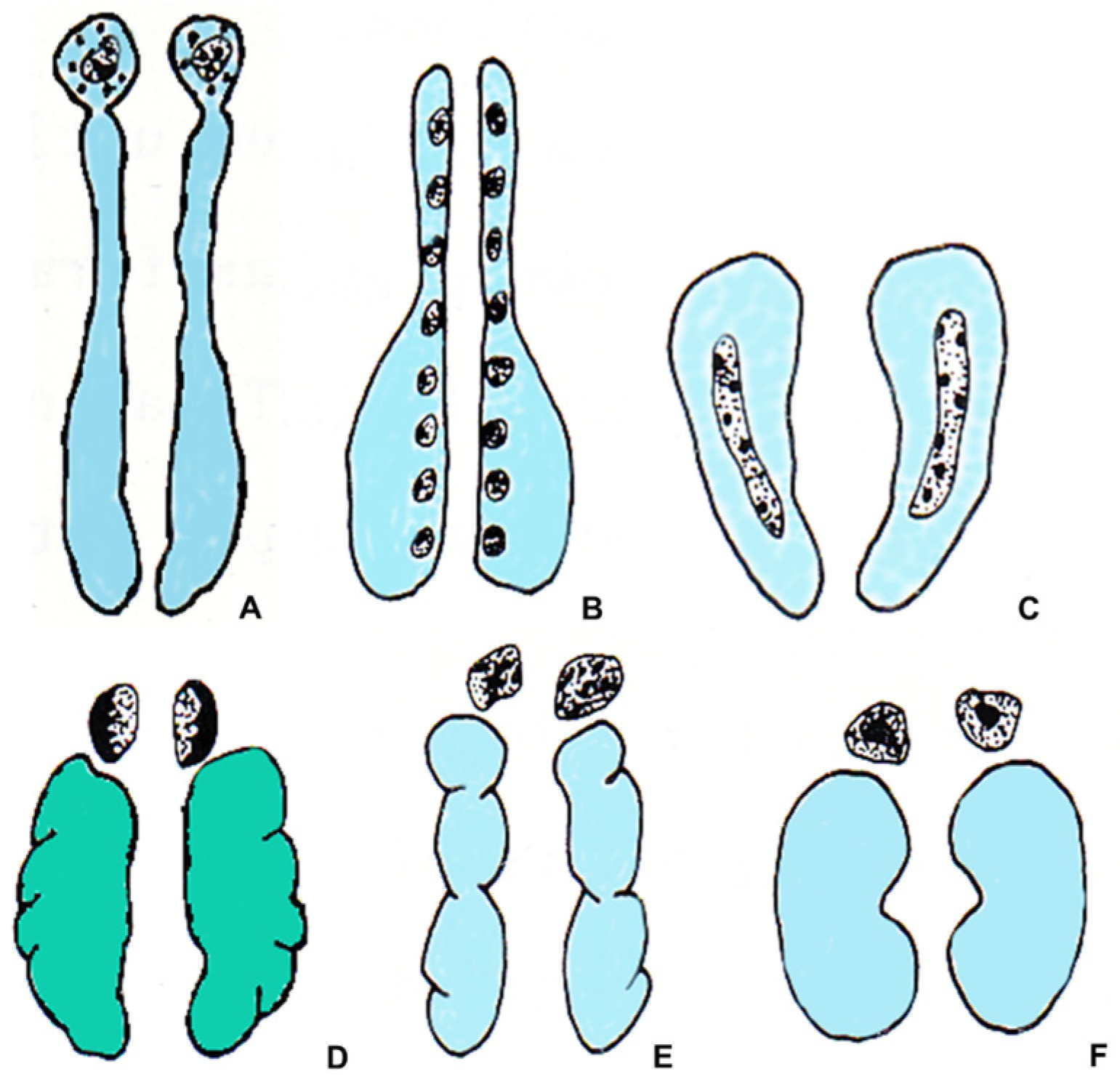
Figure 1. Schematic drawing showing the relationships between the steroidogenic tissue (gray), the chromaffin tissue (black), the kidneys (light blue), and the gonads (green) in vertebrates. (A) teleost fish; (B) urodele amphibian; (C) anuran amphibian; (D) lacertilian reptile; (E) bird; (F) mammal.
In amphibians, the organization of the steroidogenic and chromaffin tissues in urodeles differs from those of anurans. In urodeles, the steroidogenic tissue forms many islets, also containing sporadic chromaffin cells. The islets are scattered on the ventral surface of the mesonephric kidney, close to its medial margin, and are separated from each other. The degree of association between the chromaffin and the steroidogenic cells is variable. In Salamandridae and Plethodontidae, the two types of cells are mixed [1][4]. In anurans, the steroidogenic tissue located on the ventral surface of the kidneys forms continuous strands containing clusters of chromaffin cells. In both urodeles and anurans, the degree of compactness of the gland and the aggregation of steroidogenic and chromaffin cells is variable. Generally, it increases in the transition from basal to advanced families [1][5]. Moreover, in ranid anurans, in addition to the steroidogenic and chromaffin cells, a third type of cell has been found, the summer or Stilling cells, only present in summer and with a still unknown function [3].
In reptiles, the steroidogenic and the chromaffin tissues form a discrete gland that is in contact with the gonads and genital ducts, except for chelonians, where the gland is in contact with the ventral surface of the kidney [6]. Although steroidogenic and chromaffin tissues are generally associated, there are considerable variations between species in their distribution. It is not possible to present a valid general picture for the whole class [2][7]. A discrete gland located near the kidneys is also observed in birds and mammals. In birds, the adrenal gland may be two separate structures in contact with each other or a single median one. The steroidogenic tissue forms radially arranged cords mixed with blood vessels and chromaffin cell groups [8]. Finally, in mammals, the steroidogenic tissue forms the peripheral portion of the gland, called the cortex, surrounding an inner part formed by the chromaffin tissue, called the medulla, an arrangement allowing a considerable degree of mixing at the border between the two tissues (Figure 1) [1][2][3][7].
According to Grassi Milano [6], the different types of organization of the adrenal gland in amniotes (reptiles, birds, and mammals) are congruent with the main phyletic lines of this group. In chelonians, having a “diffused” structural pattern, the adrenal gland is placed on the ventral surface of the kidney, as in anuran amphibians. Such a condition, in which the steroidogenic cells and the chromaffin cells are mixed together, is considered plesiomorphic in comparison to other amniotes. In the other amniotes, the adrenal gland has a “compact” structural pattern, considered apomorphic. Moreover, in amniotes, three conditions can be observed in the relationships existing between the steroidogenic and the chromaffin cells: (1) The typical condition of Rhynchocephalia and Squamata, in which the chromaffin cells are present on the dorsal region of the gland; (2) the typical condition of Crocodilia and birds, in which the chromaffin cells are dispersed among the steroidogenic cells; and (3) the typical condition of mammals (except prototherians), in which the chromaffin cells form an inner medulla, surrounded by a steroidogenic cortex.
The different anatomical positions of the gland, which is made up of steroidogenic and chromaffin tissues, justify the different nomenclature of this gland in different vertebrate groups. Indeed, when the gland is located between the kidneys, as in amphibians, it is normally referred to as interrenal. Additionally, when the gland, as in most reptiles, does not have an anatomical relationship with the kidney but with gonads and genital ducts, it is preferred to call it the adrenal gland. In mammals, the gland is called suprarenal because it is generally found on the cephalic pole of the kidney. However, the term adrenal is so widespread that it is generally used, regardless of position [2].
2. Paracrine Relationships in the Adrenal Gland
Numerous experimental data have shown the existence of paracrine relationships between the steroidogenic and the chromaffin tissues. In mammals glucocorticoids stimulate the activity of the tyrosine hydroxylase (TH) enzyme, the rate-limiting enzyme in catecholamine biosynthesis [9][10] and the phenylethanolamine-N-methyltransferase (PNMT) enzyme, the last of the catecholamines biosynthetic pathway, converting NE into E [11][12][13][14][15] (Figure 2). Aldosterone elevates PNMT activity too, but with less power than glucocorticoids [16].
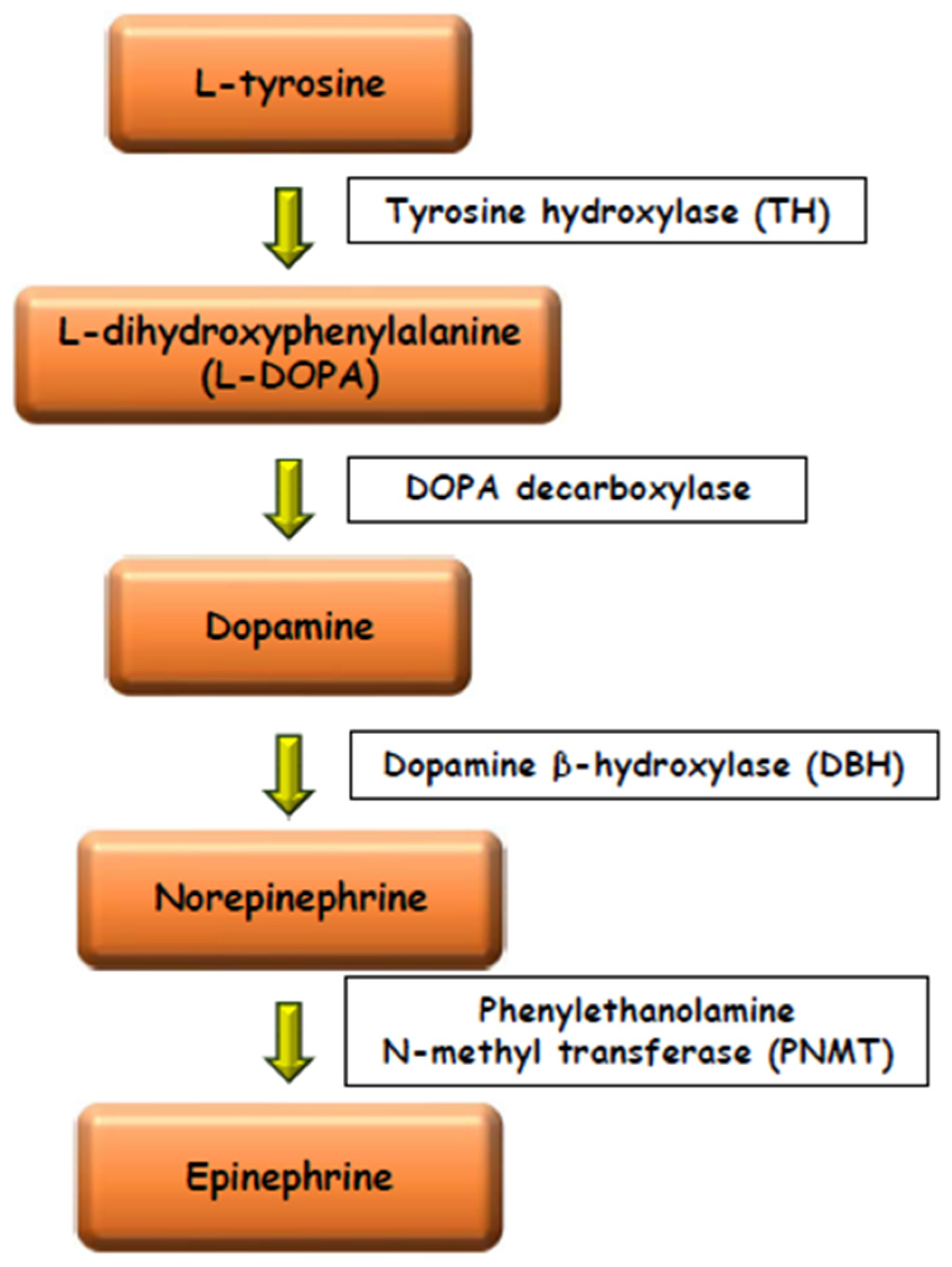
Figure 2. Schematic drawing of the catecholamines biosynthetic pathway.
Glucocorticoids are believed to upregulate catecholamine synthesis, storage, and secretion [15]. Moreover, they are essential for postnatal maintenance of adrenal and extra-adrenal chromaffin cells [17]. In birds, the adrenocorticotropic hormone (ACTH) influences TH enzyme [10], increases PNMT activity and the conversion of NE into E via intraglandular glucocorticoid production [14][18]. In reptiles, as in mammals, a stimulating action of glucocorticoids on the enzyme PNMT has been found [19][20][21] whereas in fish and amphibians such stimulating action has not been observed [1][22][23]. In turn, catecholamines and the neuropeptides co-released with them from the chromaffin tissue, may influence the synthesis and release of the steroid hormones from the steroidogenic tissue in a paracrine way. This paracrine relationship has been observed in many vertebrates, including fish and amphibians [1][9]. The resulting crosstalk that settles between the two components of the gland may be important to synchronize the response of this gland to stress [24]. From this point of view, the evolutionary tendency to the progressive coalescence of steroidogenic and chromaffin tissues has optimized the ability of the organisms to respond to stressful situations and supports the widely accepted opinion that the progressive concentration of the glandular tissues has been a selectively favorable process [25].
3. The Morphology of the Chromaffin and the Steroidogenic Tissues
The morphology of the chromaffin and the steroidogenic tissues can be studied with techniques utilized in light and electron microscopy. As for chromaffin tissue, it should be noted that two situations have been observed: the presence of two types of chromaffin cells, one of which contains NE and the other E, or the presence of only one type of chromaffin cell that produces both catecholamines. The first type of organization is generally present: (a) in fishes; (b) in anurans amphibians, where NE and E cells are usually intermingled; (c) in reptiles, where the distribution of NE and E cells is different in the different species; and (d) in birds, where the chromaffin cells are usually mixed and do not have any preferential location. As regards urodele amphibians, two subtypes of chromaffin cells were observed [4], whereas in Triturus carnifex, a single type of chromaffin cell was found, producing both NE and E, correlated to the environmental temperature [26][27] and the reproductive cycle [28]. In mammals, generally NE and E cells have been found, but their relative quantity and their topographical location are different in different species. For example, about 75% of the chromaffin cells in humans and bovine animals, and 80–85% in the rat, are E cells. In the rat, but not in bovine animals, the NE cells are generally located at the boundary between the cortex and the medulla, whereas in other species the E cells are peripherally located [2][29][30][31][32].
The two types of chromaffin cells can be distinguished with histochemical staining, using a fixative based on potassium dichromate, the Wood’s fixative, a mixture of 2.5% potassium dichromate and 1% sodium sulphate (buffered at pH 4.1 with 5 M acetate buffer) and 10% formaldehyde. Indeed, the oxidation with potassium dichromate produces brown insoluble pigments from both NE and E, which can be subsequently distinguished using two histochemical stains: the Wood stain and the Giemsa stain [33][34]. The Wood stain, a mixture of eosin and aniline blue, buffered at pH 4 with 5 M acetate buffer, stains the NE cells gold and the E cells orange red. A similar staining is also obtained with a trichromic histological stain, the Mallory staining, with which the NE cells appear gold yellow, and the E cells appear red. Moreover, this staining has in addition the advantage of staining well also the steroidogenic tissue, that appears light pink. Finally, the Giemsa solution, modified according to Pearse [34], a mixture of eosin blue and methylene blue, stains the NE cells dark green and the E cells light green [20][21] (Figure 3).
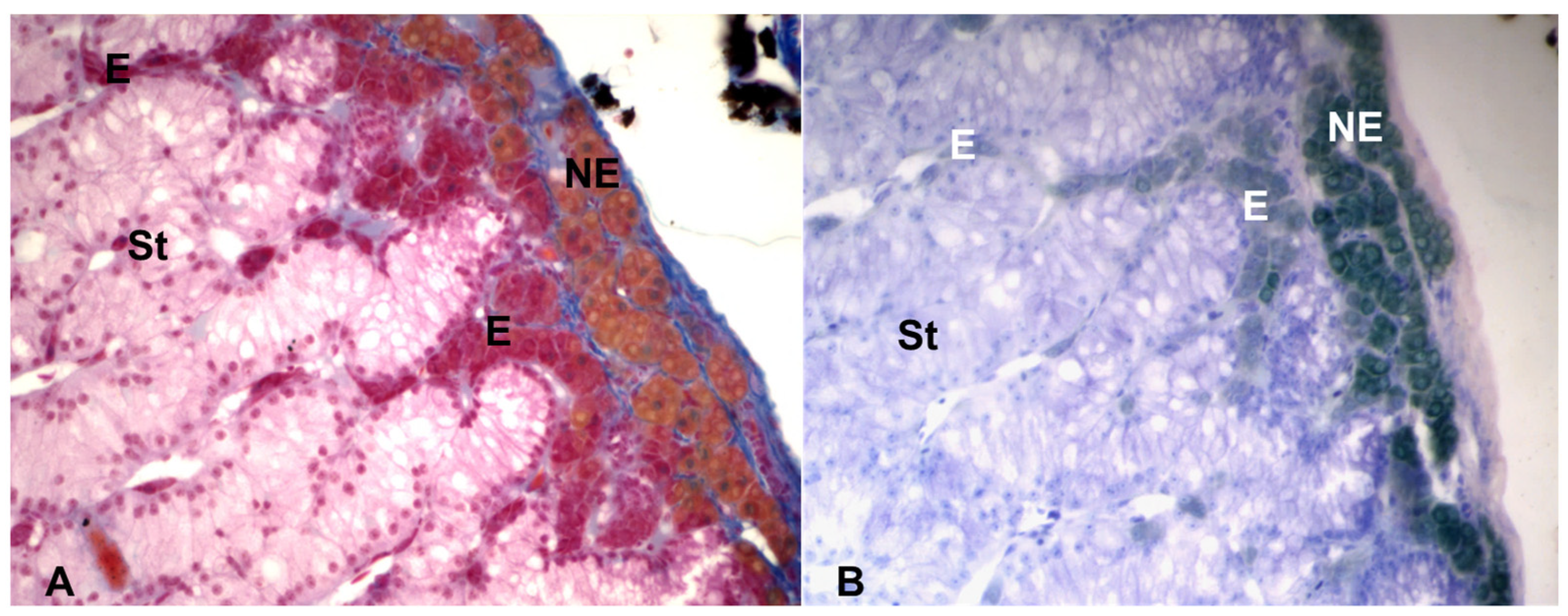
Figure 3. Light microscopy of the adrenal gland of the lacertid, Podarcis siculus. (A) Mallory stain, showing the NE cells gold yellow, the E cells red and the steroidogenic (St) tissue light pink. (B) Giemsa stain, showing the NE cells dark green and the E cells light green. Magnification: 400×.
When only one type of chromaffin cell, synthesizing both catecholamines, is present, the histochemical stains produce a mixed staining. In that case, the best method to study the chromaffin cells is electron microscopy, with which the simultaneous presence of the two types of granules in the same cell, as well as the presence of separate NE and E chromaffin cells, can be observed. Indeed, such a distinction can be obtained by using a first fixation with glutaraldehyde followed by a post-fixation with osmium tetroxide. Glutaraldehyde induces the formation of an insoluble complex with NE, which will be subsequently stained with osmium tetroxide. On the contrary, E is largely lost during the fixation and dehydration because it does not form such a complex. Therefore, E granules show only a light electron-density [35]. Under the electron microscope, the NE granules appear polymorphic and very electron-dense, with a core closely adhering to the limiting membrane, whereas the E granules are roundish, moderately electron-dense and characterized by a clear halo between the core and the limiting membrane [36][37] (Figure 4). A study, performed in E-deficient mice, generated by knocking out PNMT gene, has recently shown that the E granules still retain their shape and general appearance, despite the lack of E [38].
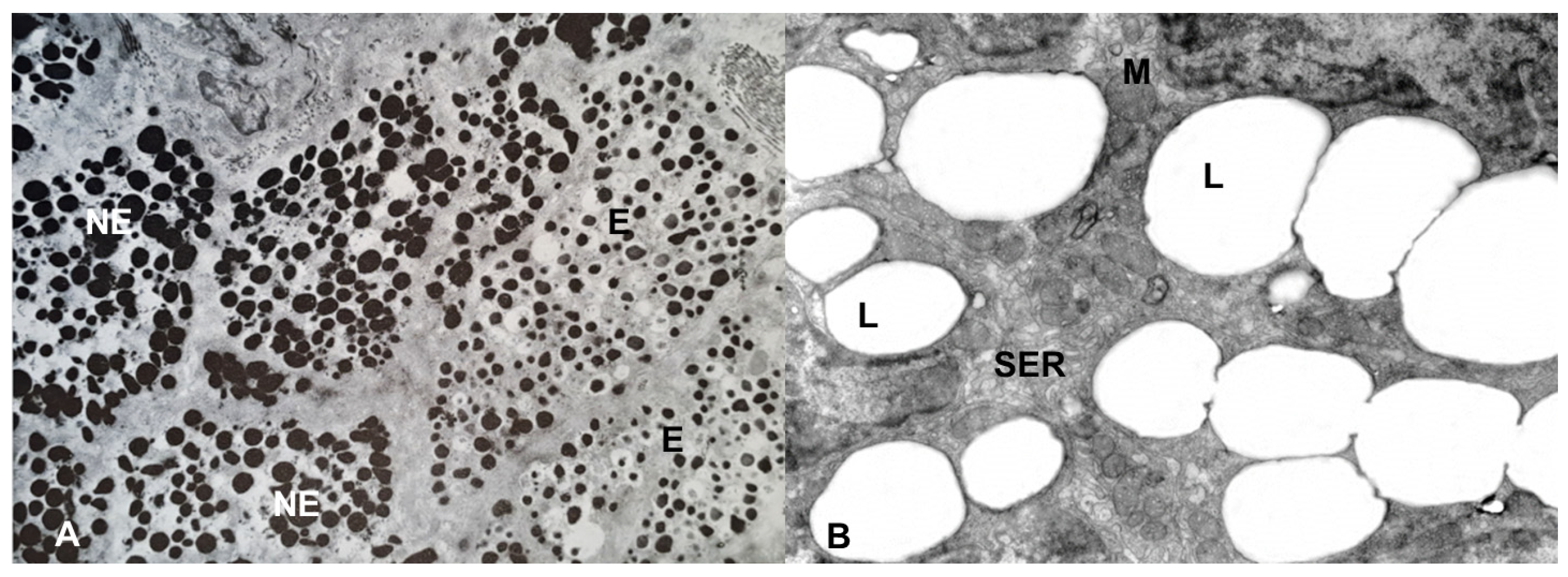
Figure 4. Electron microscopy of the adrenal gland of the lacertid, Podarcis siculus. (A) the NE cells have electron-dense, polymorphic granules, whereas the E cells have roundish, moderately electron-dense granules, characterized by a clear halo between the core and the limiting membrane. Magnification: 5000×. (B) the cytoplasm of the steroidogenic cells is mainly characterized by lipid (L) droplets, smooth endoplasmic reticulum (SER) and mitochondria (M). Magnification: 4000×.
However, the chromaffin cells can also be studied with many other histological, histochemical and immunocytochemical methods [39][40]; for example, the NE cells can be identified through the immunopositivity to the enzyme, tyrosine hydroxylase (TH), converting l-tyrosine to l-3,4-dihydroxyphenylalanine (l-DOPA) (Figure 2), and by staining with Harris hematoxylin after treatment with citrate buffer at pH 6 [32]. The E cells possess the enzyme PNMT, converting NE into E, and therefore can be identified through the immunopositivity to this enzyme. An exception is represented by birds, in which all chromaffin cells possess the enzyme PNMT, but it is active only in the E cells. Therefore, in birds the PNMT enzyme cannot be used to discriminate the E cells from the NE cells [14].
Under light microscopy, the steroidogenic tissue is characterized by cells with a generally weakly stained and finely spongy cytoplasm, due to the presence of lipid droplets, composed of esters of cholesterol, the precursor of steroid hormones (Figure 3). At the electron microscopic level, the steroidogenic cells are generally characterized by a poor rough endoplasmic reticulum and few free ribosomes while they are rich in smooth endoplasmic reticulum, Golgi complex and mitochondria, having inner membranes forming tubular or vesicular cristae. Moreover, the steroidogenic cells have lipid droplets, the quantity of which varies according to the species and the functional stage of the tissue [41] (Figure 4). In non-mammalian vertebrates, the steroidogenic tissue is usually arranged in cords forming interconnected networks, without preferential organization. However, in birds and in some reptiles, just below the connective capsule, the cords of steroidogenic cells assume a ring or basket arrangement at the periphery of the gland, and parallel more deeply, suggesting a functional zonation, that has been observed in birds [8][42][43] and reptiles, in which the existence of several sub-populations of adrenocortical cells is supposed [8][44]. In mammals, the adrenal cortex is organized in an outer glomerular zone, mainly producing mineralocorticoid hormones; a deep fasciculata zone, mainly producing glucocorticoid hormones, and an inner reticularis zone, mainly producing androgen hormones. However, this standard model of adrenocortical zonation may be variable; in the rat, for example, a white, or undifferentiated zone (ZU) was observed between glomerular and fasciculata zones. The ZU zone was considered a stem/progenitor cell zone [45], but its presence in other species is not clear [46].
4. The NE/E Cell Ratio
The possibility of distinguishing NE cells from E cells by light microscopy makes it possible to calculate the numerical ratio between NE and E cells, or NE/E cell ratio. This ratio reflects the proximity relationships between the steroidogenic and the chromaffin tissues, since, in mammals, birds and reptiles, glucocorticoids stimulate the activity of the enzyme PNMT, that methylates NE converting it into E (Figure 2). Therefore, high E levels indicate an efficient methylation process due to a close contact between the chromaffin and the steroidogenic cells, and therefore high glucocorticoid levels available to stimulate the enzyme PNMT [24]. The values of this ratio are very variable in birds and reptiles, and attempts were made to attribute a phylogenetic significance to its variations. For example, it was found that birds with more primitive ancestry, as cormorant and egret, had more NE, while recently evolved species, as passerine birds, had more E; a NE/E cell ratio around 1/1 was typical of species, as pigeon and cuckoo, having an intermediate evolutionary position [47]. However, this opinion is no more supported by recent bird phylogenetic reconstructions [48]. Also in reptiles, the NE/E cell ratio is highly variable. For example, in Squamata (lizards, snakes and amphisbaenians), high values of this ratio correspond to a high degree of separation between the steroidogenic and the chromaffin tissues, whereas low values of this ratio correspond to a higher degree of admixing of the two tissues. As for birds, a correlation between the value of NE/E cell ratio and the phylogenetic history of different species was assumed [2][47]. This correlation was confirmed by numerous biochemical, karyological and paleontological data [49][50][51][52]. However, in recent years the hypotheses on Squamata phylogeny have changed radically, especially if both morphological and molecular data are considered [53][54][55][56][57][58]. Therefore, the hypothesis of a correspondence between the value of the NE/E cell ratio and the greater or lesser antiquity of the species in Squamata should be verified with further studies.
5. The Adrenal Gland of Reptiles
Before describing the adrenal gland of reptiles, it must be emphasized that, from a systematic point of view, there is no agreement on the taxon Reptilia, since morphological and molecular studies have questioned its composition and its nomenclature [59]. The discussion on the systematic position and the nomenclature of this taxon goes beyond the scope of this resesrch, in which the traditional and better-known term of “reptiles” will still be used. Moreover, it must be said that in this research are considered also papers published many years ago, relating to species whose nomenclature has changed over time. Where possible, the current nomenclature in brackets right after the specific name, utilized in the mentioned papers, will be quoted [60].
In reptiles, the steroidogenic and the chromaffin tissues are associated to form a gland in close relationship with the gonads and the genital ducts (Figure 1), except for chelonians, where the gland is related to the ventral surface of the kidney. The degree of admixing of the two tissues is highly variable and it is not possible to define a scheme valid for all reptiles [1][2][7]. Indeed, in Chelonia and Crocodilia, a close intermingling of the steroidogenic and the chromaffin cells, without any concentration of chromaffin tissue on the dorsal region of adrenal gland, can be observed [6][61][62]. The arrangement of the adrenal gland of Chelonia, recently considered diapsid reptiles (for review see [59]), is like that of amphibians, forming a stripe on the ventral surface of the kidney, whereas the arrangement of the adrenal gland of Crocodilia, characterized by strands of chromaffin cells intermingled with interrenal cords, is like that of birds, where the chromaffin tissue forms clumps or strands of cells mixed with blood vessels and interrenal steroidogenic cords, both in subcapsular zone and inner part of the gland [6]. In Rhynchocephalia and Squamata, the chromaffin tissue is mainly distributed on dorsal part of the gland. The adrenal gland of Rhynchocephalia has a parenchyma of steroidogenic tissue also containing islets of chromaffin cells; a major part of chromaffin tissue forms a dorsal mass that sends expansions into the steroidogenic parenchyma, whereas some clusters of chromaffin cells are also present on the ventral surface of the gland [2][6][63].
6. The Adrenal Gland of Squamata
In Squamata (lizards, snakes and amphisbaenians), the general arrangement of the adrenal gland is like that of Rhynchocephalia; indeed, the adrenal gland shows a steroidogenic parenchyma and a dorsal mass made of chromaffin tissue. The latter is often present also in the parenchyma, where it forms islets, more or less numerous, having few chromaffin cells. Unlike Rhynchocephalia, usually the adrenal gland of Squamata does not show chromaffin tissue on the ventral surface of the gland [61][62][63][64][65]. However, there are many differences in the distribution of the chromaffin cells, which can form a continuous envelope around the steroidogenic parenchyma, with few or no inner islets, as well as they can be concentrated to form islets scattered in the parenchyma and a very reduced dorsal mass. Above all, this great variability in the distribution of the two types of tissue can be observed not only between different families, but also within the same family or within the same genus, as has been observed studying many species belonging to different families, subfamilies, orders, and infraorders (for review see [2]).
References
- Perry, F.S.; Capaldo, A. The autonomic nervous system and chromaffin tissue: Neuroendocrine regulation of catecholamine secretion in non-mammalian vertebrates. Auton. Neurosci. Basic Clin. 2011, 165, 54–66.
- Varano, L.; Laforgia, V. Evolutionary trends in the adrenal gland of reptiles. In Symposium on the Evolution of Terrestrial Vertebrates; Ghiara, G., Angelini, F., Olmo, E., Varano, L., Eds.; Mucchi: Modena, Italy, 1991; Volume 4, pp. 291–303.
- Norris, D.O. Vertebrate Endocrinology, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2007.
- Accordi, F. The chromaffin cells of urodele amphibians. J. Anat. 1991, 179, 1–8.
- Grassi Milano, E.; Accordi, F. Evolutionary trends in adrenal gland of anurans and urodeles. J. Morphol. 1986, 189, 249–259.
- Grassi Milano, E. Development of the adrenal gland in amniotes: A comparison between chelonians and birds. Boll. Zool. 1991, 58, 205–209.
- Di Lorenzo, M.; Barra, T.; Rosati, L.; Valiante, S.; Capaldo, A.; De Falco, M.; Laforgia, V. Adrenal gland response to endocrine disrupting chemicals in fishes, amphibians and reptiles: A comparative overview. Gen. Comp. Endocrinol. 2020, 297, 113550.
- Carsia, R.V.; McIlroy, P.J.; John-Alder, H.B. Adrenocortical function in avian and non-avian reptiles: Insights from dispersed adrenocortical cells. Comp. Biochem. Physiol. A 2023, 281, 111424.
- Ehrart-Bornstein, M.; Hinson, J.P.; Bornstein, S.R.; Scherbaum, W.A.; Vinson, G.P. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr. Rev. 1998, 19, 101–143.
- Rao, F.; Zhang, L.; Wessel, J.; Zhang, K.; Wen, G.; Kennedy, B.P.; Rana, B.K.; Das, M.; Rodriguez-Flores, J.L.; Smith, D.W.; et al. Tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis: Discovery of common human genetic variants governing transcription, autonomic activity, and blood pressure in vivo. Circulation 2007, 116, 993–1006.
- Lucas, C.A.; Thoenen, H. Selective induction by glucocorticoids of tyrosine hydroxylase in organ cultures of rat pheochromocytoma. Neuroscience 1977, 2, 1095–1101.
- Kelner, K.L.; Pollard, H.B. Glucocorticoid receptors and regulation of phenylethanolamine-N- methyltransferase activity in cultured chromaffin cells. J. Neurosci. 1985, 5, 2161–2168.
- Evinger, M.J.; Towle, A.C.; Park, D.H.; Lee, P.; Joh, T.H. Glucocorticoids stimulate transcription of the rat phenylethanolamine N-methyltransferase (PNMT) gene in vivo and in vitro. Cell. Mol. Neurobiol. 1992, 12, 193–215.
- Ghosh, A.; Carmichael, S.W.; Mukherjee, M. Avian adrenal medulla: Cytomorphology and function. Acta Biol. Szeged. 2001, 45, 1–11.
- Hodel, A. Effects of glucocorticoids on adrenal chromaffin cells. J. Neuroendocr. 2001, 13, 216–220.
- Wurtman, R.J. Control of epinephrine synthesis in the adrenal medulla by the adrenal cortex: Hormonal specificity and dose-response characteristics. Endocrinology 1966, 79, 392–394.
- Unsicker, K.; Huber, K.; Schober, A.; Kalcheim, C. Resolved and open issues in chromaffin cell development. Mech. Dev. 2013, 130, 324–329.
- Zachariasen, R.D.; Newcomer, W.S. Phenylethanolamine-N-methyltransferase activity in the avian adrenal following immobilization or adrenocorticotropin. Gen. Comp. Endocrinol. 1974, 23, 193–198.
- Laforgia, V.; Varano, L. The influence of the interrenal steroidogenic tissue on the chromaffin cells of the adrenal gland of Lacerta s. sicula Raf.: Effects of ACTH administration during the winter. Cell. Mol. Biol. 1978, 23, 379–390.
- Capaldo, A.; Laforgia, V.; Sciarrillo, R.; De Falco, M.; Valiante, S.; Gay, F.; Virgilio, F.; Varano, L. Effects of dopamine on the adrenal gland of Podarcis sicula (Reptilia, Lacertidae). Gen. Comp. Endocrinol. 2004, 135, 17–24.
- Capaldo, A.; Sciarrillo, R.; Valiante, S.; Gay, F.; Virgilio, F.; Varlese, M.G.; Laforgia, V.; Varano, L. Neuropeptide Y (NPY) modulates pituitary-adrenal axis activity in the lizard, Podarcis sicula. Gen. Comp. Endocrinol. 2004, 137, 237–247.
- Reid, S.G.; Vijayan, M.M.; Perry, S.F. Modulation of catecholamine storage and release by the pituitary-interrenal axis in the rainbow trout, Oncorhynchus Mykiss. J. Comp. Physiol. B 1996, 165, 665–676.
- Capaldo, A.; Gay, F.; De Falco, M.; Virgilio, F.; Laforgia, V.; Varano, L. The adrenal gland of newt Triturus carnifex (Amphibia, Urodela) following in vivo betamethasone administration. Anat. Embriol. 2006, 211, 577–584.
- Ehrhart-Bornstein, M.; Bornstein, S.R. Cross-talk between adrenal medulla and adrenal cortex in stress. Ann. N. Y. Acad. Sci. 2008, 1148, 112–117.
- Hanke, W. Changes of the biology of the adrenal cortex in the Vertebrate evolution. Nova Acta Leopold. NF 1984, 56, 363–378.
- Laforgia, V.; Capaldo, A. Annual cycle of the chromaffin cells of Triturus cristatus. J. Morphol. 1991, 208, 83–90.
- Gay, F.; Valiante, S.; Sciarrillo, R.; De Falco, M.; Laforgia, V.; Capaldo, A. Annual and daily serum aldosterone and catecholamine patterns in males of the Italian crested newt, Triturus carnifex (Amphibia, Urodela). Ital. J. Zool. 2010, 77, 384–390.
- Gay, F.; Laforgia, V.; Capaldo, A. Human follicle-stimulating hormone modulation of adrenal gland activity in the Italian crested newt, Triturus carnifex (Amphibia, Urodela). Comp. Biochem. Physiol. A-Mol. Int. Physiol. 2008, 151, 126–132.
- Coupland, R.E. The natural history of the chromaffin cell. Twenty-five years on the beginning. Arch. Histol. Cytol. 1989, 52, 331–341.
- Giordano-Lanza, G. Sulla istofisiologia degli elementi cellulari della parte midollare del surrene. Quad. Anat. Prat. S 1961, 17, 251–284.
- Brown, W.J.; Barajas, L.; Latta, H. The ultrastructure of the human adrenal medulla with comparative studies of white rat. Anat. Rec. 1971, 169, 173–184.
- Khan, M.B.; Lee, B.R.; Kamitani, T. A simple and sensitive method for the demonstration of norepinephrine-storing adrenomedullary chromaffin cells. Histochem. Cell Biol. 2012, 138, 155–165.
- Wood, J.G. Identification of and observations on epinephrine and norepinephrine containing cells in the adrenal medulla. Am. J. Anat. 1963, 112, 285–304.
- Pearse, A.G.E. Histochemistry Theoretical and Applied; Little, Brown & Co.: Boston, MA, USA, 1960.
- Tramezzani, J.H.; Chiocchio, S.; Wasserman, G.F. A technique for light and electron microscopic identification of adrenaline and noradrenaline storing cells. Cytochemistry 1964, 12, 890–899.
- Capaldo, A.; Laforgia, V.; Sciarrillo, R.; Valiante, S.; Gay, F.; Varano, L. Localization and role of serotonin in the adrenal gland of Podarcis sicula (Reptilia, Lacertidae). Gen. Comp. Endocrinol. 2003, 132, 66–76.
- Capaldo, A.; De Falco, M.; Rosati, L.; Laforgia, V. Transmission electron microscopy: A method for studying the adrenal chromaffin cells. In Chromaffin Cells. Methods in Molecular Biology; Borges, R., Ed.; Humana: New York, NY, USA, 2023; Volume 2565, pp. 43–55.
- Gonzàlez-Santana, A.; Castañeira, L.; Baz-Dàvila, R.; Estévez-Herrera, J.; Domìnguez, N.; Méndez-Lòpez, I.; Padìn, J.F.; Castañeira, A.; Machado, J.D.; Ebert, S.N.; et al. Adrenergic chromaffin cells are adrenergic even in the absence of epinephrine. J. Neurochem. 2020, 152, 299–314.
- Moawad, U.K.; Soliman, S.M.M.; Mazher, K.M.; Hassan, R.M.; Nabil, T.M. Histological, histochemical, ultrastructural and immunohistochemical identification and characterization of the neurosecretory cells of the adult rabbit’s adrenal medulla. Anat. Histol. Embryol. 2022, 51, 280–288.
- Maneu, V.; Borges, R.; Gandìa, L.; Garcìa, A.G. Forty years of the adrenal chromaffin cell through ISCCB meetings around the world. Pflug. Arch. Eur. J. Physiol. 2023, 475, 667–690.
- Idelman, S. The structure of the mammalian adrenal cortex. In General, Comparative and Clinical Endocrinology of the Adrenal Cortex; Chester Jones, I., Henderson, I.W., Eds.; Academic Press: London, UK; New York, NY, USA; San Francisco, CA, USA, 1978; pp. 1–199.
- Klingbeil, C.K.; Holmes, W.N.; Pearce, R.B.; Cronshaw, J. Functional significance of interrenal cell zonation in the adrenal gland of the duck (Anas platyrhynchos). Cell Tissue Res. 1979, 201, 23–36.
- Holmes, W.N.; Cronshaw, J. Adrenal gland: Some evidence for the structural and functional zonation of the steroidogenic tissues. J. Exper. Zool. 1984, 232, 627–631.
- Lofts, B.; Phillips, J.G. Some aspects of the structure of the adrenal gland in snakes. J. Endocrinol. 1965, 33, 327–328.
- Mitani, F. Functional zonation of the rat adrenal cortex: The development and maintenance. Proc. Jpn. Acad. Ser. B 2016, 90, 163–183.
- Vinson, G.P. Functional Zonation of the Adult Mammalian Adrenal Cortex. Front. Neurosci. 2016, 10, 238.
- Ghosh, A. Cytophysiology of the avian adrenal medulla. In International Review of Cytology; Bourne, G., Danielli, J.P., Eds.; Academic Press: New York, NY, USA, 1977; pp. 253–284.
- Braun, E.L.; Kimball, R.T. Data types and the phylogeny of Neoaves. Birds 2021, 2, 1–22.
- Arnold, E.N. Relationships of the paleartctic lizards assigned to the genera Lacerta, Algyroides and Psammodromus (Reptilia: Lacertidae). Bull. Br. Mus. (Nat. Hist.) Zool. 1973, 25, 291–366.
- Lutz, B.; Cei, J.M. Immunological data on the taxonomy of some Italian lizards (Reptilia, Lacertidae). Mon. Zool. Ital. 1977, 11, 231–236.
- Lanza, B.; Cei, J.M.; Crespo, E.G. Immunological investigations on the taxonomic status of some Mediterranean lizards (Reptilia, Lacertidae). Mon. Zool. Ital. 1977, 11, 211–221.
- Lutz, D.; Mayer, W. Albumin evolution and its phylogenetic and taxonomic implications in several lacertid lizards. Amphibia-Reptilia 1985, 6, 53–61.
- Conrad, J.L. Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bull. Am. Mus. Nat. Hist. 2008, 310, 1–182.
- Mulcahy, D.G.; Noonan, B.P.; Moss, T.; Townsend, T.M.; Reeder, T.W.; Sites, J.W., Jr.; Wiens, J.J. Estimating divergence times and evaluating dating methods using phylogenomic and mitochondrial data in squamate reptiles. Mol. Phylogenet Evol. 2012, 65, 974–991.
- Townsend, T.M.; Larson, A.; Louis, E.; Macey, J.R. Molecular phylogenetics of Squamata: The position of snakes, amphisbaeninans, and dibamids, and the root of the squamate tree. Syst. Biol. 2004, 53, 735–757.
- Vidal, N.; Hedges, S.B. The phylogeny of squamate reptiles (lizards, snakes, and amphisbaenians) inferred from nine nuclear protein-coding genes. CR Biol. 2005, 328, 1000–1008.
- Wiens, J.J.; Hutter, C.R.; Mulcahy, D.G.; Noonan, B.P.; Townsend, T.M.; Sites, J.W.; Reeder, T.W. Resolving the phylogeny of lizard and snakes (Squamata) with extensive sampling of genes and species. Biol. Lett. 2012, 8, 1043–1046.
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 93.
- Modesto, S.P.; Anderson, J.S. The phylogenetic definition of Reptilia. Syst. Biol. 2004, 53, 815–821.
- The NCBI Taxonomy Database. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi (accessed on 30 June 2023).
- Gabe, M.; Martoja, M. Contribution à l’histologie de la glande surrénale des Squamata. Archs Anat. Microsc. 1961, 50, 1.
- Gabe, M.; Rancurel, P. Contribution à l’histologie de la glande surrénale de Crocodylus niloticus Laur. Archs Anat. Microsc. 1964, 53, 225–240.
- Gabe, M.; Saint Girons, H. Particularités histologiques de la glande surrénale chez Sphenodon punctatus Gray. C. R. Hebd. SÉAnc Acad. Sci. Paris 1964, 258, 3559–3562.
- Wasserman, G.F.; Tramezzani, J.H. Separate distribution of adrenaline and noradrenaline secreting cells in the adrenal of snakes. Gen. Comp. Endocrinol. 1963, 3, 480–489.
- Varano, L.; Della Corte, F.; Galgano, M. Brevi note sulla ultrastruttura delle cellule a catecolamine di Lacerta s. sicula Raf. Atti Soc. Pel. Sci. Fis. Mat. Nat. 1969, 15, 39–44.
More
Information
Subjects:
Anatomy & Morphology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
719
Revisions:
2 times
(View History)
Update Date:
31 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




