
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ping Zhang | -- | 5165 | 2023-08-30 10:15:54 | | | |
| 2 | Lindsay Dong | Meta information modification | 5165 | 2023-08-31 02:54:51 | | |
Video Upload Options
Allergic diseases are a set of chronic inflammatory disorders of lung, skin, and nose epithelium characterized by aberrant IgE and Th2 cytokine-mediated immune responses to exposed allergens. The prevalence of allergic diseases, including asthma, allergic rhinitis, and atopic dermatitis, has increased dramatically worldwide in the past several decades. Evidence suggests that diet and nutrition play a key role in the development and severity of allergic diseases. Dietary components can differentially regulate allergic inflammation pathways through host and gut microbiota-derived metabolites, therefore influencing allergy outcomes in positive or negative ways. A broad range of nutrients and dietary components (vitamins A, D, and E, minerals Zn, Iron, and Se, dietary fiber, fatty acids, and phytochemicals) are found to be effective in the prevention or treatment of allergic diseases through the suppression of type 2 inflammation.
1. Introduction
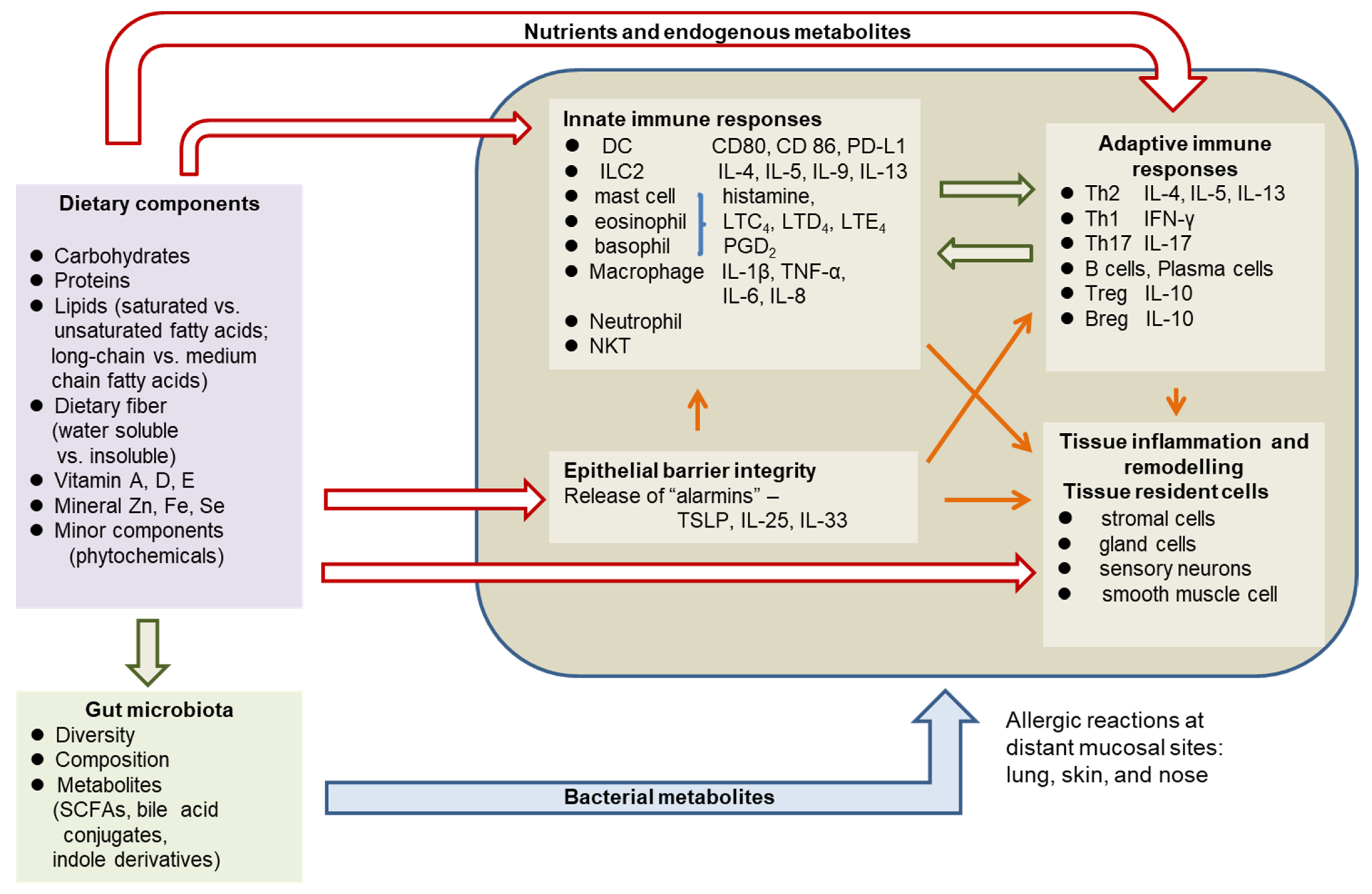
2. Pathophysiology of Allergic Diseases
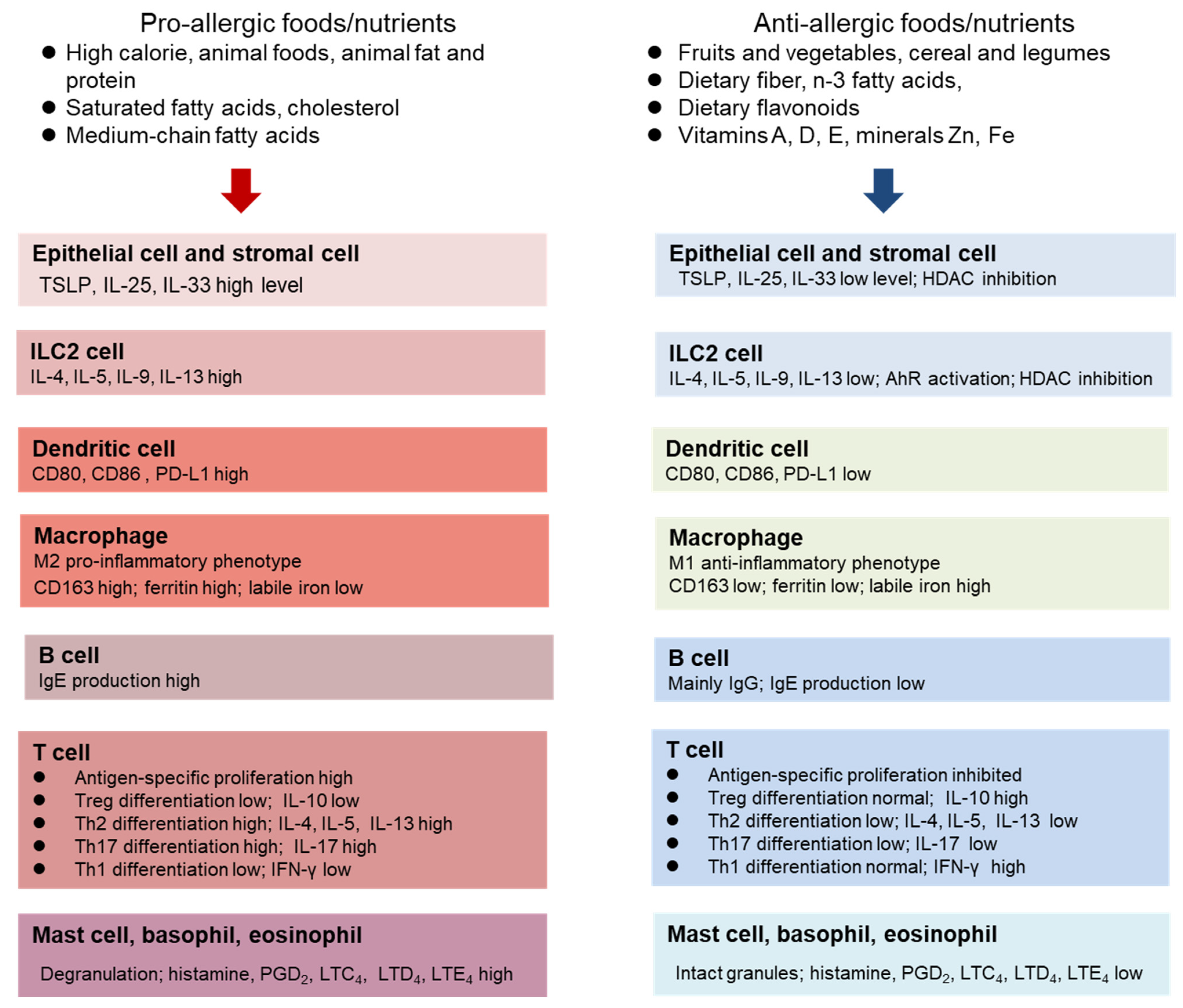
3. The Role of Diet and Nutritional Status in Allergy
- High energy
- High protein
- High saturated fat, n-6 fatty acids, medium-chain fatty acids, cholesterol
- Low total dietary fiber
- Low vegetables and fruits
- High simple sugar and processed foods
- Low level of Zn, Fe, Vitamins A, D, E
3.1. Dietary protein, Amino Acids, and Energy
3.2. Dietary Lipids
3.3. Dietary Fiber
3.4. Dietary Flavonoids and Other Phytochemicals
3.5. Vitamins and Minerals
4. Obesity and Allergy
Dietary interventions producing weight loss in obese patients have been shown to be effective in improving asthma control [80]. Randomized controlled trials on dietary intervention showed that weight loss through restrictive diets with low energy is effective in improving asthma outcomes [81] and reducing airway inflammation in obese patients [82]. Even a normal caloric diet with a reduced content of fat, particularly saturated fat, was associated with reduced body weight and improvement of asthma-related quality of life in obese pubertal adolescents [83]. Although there are very limited studies, weight loss is associated with improved symptoms in atopic dermatitis.
Plant-based diets are effective for weight loss [84][85][86] and can be an effective strategy for weight control, as well as in the treatment of obesity [86]. A plant-based vegan diet excludes all animal products, mainly consisting of grains, legumes, and vegetables and fruits; while in comparison, a vegetarian diet does not eliminate all animal products but emphasizes the consumption of fruits, vegetables, and nuts [86]. The weight reduction effect of such diets may be attributed to reduced calories and low fat intake [86]. Plant protein, as part of a plant-based diet, has recently been shown to be a contributing factor for weight control in overweight individuals [84]. An increased intake of protein and a decreased intake of animal protein are associated with a decrease in body fat mass. Plant-based diets are nutritionally adequate if planned well [85]. However, nutrient intake in the long term can be a concern, as revealed in a study of the weight-loss effects of a vegan diet in overweight postmenopausal women. The adoption of a low-fat vegan diet for 14 weeks leads to changes in macronutrients such as decreased intake of total fat, saturated fat and cholesterol, protein, and increased carbohydrate and fiber intake [85]. In terms of micronutrients, the vegan diet increased intakes of total vitamin A, β-carotene, thiamine, vitamin B6, folic acid, vitamin C, magnesium, and potassium, but decreased intakes of vitamin D, vitamin B12, calcium, phosphorous, selenium, and zinc [85]. Fortified food or supplements may help those following a vegan diet to meet the requirements of micronutrient intakes.
5. Conclusions
References
- Undem, B.J.; Taylor-Clark, T. Mechanisms underlying the neuronal-based symptoms of allergy. J. Allergy Clin. Immunol. 2014, 133, 1521–1534.
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025.
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Canonica, C.W.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic rhinitis. Nat. Rev. Dis. Primers 2020, 6, 95.
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122.
- Julia, V.; Macia, L.; Dombrowicz, D. The impact of diet on asthma and allergic diseases. Nature 2015, 15, 308–322.
- Lin, Y.P.; Kao, Y.C.; Pan, W.H.; Yang, Y.H.; Chen, Y.C.; Lee, Y.L. Associations between respiratory diseases and dietary patterns derived by factors analysis and reduced rank regression. Ann. Nutr. Metab. 2016, 68, 306–314.
- Netting, M.J.; Middleton, P.F.; Markrides, M. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systemic review of food-based approaches. Nutrition 2014, 30, 1225–1241.
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “Western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842.
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Longo, M.N.L.; Luerigo, O.; Manso, L.; Matheu, V.; Seoane, E.; Zamorano, M.; et al. Microbiome and allergic diseases. Front. Immunol. 2018, 9, 1584.
- McKenzie, C.; Tan, J.; Macia, L.; Mackay, C.R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 2017, 278, 277–295.
- Sugihara, K.; Kamada, N. Diet-microbiota interactions in inflammatory bowel disease. Nutrients 2021, 13, 1533.
- Zhang, P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 2022, 23, 9588.
- Arifuzzaman, M.; Won, T.H.; Li, T.T.; Yano, H.; Digumarthi, S.; Heras, A.F.; Zhang, W.; Parkhurst, C.N.; Kashyap, S.; Jin, W.B.; et al. Inulin fiber promotes microbiota-derived bile acids and type 2 inflammation. Nature 2022, 611, 578–584.
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Domingo, J.S.; Camacho-Muñoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P.; et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022, 15, 908–926.
- Palomares, O.; Akdis, M.; Martin-Frontecha, M.; Akdis, C.A. Mechanisms of immune regulation in allergic diseases: The role of regulatory T and B cells. Immunol. Rev. 2017, 278, 219–236.
- Roan, F.; Obata-Ninomiya, K.; Ziegler, S.F. Epithelial cell-derived cytokines: More than just signaling the alarm. J. Clin. Investig. 2019, 129, 1441–1451.
- Dahlgren, M.W.; Jones, S.W.; Cautivo, K.M.; Dubinin, A.; Oritiz-Carpena, J.F.; Farhat, S.; Yu, K.S.; Lee, K.; Wang, C.Q.; Molofsky, A.V.; et al. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity 2019, 50, 702–722.
- Klose, C.S.N.; Artis, D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 2020, 30, 475–491.
- Kiss, E.A.; Vonarbourg, C.; Kopfmann, S.; Hobeika, E.; Finke, D.; Esser, C.; Diefenbach, A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011, 334, 1561–1565.
- Li, S.; Bostick, J.W.; Ye, J.; Qiu, J.; Zhang, B.; Urban, J.F.; Auram, D.; Zhou, L. Aryl hydrocarbon receptor signaling cell intrinsically inhibits intestinal group innate lymphoid cell function. Immunity 2018, 49, 915–928.
- Van der Marel, A.P.J.; Samsom, J.N.; Greuter, M.; van Berkel, L.A.; O’Toole, T.; Kraal, G.; Mebius, R.E. Blockade of IDO inhibits nasal tolerance induction. J. Immunol. 2007, 179, 894–900.
- Ünüvar, S.; Erge, D.; Kiliçarslan, B.; Bağ, H.G.G.; Çatal, F.; Girgin, G.; Baydar, T. Neopterin levels and indoleamine 2,3-dioxygenase activity as biomarkers of immune system activation and childhood allergic diseases. Ann. Lab. Med. 2019, 39, 284–290.
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. AR and its impact on asthma (ARIA) 2008 update. Allergy 2008, 63 (Suppl. S86), 8–160.
- Leung, D.Y.M.; Berdyshev, E.; Gloeva, E. Cutaneous barrier dysfunction in allergic diseases. J. Allergy Clin. Immnol. 2020, 145, 1485–1497.
- Kraft, M.T.; Prince, B.T. AD is a barrier issue, not an allergy issue. Immunol. Allergy Clin. N. Am. 2019, 39, 507–519.
- Urrutia-Pereira, M.; Mocelin, L.P.; Ellwood, P.; Garcia-Marcos, L.; Simon, L.; Rinelli, P.; Chong-Neto, H.J.; Solé, D. Prevalence of rhinitis and associated factors in adolescents and adults: A global asthma network study. Rev. Paul. Pediatr. 2023, 41, e2021400.
- Peroni, D.G.; Hufnagl, K.; Comberiati, P.; Roth-Walter, F. Lack of iron, zinc, and vitamins as contributor to the etiology of atopic diseases. Front. Nutr. 2023, 9, 1032481.
- Andrianasolo, R.M.; Hercberg, S.; Kesse-Guyot, E.; Druesne-Pecollo, N.; Touvier, M.; Galan, P.; Varraso, R. Association between dietary fiber intake and asthma (symptoms and control) results from the French national e-cohort NutriNet-Santé. Brit. J. Nutr. 2019, 122, 1040–1051.
- Cazzoletti, L.; Zanolin, M.E.; Speita, F.; Bono, R.; Chamitava, L.; Cerveri, I.; Garcia-Larsen, V.; Grosso, A.; Mattioli, V.; Pirina, P.; et al. Dietary fats, olive oil and respiratory diseases in Italian adults: A population-based study. Clin. Exp. Allergy 2019, 49, 799–807.
- Zhou, Y.J.; Li, L.S.; Sun, J.L.; Guan, K.; Wei, J.F. 1H NMR-based metabolomics study of metabolic profiling for pollinosis. World Allergy Org. J. 2019, 12, 100005.
- Ma, G.C.; Wang, T.S.; Wang, J.; Ma, Z.J.; Pu, S.B. Serum metabolomics of patients with AR. Biomed. Chromatogr. 2020, 34, e4739.
- Yoshino, K.; Sakai, K.; Okada, H.; Sakai, T.; Yamamoto, S. IgE responses in mice fed moderate protein deficient and high protein diets. J. Nutr. Sci. Vitaminol. 2003, 49, 172–178.
- Fan, W.Y.; Kouda, K.; Nakamura, H.; Takeuchi, H. Effects of dietary restriction on spontaneous dermatitis in NC/Nga mice. Exp. Biol. Med. 2001, 226, 1045–1050.
- Kositz, C.; Schroecksnadel, K.; Grander, G.; Schennach, H.; Kofler, H.; Fuchs, D. Serum tryptophan concentration in patients predicts outcome of specific immunotherapy with pollen extracts. Int. Arch. Allergy Immunol. 2008, 147, 35–40.
- Licari, A.; Fuchs, D.; Marseglia, G.; Ciprandi, G. Tryptophan metabolic pathway and neopterin in asthmatic children in clinical practice. Ital. J. Pediatr. 2019, 45, 11.
- Luukkainen, A.; Karjalainen, J.; Hurme, M.; Paavonen, T.; Toppila-salmi, S. Relationships of indoleamine 2,3-dioxygenase activity and cofactors with asthma and nasal polyps. Am. J. Rhinil. Allergy 2014, 28, e5–e10.
- Gostner, J.M.; Becker, K.; Kofler, H.; Strasser, B.; Fuchs, D. Tryptophan metabolism in allergic disorders. Int. Arch. Allergy Immunol. 2016, 169, 203–215.
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newshoime, P. Glutamine: Metabolism and immune function, supplementation and clinical transition. Nutrients 2018, 10, 1564.
- Moon, P.D.; Han, N.R.; Kim, H.M.; Jeong, H.J. High-fat diet exacerbates dermatitis through up-regulation of TSLP. J. Investig. Dermatol. 2019, 139, 1198–1201.
- Jena, P.K.; Sheng, L.; McNeil, K.; Chau, T.Q.; Yu, S.; Kiuru, M.; Fung, M.A.; Hwang, S.T.; Wan, Y.J.Y. Long-term western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice. J. Dermatol. Sci. 2019, 95, 13–20.
- Li, J.; Wang, Y.; Tang, L.; de Villiers, W.J.S.; Cohen, D.; Woodward, J.; Finkelman, F.D.; Eckhardt, E.R.M. Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice. J. Allergy Clin. Immunol. 2013, 131, 442–450.
- Iwamoto, A.; Hamajima, H.; Tsuge, K.; Tsuruta, Y.; Nagata, Y.; Yotsumoto, H.; Yanagita, T. Inhibitory effects of green asparagus extract, especially phospholipids, on allergic responses in vitro and in vivo. J. Agric. Food Chem. 2020, 68, 15199–15207.
- Radzikowska, U.; Rinaldi, A.O.; Sözener, Z.Ç.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The influence of dietary fatty acids on immune responses. Nutrients 2019, 11, 2990.
- Fujii, M.; Nakashima, J.; Tomozawa, J.; Shimazaki, Y.; Ohyanagi, N.; Kawaguchi, S.; Ohya, S.; Kohno, S.; Nabe, T. Deficiency of n-6 polyunsaturated fatty acids is mainly responsible for AD-like pruritic skin inflammation in special diet-fed hairless mice. Exp. Dermatol. 2013, 22, 272–277.
- Sawane, K.; Nagatake, T.; Hosomi, K.; Hirata, S.; Adachi, J.; Abe, Y.; Isoyama, J.; Suzuki, H.; Matsunaga, A.; Kunisawa, J.; et al. Dietary omega-3 fatty acid dampens AR via eosinophilic production of the anti-allergic lipid mediator 15-hydroxyeicosapentaenoic acid in mice. Nutrients 2019, 11, 2868.
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Harris, N.L.; Marsland, B.J.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166.
- Cait, A.; Huges, M.R.; Antignano, F.; Cait, T.; Dimitriu, P.A.; Maas, K.R.; Reynolds, L.A.; Hacker, L.; Mohr, J.; Finlay, B.B.; et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018, 11, 785–795.
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying AD. J. Allergy Clin. Immunol. 2016, 137, 852–860.
- Chiu, C.Y.; Cheng, M.L.; Chiang, M.H.; Kuo, Y.L.; Tsai, M.H.; Chiu, C.C.; Lin, G. gut microbial-derived butyrate is inversely associated with IgE responses to allergens in childhood asthma. Pediatr. Allergy Immunol. 2019, 30, 689–697.
- Thio, C.L.P.; Chi, P.Y.; Lai, A.C.Y.; Chang, Y.J. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J. Allergy Clin. Immunol. 2018, 142, 1867–1883.
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-inflammatory and anti-allergic potential of dietary flavonoids. Biomed. Pharmacother. 2022, 156, 113945.
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124.
- Jafarinia, M.; Hosseini, M.S.; Kasiri, N.; Fazel, N.; Fathi, F.; Hakemi, M.G.; Eskandari, N. Quercetin with the potential on allergic diseases. Allergy Asthma Clin. Immunol. 2020, 16, 36.
- Okumo, T.; Furuta, A.; Kimura, T.; Yusa, K.; Asano, K.; Sunagawa, M. Inhibition of angiogenic factor productions by quercetin in vitro and in vivo. Medicines 2021, 8, 22.
- Gong, J.H.; Cho, I.H.; Shin, D.; Han, S.Y.; Park, S.H.; Kang, Y.H. Inhibition of airway epithelial-to-mesenchymal transition and fibrosis in endotoxin-induced epithelial cells and ovalbumin-sensitized mice. Lab. Investig. 2014, 94, 297–308.
- Lee, H.S.; Jeong, G.S. Therapeutic effect of kaempferol on AD by attenuation of T cell activity via interaction with multidrug-associated protein. Br. J. Pharmacol. 2021, 178, 1772–1788.
- Park, S.; Bong, S.K.; Lee, J.W.; Park, N.J.; Choi, Y.; Kim, S.M.; Yang, M.H.; Kim, Y.K.; Kim, S.N. Diosmetin and its glycoside, diosmin, improves AD-like lesions in 2,4-dinitrochlorobenzene-induced murine models. Biomol. Ther. 2020, 28, 542–548.
- Sahin, A.; Sakat, M.S.; Kilic, K.; Aktan, B.; Yildirim, S.; Kandemir, F.M.; Dortbudak, M.B.; Kucukler, S. The protective effect of naringenin against ovalbumin-induced AR in rats. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 4839–4846.
- Lee, H.S.; Kim, J.; Choi, H.G.; Kim, E.K.; Jun, C.D. Licoricidin abrogates T-cell activation by modulating PTPN1 activity and attenuates AD in vivo. J. Investig. Dermatol. 2021, 141, 2490–2498.
- Civelek, M.; Bilotta, S.; Lorentz, A. Resveratrol attenuates mast cell mediated allergic reactions: Potential for use as a nutraceutical in allergic diseases. Mol. Nutr. Food Res. 2022, 66, 2200170.
- Reynolds, K.A.; Juhasz, M.L.W.; Mesinkovska, N.A. The role of oral vitamins and supplements in the management of AD: A systematic review. Int. J. Dermatol. 2019, 58, 1371–1376.
- Sanchez-Armendariz, K.; Garcia-Gil, A.; Romero, C.A.; Contreras-Ruiz, J.; Karam-Orante, M.; Balcazar-Antonio, D.; Dominguez-Cherit, J. Oral vitamin D3 5000 IU/day as an adjuvant in the treatment of AD: A randomized control trial. Int. J. Dermatol. 2018, 57, 1516–1520.
- The role of vitamin D supplementation on airway remodeling in asthma: A systemic review. Nutrients 2023, 15, 2477.
- Khan, A.; Adalsteinsson, J.; Whitaker-Worth, D.L. AD and nutrition. Clin. Dermatol. 2022, 40, 135–144.
- Truong-Tran, A.Q.; Ruffin, R.E.; Foster, P.S.; Koskinen, A.M.; Coyle, P.; Philox, J.C.; Rofe, A.M.; Zalewski, P.D. Altered zinc homeostasis and caspase-3 activity in murine allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 2002, 27, 286–296.
- Kulik, L.; Maywald, M.; Kloubert, V.; Wessels, I.; Rink, L. Zinc deficiency drives Th17 polarization and promotes loss of Treg cell function. J. Nutr. Biochem. 2019, 63, 11–18.
- Tsai, Y.L.; Ko, W.S.; Hsino, J.L.; Pan, H.H.; Chiou, Y.L. zinc sulfate improved the unbalanced T cell profiles in Der p-allergic asthma: An ex vivo study. Clin. Respir. J. 2018, 12, 563–571.
- Rosenkranz, E.; Hilgers, R.D.; Uciechowski, P.; Petersen, A.; Plümäkers, B.; Rink, L. Zinc enhances the number of regulatory T cells in allergen-stimulated cells from atopic subjects. Eur. J. Nutr. 2017, 56, 557–567.
- Maywald, M.; Meurer, S.K.; Weiskirchen, R.; Rink, L. Zinc supplementation augments TGF-β1-depedent regulatory T cell induction. Mol. Nutr. Food Res. 2017, 61, 1600493.
- Roth-Walter, F. Iron-deficiency in atopic diseases: Innate immune priming by allergens and siderophores. Front. Allergy 2022, 3, 859922.
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39.
- Petje, L.M.; Jensen, S.A.; Szikora, S.; Sulzbacher, M.; Bartosik, T.; Pjevac, P.; Hausmann, B.; Hufnagi, K.; Untersmayr, E.; Fischer, L.; et al. Functional iron-deficiency in women with AR is associated with symptoms after nasal provocation and lack of iron-sequestering microbes. Allergy 2021, 76, 2882–2923.
- Sahoyama, Y.; Hamazato, F.; Shiozawa, M.; Nakagawa, T.; Suda, W.; Ogata, Y.; Hachiya, T.; Kawakami, E.; Hattori, M. Multiple nutritional and gut microbial factors associated with AR: The Hitachi Health Study. Sci. Rep. 2022, 12, 3359.
- Podlecka, D.; Jerzynska, J.; Sanad, K.; Polanska, K.; Bobrow-Korzeniowska, M.; Stelmach, I.; Brzozowska, A. Micronutrients and the risks of allergic diseases in school children. Intl. J. Environ. Res. Public Health 2022, 19, 12187.
- Norton, R.L.; Hoffmann, P.R. Selenium and Asthma. Mol. Asp. Med. 2012, 33, 98–106.
- Gozzi-Silva, S.C.; Teixeira, F.M.E.; Duarte, A.J.S.; Sato, M.N.; de Oliveira, L.M. Immunomodulatory role of nutrients: How can pulmonary dysfunctions improve? Front. Nutr. 2021, 8, 674258.
- Chen, M.; Sun, Y.; Wu, Y.L. Lower circulating zinc and selenium levels are associated with an increased risk of asthma: Evidence from a meta-analysis. Public Health Nutr. 2019, 23, 1555–1562.
- Kuti, B.P.; Kuti, D.K.K.; Smith, O.S. Serum zinc, selenium and total antioxidant contents of Nigerian children with asthma: Association with disease severity and symptoms control. J. Trop. Pediatr. 2020, 66, 395–402.
- Jiang, J.; Nasab, E.M.; Athari, S.M.; Athari, S.S. Effects of vitamin E and selenium on AR and asthma pathophysiology. Respir. Physiol. Neurobiol. 2021, 286, 103614.
- Alwarith, J.; Kahleova, H.; Crosby, L.; Brooks, A.; Brandon, L.; Levin, S.M.; Barnard, N.D. The role of nutrition in asthma prevention and treatment. Nutr. Rev. 2020, 78, 928–938.
- Jensen, M.E.; Gibson, P.G.; Collins, C.E.; Hilton, J.M.; Wood, L.G. Diet-induced weight loss in obese children with asthma: A randomized controlled trial. Clin. Exp. Allergy 2013, 43, 775–784.
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Pretto, J.J.; Morgan, P.J.; Callister, R.; Wood, L.G. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: A randomized trial. Clin. Exp. Allergy 2013, 43, 36–49.
- Luna-Pech, J.A.; Torres-Mendoza, B.M.; Luna-Pech, J.A.; Garcia-Cobas, C.Y.; Navarrete-Navarro, S.; Elizalde-Lozano, A.M. Normocaloric diet improves asthma-related quality of life in obese pubertal adolescents. Int. Arch. Allergy Immunol. 2014, 163, 252–258.
- Kahleova, H.; Dort, S.; Holubkov, R.; Barnard, N.D. A Plant-Based High-Carbohydrate, Low-Fat Diet in Overweight Individuals in a 16-Week Randomized Clinical Trial: The Role of Carbohydrates. Nutrients 2018, 8, 58.
- Turner-McGrievy, G.; Barnard, N.D.; Scialli, A.R.; Lanou, A.J. Effects of a low-fat vegan diet and a Step II diet on macro- and micronutrient intakes in overweight postmenopausal women. Nutrition 2004, 20, 738–746.
- Ivanova, S.; Delattre, C.; Karcheva-Bahchevanska, D.; Benbasat, N.; Nalbantova, V.; Ivanov, K. Plant-Based Diet as a Strategy for Weight Control. Foods 2021, 10, 3052.




