
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francisca Sepúlveda | -- | 1827 | 2023-08-30 17:33:17 | | | |
| 2 | Wendy Huang | Meta information modification | 1827 | 2023-08-31 08:31:14 | | |
Video Upload Options
Cancer research has prioritized the study of the tumor microenvironment (TME) as a crucial area of investigation. Understanding the communication between tumor cells and the various cell types within the TME has become a focal point. Bidirectional communication processes between these cells support cellular transformation, as well as the survival, invasion, and metastatic dissemination of tumor cells. Extracellular vesicles are lipid bilayer structures secreted by cells that emerge as important mediators of this cell-to-cell communication. EVs transfer their molecular cargo, including proteins and nucleic acids, and particularly microRNAs, which play critical roles in intercellular communication. Adipocytes, a significant component of the breast stroma, exhibit high EV secretory activity, which can then modulate metabolic processes, promoting the growth, proliferation, and migration of tumor cells.
1. Introduction
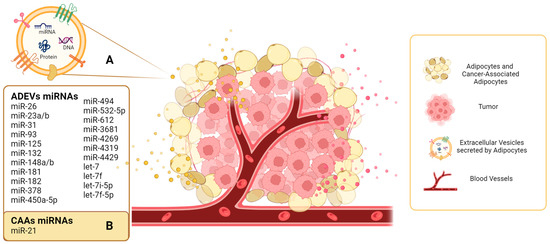
2. Adipocytes as an Important Source of EVs in the TME
| miRNAs | Source of EVs | Description | References |
|---|---|---|---|
| miR-148a let-7f miR-532-5p miR-378 |
Porcine adipose-derived stem cells | Characterized the cargo of EVs by high-throughput RNA sequencing. At least 386 annotated miRNAs were read but four were enriched in EVs. | [32] |
| miR-23b miR-148b miR-182 miR-3681 miR-4269 miR-4319 miR-4429 |
Visceral and subcutaneous adipose samples from obese and lean patients | Compared miRNA levels between obese and lean visceral exosomes. | [33] |
| miR-31 | Human ADSCs | miR-31 promote angiogenesis in HUVECs by targeting factor-inhibiting HIF-1 (FIH1). | [42] |
| miR-21 | Normal and cancer-associated adipocytes from ovarian cancer patients | miR-21 is transferred from cancer-associated adipocytes to cancer cells and confers chemoresistance. | [39] |
| miR-450a-5p | Rat adipose tissue and ADSCs | miR-450a-5p mediates adipogenic differentiation. | [43] |
| miR-132 | Human adipose-derived stem cells (ADSCs) | miR-132 was transferred from ADSCs to lymphatic endothelial cells and promoted proliferation, migration, and tube formation. | [44] |
| miR-23a/b | 3T3-L1 cells, serum, and tumor tissues of hepatocellular carcinoma patients. | miR-23a/b was upregulated in serum exosomes and tumor tissue. Results suggested that miR-23a/b was derived from adipocytes and transported into cancer cells, conferring chemoresistance. | [35] |
| let-7 | Human ADSCs | Human ADSC-EVs contribute to angiogenesis via let-7. | [45] |
| let-7i-5p, let-7f-5p |
Human ADSCs | Human ADSC-EVs promote migration and invasion of endothelial cells. | [46] |
| miR-93, miR-125, miR-16, let7, miR-612, miR-494, miR-181 | Human adipose tissue | miRNAs contained in ADEVs upregulate genes that may impact increased proliferation and deregulate genes that reduce invasion of prostate cancer cells. | [36] |
References
- Baghban Roghayyeh et aBaghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P.L. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signal. 2020, 18, 1–19.
- Nicole, M.A.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, 905–931.
- Bouche, C.; Quail, D.F. Fueling the Tumor Microenvironment with Cancer-Associated Adipocytes. Cancer Res. 2023, 83, 1170–1172.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46.
- Pinedo, M.; de la Canal, L.; de Marcos Lousa, C. A call for Rigor and standardization in plant extracellular vesicle research. J. Extracell Vesicles 2021, 10, e12048.
- Pezzicoli, G.; Tucci, M.; Lovero, D.; Silvestris, F.; Porta, C.; Mannavola, F. Large Extracellular Vesicles—A New Frontier of Liquid Biopsy in Oncology. Int. J. Mol. Sci. 2020, 21, 6543.
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A Compendium of RNA, Proteins, Lipids and Metabolites in Extracellular Vesicles. Nucleic Acids Res. 2019, 47, D516–D519.
- Lana, G.; Billan, S.; Fridman, E.; Gil, Z. Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate. Cells 2022, 11, 1375.
- Shao, J.; Zaro, J.; Shen, Y. Advances in Exosome-Based Drug Delivery and Tumor Targeting: From Tissue Distribution to Intracellular Fate. Int. J. Nanomed. 2020, 15, 9355–9371.
- Jafari, A.; Babajani, A.; Abdollahpour-Alitappeh, M.; Ahmadi, N.; Rezaei-Tavirani, M. Exosomes and Cancer: From Molecular Mechanisms to Clinical Applications. Med. Oncol. 2021, 38, 45.
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465.
- Hemmatzadeh, M.; Mohammadi, H.; Jadidi-Niaragh, F.; Asghari, F.; Yousefi, M. The Role of Oncomirs in the Pathogenesis and Treatment of Breast Cancer. Biomed. Pharmacother. 2016, 78, 129–139.
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Res. 2016, 76, 3666–3670.
- Soheilifar, M.H.; Masoudi-Khoram, N.; Madadi, S.; Nobari, S.; Maadi, H.; Neghab, H.K.; Amini, R.; Pishnamazi, M. Angioregulatory MicroRNAs in Breast Cancer: Molecular Mechanistic Basis and Implications for Therapeutic Strategies. J. Adv. Res. 2022, 37, 235–253.
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted MiR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell 2014, 25, 501–515.
- Gwak, J.M.; Kim, H.J.; Kim, E.J.; Chung, Y.R.; Yun, S.; Seo, A.N.; Lee, H.J.; Park, S.Y. MicroRNA-9 Is Associated with Epithelial-Mesenchymal Transition, Breast Cancer Stem Cell Phenotype, and Tumor Progression in Breast Cancer. Breast Cancer Res. Treat. 2014, 147, 39–49.
- Wright, J.A.; Richer, J.K.; Goodall, G.J. microRNAs and EMT in mammary cells and breast cancer. J. Mammary Gland Biol. Neoplasia 2010, 15, 213–223.
- Maryam, Z.; Bastami, M.; Solali, S.; Alivand, M.R. Aberrant MiRNA Promoter Methylation and EMT-Involving MiRNAs in Breast Cancer Metastasis: Diagnosis and Therapeutic Implications. J. Cell. Physiol. 2018, 233, 3729–3744.
- Min, Z.; Ang, L.; Huang, J.; Wang, J. MicroRNAs Regulate the Epithelial–Mesenchymal Transition and Influence Breast Cancer Invasion and Metastasis. Tumor Biol. 2017, 39, 1–8.
- Cho, Y.K.; Son, Y.; Kim, S.N.; Song, H.D.; Kim, M.; Park, J.H.; Jung, Y.S.; Ahn, S.Y.; Saha, A.; Granneman, J.G.; et al. MicroRNA-10a-5p Regulates Macrophage Polarization and Promotes Therapeutic Adipose Tissue Remodeling. Mol. Metab. 2019, 29, 86–98.
- Heyn, G.S.; Corrêa, L.H.; Magalhães, K.G. The Impact of Adipose Tissue-Derived miRNAs in Metabolic Syndrome, Obesity, and Cancer. Front. Endocrinol. 2020, 11, 563816.
- Kurylowicz, A. microRNAs in Human Adipose Tissue Physiology and Dysfunction. Cells 2021, 10, 3342.
- Rybinska, I.; Agresti, R.; Trapani, A.; Tagliabue, E.; Triulzi, T. Adipocytes in Breast Cancer, the Thick and the Thin. Cells 2020, 9, 560.
- Kristin, M.N.; Romero, I.L.; Van Houten, B.; Lengyel, E. Adipose Tissue and Adipocytes Support Tumorigenesis and Metastasis. Biochim. Et Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 1533–1541.
- Zhe, H.; Xu, A. Adipose Extracellular Vesicles in Intercellular and Inter-Organ Crosstalk in Metabolic Health and Diseases. Front. Immunol. 2021, 12, 608680.
- Le Lay, S.; Rome, S.; Loyer, X.; Nieto, L. Adipocyte-derived extracellular vesicles in health and diseases: Nano-packages with vast biological properties. FASEB Bioadv. 2021, 3, 407–419.
- Connolly, K.D.; Guschina, I.A.; Yeung, V.; Clayton, A.; Draman, M.S.; Von Ruhland, C.; Ludgate, M.; James, P.E.; Rees, D.A. Characterization of Adipocyte-Derived Extracellular Vesicles Released Pre-and Post-Adipogenesis. J. Extracell. Vesicles 2015, 4, 29159.
- Durcin, M.; Fleury, A.; Taillebois, E.; Hilairet, G.; Krupova, Z.; Henry, C.; Truchet, S.; Trötzmüller, M.; Köfeler, H.; Mabilleau, G.; et al. Characterisation of Adipocyte-Derived Extracellular Vesicle Subtypes Identifies Distinct Protein and Lipid Signatures for Large and Small Extracellular Vesicles. J. Extracell. Vesicles 2017, 6, 1305677.
- Bond, S.T.; Calkin, A.C.; Drew, B.G. Adipose-Derived Extracellular Vesicles: Systemic Messengers and Metabolic Regulators in Health and Disease. Front. Physiol. 2022, 13, 837001.
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-Derived Circulating MiRNAs Regulate Gene Expression in Other Tissues. Nature 2017, 542, 450–455.
- Ogawa, R.; Tanaka, C.; Sato, M.; Nagasaki, H.; Sugimura, K.; Okumura, K.; Nakagawa, Y.; Aoki, N. Adipocyte-Derived Microvesicles Contain RNA That Is Transported into Macrophages and Might Be Secreted into Blood Circulation. Biochem. Biophys. Res. Commun. 2010, 398, 723–729.
- Eirin, A.; Riester, S.M.; Zhu, X.Y.; Tang, H.; Evans, J.M.; O’Brien, D.; van Wijnen, A.J.; Lerman, L.O. MicroRNA and MRNA Cargo of Extracellular Vesicles from Porcine Adipose Tissue-Derived Mesenchymal Stem Cells. Gene 2014, 551, 55–64.
- Ferrante, S.C.; Nadler, E.P.; Pillai, D.K.; Hubal, M.J.; Wang, Z.; Wang, J.M.; Gordish-Dressman, H.; Koeck, E.; Sevilla, S.; Wiles, A.A.; et al. Adipocyte-Derived Exosomal MiRNAs: A Novel Mechanism for Obesity-Related Disease. Pediatr. Res. 2015, 77, 447–454.
- Moraes, J.A.; Encarnação, C.; Franco, V.A.; Xavier Botelho, L.G.; Rodrigues, G.P.; Ramos-Andrade, I.; Barja-Fidalgo, C.; Renovato-Martins, M. Adipose Tissue-Derived Extracellular Vesicles and the Tumor Microenvironment: Revisiting the Hallmarks of Cancer. Cancers 2021, 13, 3328.
- Liu, Y.; Tan, J.; Ou, S.; Chen, J.; Chen, L. Adipose-Derived Exosomes Deliver MiR-23a/b to Regulate Tumor Growth in Hepatocellular Cancer by Targeting the VHL/HIF Axis. J. Physiol. Biochem. 2019, 75, 391–401.
- Mathiesen, A.; Haynes, B.; Huyck, R.; Brown, M.; Dobrian, A. Adipose Tissue-Derived Extracellular Vesicles Contribute to Phenotypic Plasticity of Prostate Cancer Cells. Int. J. Mol. Sci. 2023, 24, 1229.
- François, D.J.; Brisson, L. Interaction between Adipose Tissue and Cancer Cells: Role for Cancer Progression. Cancer Metastasis Rev. 2021, 40, 31–46.
- Simona, H.-S.; Scherer, P.E. Adipocytes: Impact on Tumor Growth and Potential Sites for Therapeutic Intervention. Pharmacol. Ther. 2013, 138, 197–210.
- Au Yeung, C.L.; Co, N.N.; Tsuruga, T.; Yeung, T.L.; Kwan, S.Y.; Leung, C.S.; Li, Y.; Lu, E.S.; Kwan, K.; Wong, K.K.; et al. Exosomal Transfer of Stroma-Derived MiR21 Confers Paclitaxel Resistance in Ovarian Cancer Cells through Targeting APAF1. Nat. Commun. 2016, 7, 11150.
- Tang, Y.; Zhang, W.; Sheng, T.; He, X.; Xiong, X. Overview of the Molecular Mechanisms Contributing to the Formation of Cancer-associated Adipocytes (Review). Mol. Med. Rep. 2021, 24, 1–11.
- Huihui, Y.; He, S. Multi-Faceted Role of Cancer-Associated Adipocytes in the Tumor Microenvironment (Review). Mol. Med. Rep. 2021, 24, 866.
- Kang, T.; Jones, T.M.; Naddell, C.; Bacanamwo, M.; Calvert, J.W.; Thompson, W.E.; Bond, V.C.; Chen, Y.E.; Liu, D. Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of MiRNA-31. Stem Cells Transl. Med. 2016, 5, 440–450.
- Zhang, Y.; Yu, M.; Dai, M.; Chen, C.; Tang, Q.; Jing, W.; Wang, H.; Tian, W. MiR-450a-5p within Rat Adipose Tissue Exosome-like Vesicles Promotes Adipogenic Differentiation by Targeting WISP2. J. Cell Sci. 2017, 130, 1158–1168.
- Xiaolei, W.; Wang, H.; Cao, J.; Ye, C. Exosomes from Adipose-Derived Stem Cells Promotes VEGF-C-Dependent Lymphangiogenesis by Regulating MiRNA-132/TGF-β Pathway. Cell. Physiol. Biochem. 2018, 49, 160–171.
- Zhu, Y.; Zhang, J.; Hu, X.; Wang, Z.; Wu, S.; Yi, Y. Extracellular Vesicles Derived from Human Adipose-Derived Stem Cells Promote the Exogenous Angiogenesis of Fat Grafts via the Let-7/AGO1/VEGF Signalling Pathway. Sci. Rep. 2020, 10, 5313.
- Huang, B.; Huang, L.F.; Zhao, L.; Zeng, Z.; Wang, X.; Cao, D.; Yang, L.; Ye, Z.; Chen, X.; Liu, B.; et al. Microvesicles (MIVs) Secreted from Adipose-Derived Stem Cells (ADSCs) Contain Multiple MicroRNAs and Promote the Migration and Invasion of Endothelial Cells. Genes Dis. 2020, 7, 225–234.
- Junjeong, C.; Cha, Y.J.; Koo, J.S. Adipocyte Biology in Breast Cancer: From Silent Bystander to Active Facilitator. Prog. Lipid Res. 2018, 69, 11–20.




