
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Margarita L. Martinez-Fierro | -- | 3541 | 2023-08-30 05:24:27 | | | |
| 2 | Alfred Zheng | Meta information modification | 3541 | 2023-08-30 06:03:59 | | |
Video Upload Options
Acute lymphoblastic leukemia (ALL) is a hematological disease characterized by the dysfunction of the hematopoietic system that leads to arrest at a specific stage of stem cells development, suppressing the average production of cellular hematologic components. BCP (B-cell progenitor)-ALL is a neoplasm of the B-cell lineage progenitor. BCP-ALL is caused and perpetuated by several mechanisms that provide the disease with its tumor potential and genetic and cytological characteristics. These pathological features are used for diagnosis and the prognostication of BCP-ALL. The BCP-ALL diagnostic protocol is well established. Firstly, it is necessary to demonstrate ≥ 20% lymphoblasts in bone marrow (BM) based on a BPM. Second, a hematopathological review is performed; it comprises a morphological assessment, and flow cytometric and genetic characterization.
1. Introduction
2. Diagnostic Biomarkers for BCP-ALL
2.1. Proteins Type BCP-ALL Biomarkers
| Biomarker | Blood Sample | Leukemia Type | Area under the ROC Curve | Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Reference |
|---|---|---|---|---|---|---|---|---|
| Smad 7 | Serum | ALL | 0.81 | 63 | 100 | 100 | 73 | |
| TGF-β1 | Serum | ALL | 0.79 | 57 | 93 | 89.5 | 68 | [16] |
| Smad 7 TGF-β1 miR-181a |
Serum | ALL | - | 100 | 93 | 93.7 | 100 | |
| IGF-I | Serum | ALL | - | 60.6 | 73.3 | - | - | [40] |
| IGF-II | Serum | ALL | - | 72.2 | 73.3 | - | - | |
| IGFBP-2 | Serum | ALL | - | 72.2 | 86.7 | - | - | |
| IGFBP-3 | Serum | ALL | - | 93.9 | 93.9 | - | - | |
| Anti-9-0AcSGs | Serum | ALL-GI | - | 98.9 | 92.1 | 96.8 | 97.2 | [41] |
| Anti-9-0AcSGs | Serum | ALL-GI | - | 96.8 | 95.9 | 96.8 | 95.9 | |
| PF4 CTAP-II |
Serum | ALL | - | 91.8 | 90 | - | - | [7] |
| C3f | Serum | AL | 0.99 | 97 | 100 | - | - | [42] |
| TNF-α | Serum | ALL | 0.94 | 91.7 | 100 | - | - | [3] |
| Survivin | Serum | ALL | 0.98 | 90 | 80 | - | - | |
| p53 | Serum | AL | 0.8 | 52 | 100 | - | - | [43] |
| EGFR | Serum | AL | 0.93 | 73.9 | 95.8 | - | - | |
| Pseudouridine | Serum | ALL | - | 90 | 97.5 | - | - | [44] |
| ADAM 17 | Plasma | BCP-ALL | 0.98 | 100 | 100 | - | - | [45] |
| ATG3 | Plasma | BCP-ALL | 0.95 | 100 | 100 | - | - | |
| AC133 * | Whole blood | ALL | - | 100 | 100 | 100 | 100 | [46] |
| miR-181a | Serum | ALL | 0.93 | 86.5 | 93.3 | 92.8 | 87.5 | [16] |
| miR-146a | Plasma | BCP-ALL | 1 | 100 | 100 | - | - | [9] |
| mRNA Survivin | Whole blood | BCP-ALL | 0.85 | 95 | 95 | - | - | [47] |
| mRNA HLA-G | PBMC | ALL | - | 74 | 100 | - | - | [48] |
| miR-125b-1 | Serum | ALL | 0.85 | 83.7 | 100 | - | - | [49] |
| miR-203 | Serum | ALL | 0.87 | 97.7 | 87 | - | - | |
| miR-100 | PBMC | ALL | 0.87 | 82.7 | 100 | - | - | [15] |
| miR-196a | PBMC | ALL | 0.537 | 46.6 | 100 | - | - | |
| miR-146a | PBMC | ALL | 1 | 100 | 100 | - | - | |
| miR-511 | Plasma | BCP-ALL | 1 | 100 | 100 | 1 | 1 | [50] |
| miR-34a | Plasma | BCP-ALL | 0.98 | 92 | 100 | 1 | 0.70 | |
| miR-22 | Plasma | BCP-ALL | 0.91 | 79 | 100 | 1 | 0.54 | |
| miR-26a | Plasma | BCP-ALL | 0.91 | 79 | 100 | 1 | 0.47 | |
| miR-221 | Plasma | BCP-ALL | 0.92 | 83 | 100 | 1 | 0.54 | |
| miR-223 | Plasma | BCP-ALL | 0.93 | 89 | 100 | 1 | 0.64 | |
| miR-21 | PBMC | ALL | 0.565 | 44 | 55 | - | - | [51] |
| miR-26 | PBMC | ALL | 0.464 | 54 | 50 | - | - | |
| miR-148a | PBMC | ALL | 0.719 | 74 | 79 | - | - | |
| miR-133b | PBMC | ALL | 0.669 | 70 | 60 | - | - | |
| miR-24 | PBMC | ALL | 0.785 | 72 | 81 | - | - | |
| miR-92a | PBMC and plasma | ALL | 0.99 | - | - | - | - | [52] |
| miR-92a | Plasma | ALL | 0.755 | 41.5 | 100 | 100 | 36.7 | [6] |
| miR-638 | Plasma | ALL | 0.86 | 54.7 | 100 | 100 | 42.9 | |
| miR-125b | PBMC | ALL | 0.99 | 98 | 96.7 | - | - | [53] |
| mRNA-Bcl-2 | PBMC | ALL | 0.9 | 96.7 | 70 | - | - | |
| miR-128b | PBMC | ALL | - | 75 | 87.5 | - | - | [54] |
| cf-DNA levels | Plasma | ALL, AML | 0.79 | 65 | 100 | - | - | [55] |
| cf-DNA integrity | Plasma | ALL, AML | 88 | 78 | 90 | - | - |
2.2. RNA Type BCP-ALL Biomarkers
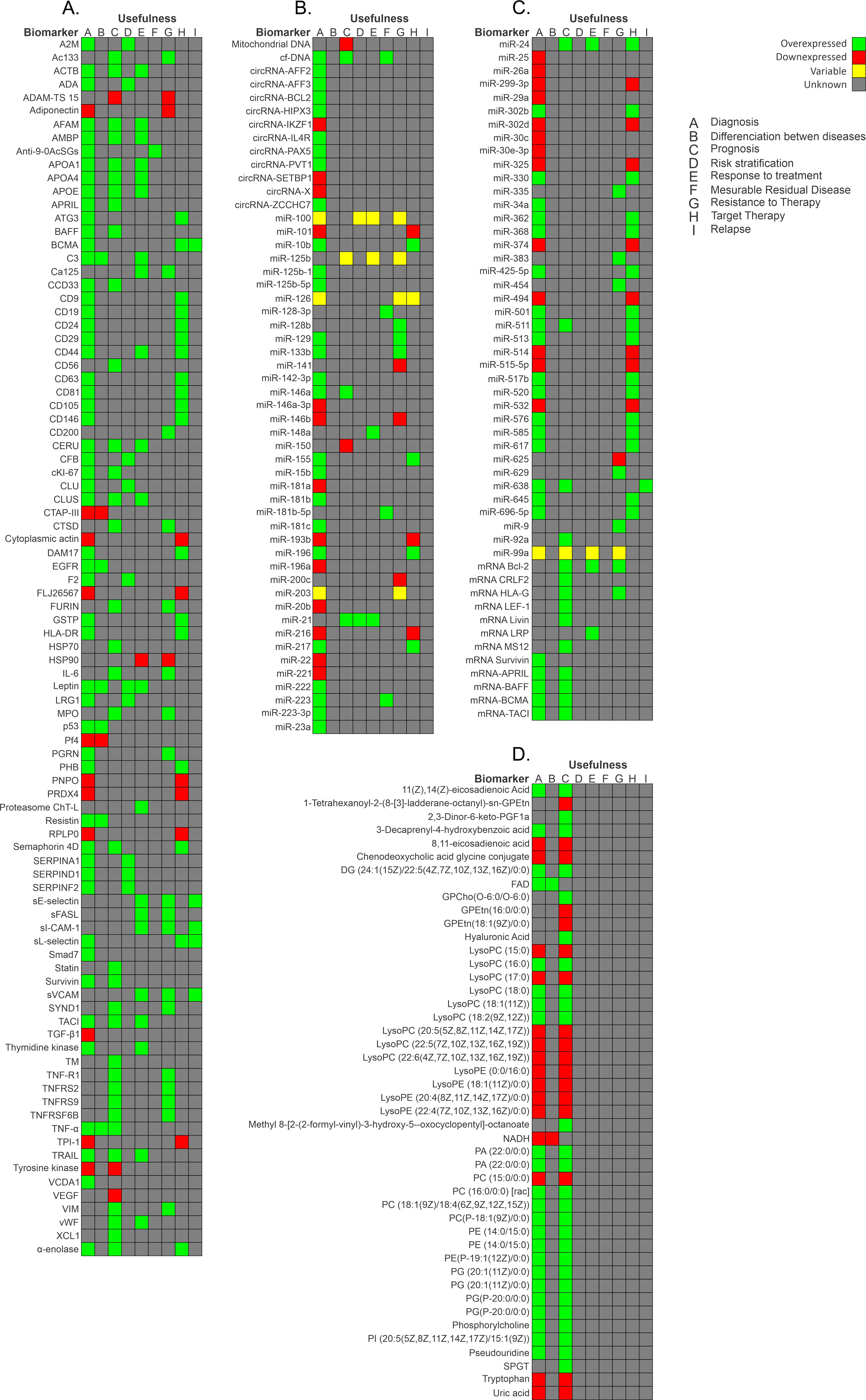
2.3. Metabolites Type BCP-ALL Biomarkers
References
- Fujita, T.C.; Sousa-Pereira, N.; Amarante, M.K.; Watanabe, M.A.E. Acute lymphoid leukemia etiopathogenesis. Mol. Biol. Rep. 2021, 48, 817–822.
- Kaplan, J.A. Leukemia in Children. Pediatr. Rev. 2019, 40, 319–331.
- Ahmed, M.B.; Shehata, H.H.; Moussa, M.; Ibrahim, T.M. Prognostic significance of survivin and tumor necrosis factor-alpha in adult acute lymphoblastic leukemia. Clin. Biochem. 2012, 45, 112–116.
- Wang, D.; Lv, Y.Q.; Liu, Y.F.; Du, X.J.; Li, B. Differential protein analysis of lymphocytes between children with acute lymphoblastic leukemia and healthy children. Leuk. Lymphoma 2013, 54, 381–386.
- Swerdlow, S. WHO Classification of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2017.
- Fayed, D.; Donia, T.; El-Shanshory, M.; Ali, E.M.M.; Mohamed, T.M. Evaluation of MicroRNA92, MicroRNA638 in Acute Lymphoblastic Leukemia of Egyptian Children. Asian Pac. J. Cancer Prev. 2021, 22, 1567–1572.
- Shi, L.; Zhang, J.; Wu, P.; Feng, K.; Li, J.; Xie, Z.; Xue, P.; Cai, T.; Cui, Z.; Chen, X.; et al. Discovery and identification of potential biomarkers of pediatric acute lymphoblastic leukemia. Proteome Sci. 2009, 7, 7.
- Masilamani, V.; Devanesan, S.; AlSalhi, M.S.; AlQahtany, F.S.; Farhat, K.H. Fluorescence spectral detection of acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML): A novel photodiagnosis strategy. Photodiag. Photodyn. Ther. 2020, 29, 101634.
- Shahid, S.; Shahid, W.; Shaheen, J.; Akhtar, M.W.; Sadaf, S. Circulating miR-146a expression as a non-invasive predictive biomarker for acute lymphoblastic leukemia. Sci. Rep. 2021, 11, 22783.
- Hassan, N.M.; Refaat, L.A.; Ismail, G.N.; Abdellateif, M.; Fadel, S.A.; AbdelAziz, R.S. Diagnostic, prognostic and predictive values of miR-100 and miR-210 in pediatric acute lymphoblastic Leukemia. Hematology 2020, 25, 405–413.
- Brown, P.; Inaba, H.; Annesley, C.; Beck, J.; Colace, S.; Dallas, M.; DeSantes, K.; Kelly, K.; Kitko, C.; Lacayo, N.; et al. Pediatric Acute Lymphoblastic Leukemia, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2020, 18, 81–112.
- Brown, P.A.; Shah, B.; Advani, A.; Aoun, P.; Boyer, M.W.; Burke, P.W.; DeAngelo, D.J.; Dinner, S.; Fathi, A.T.; Gauthier, J.; et al. Acute Lymphoblastic Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1079–1109.
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748.
- Yu, R.; Yang, S.; Liu, Y.; Zhu, Z. Identification and validation of serum autoantibodies in children with B-cell acute lymphoblastic leukemia by serological proteome analysis. Proteome Sci. 2022, 20, 3.
- Swellam, M.; El-Khazragy, N. Clinical impact of circulating microRNAs as blood-based marker in childhood acute lymphoblastic leukemia. Tumour Biol. 2016, 37, 10571–10576.
- Nabhan, M.; Louka, M.L.; Khairy, E.; Tash, F.; Ali-Labib, R.; El-Habashy, S. MicroRNA-181a and its target Smad 7 as potential biomarkers for tracking child acute lymphoblastic leukemia. Gene 2017, 628, 253–258.
- Bai, Y.; Zhang, H.; Sun, X.; Sun, C.; Ren, L. Biomarker identification and pathway analysis by serum metabolomics of childhood acute lymphoblastic leukemia. Clin. Chim. Acta 2014, 436, 207–216.
- Carceles-Alvarez, A.; Ortega-Garcia, J.A.; Lopez-Hernandez, F.A.; Fuster-Soler, J.L.; Ramis, R.; Kloosterman, N.; Castillo, L.; Sanchez-Solis, M.; Claudio, L.; Ferris-Tortajada, J. Secondhand smoke: A new and modifiable prognostic factor in childhood acute lymphoblastic leukemias. Environ. Res. 2019, 178, 108689.
- Vrooman, L.M.; Silverman, L.B. Treatment of Childhood Acute Lymphoblastic Leukemia: Prognostic Factors and Clinical Advances. Curr. Hematol. Malig. Rep. 2016, 11, 385–394.
- Aly, R.M.; Yousef, A.B. Prognostic significance of lymphoid enhancer-binding factor-1 expression in egyptian adult B-acute lymphocytic leukemia patients. Turk. J. Haematol. 2015, 32, 15–20.
- Gutierrez-Aguirre, C.H.; Flores-Jimenez, J.A.; Alatorre-Ricardo, J.; Cantu-Rodriguez, O.G.; Rosas-Taraco, A.; Salazar-Riojas, R.; Jaime-Perez, J.C.; Sanchez-Cardenas, M.; Lopez-Silva, L.; Martinez-Castilla, A.M.; et al. The prognostic significance of serum XCL1 concentration in patients with acute lymphoblastic leukemia: A pilot study. Ann. Hematol. 2017, 96, 2015–2024.
- Kruse, A.; Abdel-Azim, N.; Kim, H.N.; Ruan, Y.; Phan, V.; Ogana, H.; Wang, W.; Lee, R.; Gang, E.J.; Khazal, S.; et al. Minimal Residual Disease Detection in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2020, 21, 1054.
- Zhang, Q.; Shi, C.; Han, L.; Jain, N.; Roberts, K.G.; Ma, H.; Cai, T.; Cavazos, A.; Tabe, Y.; Jacamo, R.O.; et al. Inhibition of mTORC1/C2 signaling improves anti-leukemia efficacy of JAK/STAT blockade in CRLF2 rearranged and/or JAK driven Philadelphia chromosome-like acute B-cell lymphoblastic leukemia. Oncotarget 2018, 9, 8027–8041.
- Cante-Barrett, K.; Spijkers-Hagelstein, J.A.; Buijs-Gladdines, J.G.; Uitdehaag, J.C.; Smits, W.K.; van der Zwet, J.; Buijsman, R.C.; Zaman, G.J.; Pieters, R.; Meijerink, J.P. MEK and PI3K-AKT inhibitors synergistically block activated IL7 receptor signaling in T-cell acute lymphoblastic leukemia. Leukemia 2016, 30, 1832–1843.
- Tovar, C.F.; Zeron, H.M.; Romero, M.D.; Sanchez, Y.V.; Romero, I.T. Glycogen Synthase Kinase-3beta (GSK-3beta) and Nuclear Factor Kappa-B (NFKB) in Childhood Acute Lymphoblastic Leukemia. Adv. Clin. Exp. Med. 2016, 25, 1139–1147.
- Vrooman, L.M.; Silverman, L.B. Childhood acute lymphoblastic leukemia: Update on prognostic factors. Curr. Opin. Pediatr. 2009, 21, 1–8.
- Aly, R.M.; Ghazy, H.F. Prognostic significance of MSI2 predicts unfavorable outcome in adult B-acute lymphoblastic leukemia. Int. J. Lab. Hematol. 2015, 37, 272–278.
- Aguirre-Guillen, W.A.; Angeles-Floriano, T.; Lopez-Martinez, B.; Reyes-Morales, H.; Zlotnik, A.; Valle-Rios, R. Omics techniques and biobanks to find new biomarkers for the early detection of acute lymphoblastic leukemia in middle-income countries: A perspective from Mexico. Bol. Med. Hosp. Infant. Mex. 2017, 74, 227–232.
- Yan, M.; Liu, H.; Xu, J.; Cen, X.; Wang, Q.; Xu, W.; Wang, W.; Qiu, Z.; Ou, J.; Dong, Y.; et al. Expression of human Krüppel-like factor 3 in peripheral blood as a promising biomarker for acute leukemia. Cancer Med. 2020, 9, 2803–2811.
- Morad, H.M.; Abou-Elzahab, M.M.; Aref, S.; El-Sokkary, A.M.A. Diagnostic Value of (1)H NMR-Based Metabolomics in Acute Lymphoblastic Leukemia, Acute Myeloid Leukemia, and Breast Cancer. ACS Omega 2022, 7, 8128–8140.
- Hussan, S.S.; Maqsood, N.; Wang, Q.; Tao, S.; Sadaf, S. A panel of epigenetically dysregulated Wnt signaling pathway genes for non-invasive diagnosis of pediatric acute lymphoblastic leukemia. Cancer Biomark. 2021, 32, 459–470.
- Li, G.; Hu, J.; Hu, G. Biomarker Studies in Early Detection and Prognosis of Breast Cancer. Adv. Exp. Med. Biol. 2017, 1026, 27–39.
- Damanti, C.C.; Gaffo, E.; Lovisa, F.; Garbin, A.; Di Battista, P.; Gallingani, I.; Tosato, A.; Pillon, M.; Carraro, E.; Mascarin, M.; et al. MiR-26a-5p as a Reference to Normalize MicroRNA qRT-PCR Levels in Plasma Exosomes of Pediatric Hematological Malignancies. Cells 2021, 10, 101.
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2008; Volume 2.
- Cristea, I.M.; Gaskell, S.J.; Whetton, A.D. Proteomics techniques and their application to hematology. Blood 2004, 103, 3624–3634.
- Cui, J.W.; Wang, J.; He, K.; Jin, B.F.; Wang, H.X.; Li, W.; Kang, L.H.; Hu, M.R.; Li, H.Y.; Yu, M.; et al. Proteomic analysis of human acute leukemia cells: Insight into their classification. Clin. Cancer Res. 2004, 10, 6887–6896.
- Malcles, M.H.; Wang, H.W.; Koumi, A.; Tsai, Y.H.; Yu, M.; Godfrey, A.; Boshoff, C. Characterisation of the anti-apoptotic function of survivin-DeltaEx3 during TNFalpha-mediated cell death. Br. J. Cancer 2007, 96, 1659–1666.
- Aref, S.; Salama, O.; Shamaa, S.; El-Refaie, M.; Mourkos, H. Angiogenesis factor pattern differs in acute lymphoblastic leukemia and chronic lymphocytic leukemia. Hematology 2007, 12, 319–324.
- Potapnev, M.P.; Petyovka, N.V.; Belevtsev, M.V.; Savitskiy, V.P.; Migal, N.V. Plasma level of tumor necrosis factor-alpha (TNF-alpha) correlates with leukocytosis and biological features of leukemic cells, but not treatment response of children with acute lymphoblastic leukemia. Leuk. Lymphoma 2003, 44, 1077–1079.
- Zakhary, N.I.; Boshra, S.A.; El-Sawalhi, M.M.; Fahim, A.T.; Ebeid, E.N. Insulin-like growth factor system in Egyptian children with acute lymphoblastic leukemia. Genet. Test. Mol. Biomark. 2012, 16, 1067–1072.
- Pal, S.; Bandyopadhyay, S.; Chatterjee, M.; Bhattacharya, D.K.; Minto, L.; Hall, A.G.; Mandal, C. Antibodies against 9-O-acetylated sialoglycans: A potent marker to monitor clinical status in childhood acute lymphoblastic leukemia. Clin. Biochem. 2004, 37, 395–403.
- Liang, T.; Wang, N.; Li, W.; Li, A.; Wang, J.; Cui, J.; Liu, N.; Li, Y.; Li, L.; Yang, G.; et al. Identification of complement C3f-desArg and its derivative for acute leukemia diagnosis and minimal residual disease assessment. Proteomics 2010, 10, 90–98.
- Abdel-Aziz, M.M. Clinical significance of serum p53 and epidermal growth factor receptor in patients with acute leukemia. Asian Pac. J. Cancer Prev. 2013, 14, 4295–4299.
- Pane, F.; Savoia, M.; Fortunato, G.; Camera, A.; Rotoli, B.; Salvatore, F.; Sacchetti, L. Serum pseudouridine in the diagnosis of acute leukaemias and as a novel prognostic indicator in acute lymphoblastic leukaemia. Clin. Biochem. 1993, 26, 513–520.
- Zhu, S.; Xing, C.; Li, R.; Cheng, Z.; Deng, M.; Luo, Y.; Li, H.; Zhang, G.; Sheng, Y.; Peng, H.; et al. Proteomic profiling of plasma exosomes from patients with B-cell acute lymphoblastic leukemia. Sci. Rep. 2022, 12, 11975.
- Elgendi, H.M.; Mekawy, M.A.; Abdel Wahab, S.E.; Tawfik, L.M.; Ismail, E.A.; Adly, A.A. AC133 expression in egyptian children with acute leukemia: Impact on treatment response and disease outcome. J. Pediatr. Hematol. Oncol. 2010, 32, 286–293.
- Mohammadi, M.; Amirmahani, F.; Goharrizi, K.J.; Pakzad, R.; Dolat, H. Evaluating the expression level of Survivin gene in different groups of B-cell acute lymphoblastic leukemia patients of Iran. Mol. Biol. Rep. 2019, 46, 2679–2684.
- Alkhouly, N.; Shehata, I.; Ahmed, M.B.; Shehata, H.; Hassan, S.; Ibrahim, T. HLA-G expression in acute lymphoblastic leukemia: A significant prognostic tumor biomarker. Med. Oncol. 2013, 30, 460.
- Swellam, M.; Hashim, M.; Mahmoud, M.S.; Ramadan, A.; Hassan, N.M. Aberrant Expression of Some Circulating miRNAs in Childhood Acute Lymphoblastic Leukemia. Biochem. Genet. 2018, 56, 283–294.
- Luna-Aguirre, C.M.; de la Luz Martinez-Fierro, M.; Mar-Aguilar, F.; Garza-Veloz, I.; Trevino-Alvarado, V.; Rojas-Martinez, A.; Jaime-Perez, J.C.; Malagon-Santiago, G.I.; Gutierrez-Aguirre, C.H.; Gonzalez-Llano, O.; et al. Circulating microRNA expression profile in B-cell acute lymphoblastic leukemia. Cancer Biomark. 2015, 15, 299–310.
- El-maadawy, E.A.; Bakry, R.M.; Moussa, M.M.; El-Naby, S.; Talaat, R.M.J.C. Alteration in miRNAs expression in paediatric acute lymphocyticleukaemia: Insight into patients’ therapeutic response. Pharmacol. Pharm. 2021, 48, 35–43.
- Ohyashiki, J.H.; Umezu, T.; Kobayashi, C.; Hamamura, R.S.; Tanaka, M.; Kuroda, M.; Ohyashiki, K. Impact on cell to plasma ratio of miR-92a in patients with acute leukemia: In vivo assessment of cell to plasma ratio of miR-92a. BMC Res. Notes 2010, 3, 347.
- El-Khazragy, N.; Elshimy, A.A.; Hassan, S.S.; Matbouly, S.; Safwat, G.; Zannoun, M.; Riad, R.A. Dysregulation of miR-125b predicts poor response to therapy in pediatric acute lymphoblastic leukemia. J. Cell Biochem. 2018, 120, 7428–7438.
- Nemes, K.; Csoka, M.; Nagy, N.; Mark, A.; Varadi, Z.; Danko, T.; Kovacs, G.; Kopper, L.; Sebestyen, A. Expression of certain leukemia/lymphoma related microRNAs and its correlation with prognosis in childhood acute lymphoblastic leukemia. Pathol. Oncol. Res. 2015, 21, 597–604.
- Gao, Y.J.; He, Y.J.; Yang, Z.L.; Shao, H.Y.; Zuo, Y.; Bai, Y.; Chen, H.; Chen, X.C.; Qin, F.X.; Tan, S.; et al. Increased integrity of circulating cell-free DNA in plasma of patients with acute leukemia. Clin. Chem. Lab. Med. 2010, 48, 1651–1656.
- Abu Sabaa, A.; Shen, Q.; Lennmyr, E.B.; Enblad, A.P.; Gammelgard, G.; Molin, D.; Hein, A.; Freyhult, E.; Kamali-Moghaddam, M.; Hoglund, M.; et al. Plasma protein biomarker profiling reveals major differences between acute leukaemia, lymphoma patients and controls. N Biotechnol. 2022, 71, 21–29.
- Wik, L.; Nordberg, N.; Broberg, J.; Bjorkesten, J.; Assarsson, E.; Henriksson, S.; Grundberg, I.; Pettersson, E.; Westerberg, C.; Liljeroth, E.; et al. Proximity Extension Assay in Combination with Next-Generation Sequencing for High-throughput Proteome-wide Analysis. Mol. Cell. Proteom. MCP 2021, 20, 100168.
- Limijadi, E.K.S.; Budiwijono, I.; Samsuria, I.K.; Adhipireno, P.; Devi, W.R. Coagulation and Fibrinolysis Profiles of Acute Myeloblastic Leukemia: Preliminary Assessment of Hypercoagulability. Eur. J. Mol. Clin. Med. 2021, 8, 607–615.
- Seftalioglu, A.; Karakus, S. Syndecan-1/CD138 expression in normal myeloid, acute lymphoblastic and myeloblastic leukemia cells. Acta Histochem. 2003, 105, 213–221.
- Lenting, P.J.; Christophe, O.D.; Denis, C.V. von Willebrand factor biosynthesis, secretion, and clearance: Connecting the far ends. Blood 2015, 125, 2019–2028.
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756.
- Maimaitiyiming, Y.; Ye, L.; Yang, T.; Yu, W.; Naranmandura, H. Linear and Circular Long Non-Coding RNAs in Acute Lymphoblastic Leukemia: From Pathogenesis to Classification and Treatment. Int. J. Mol. Sci. 2022, 23, 4442.
- Li, Y.; Xu, J.; Shao, T.; Zhang, Y.; Chen, H.; Li, X. RNA Function Prediction. Methods Mol. Biol. 2017, 1654, 17–28.
- He, L.; He, X.; Lim, L.P.; de Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nature 2007, 447, 1130–1134.
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269.
- Qu, S.; Yang, X.; Li, X.; Wang, J.; Gao, Y.; Shang, R.; Sun, W.; Dou, K.; Li, H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015, 365, 141–148.
- Perez de Acha, O.; Rossi, M.; Gorospe, M. Circular RNAs in Blood Malignancies. Front. Mol. Biosci. 2020, 7, 109.
- Gaffo, E.; Boldrin, E.; Dal Molin, A.; Bresolin, S.; Bonizzato, A.; Trentin, L.; Frasson, C.; Debatin, K.-M.; Meyer, L.H.; te Kronnie, G.; et al. Circular RNA differential expression in blood cell populations and exploration of circRNA deregulation in pediatric acute lymphoblastic leukemia. Sci. Rep. 2019, 9, 14670.
- Chen, J.; Li, Y.; Zheng, Q.; Bao, C.; He, J.; Chen, B.; Lyu, D.; Zheng, B.; Xu, Y.; Long, Z.; et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017, 388, 208–219.
- Musharraf, S.G.; Siddiqui, A.J.; Shamsi, T.; Choudhary, M.I.; Rahman, A.U. Serum metabonomics of acute leukemia using nuclear magnetic resonance spectroscopy. Sci. Rep. 2016, 6, 30693.
- Bannur, Z.; Teh, L.K.; Hennesy, T.; Rosli, W.R.; Mohamad, N.; Nasir, A.; Ankathil, R.; Zakaria, Z.A.; Baba, A.; Salleh, M.Z. The differential metabolite profiles of acute lymphoblastic leukaemic patients treated with 6-mercaptopurine using untargeted metabolomics approach. Clin. Biochem. 2014, 47, 427–431.




