Acute lymphoblastic leukemia (ALL) is a hematological disease characterized by the dysfunction of the hematopoietic system that leads to arrest at a specific stage of stem cells development, suppressing the average production of cellular hematologic components. BCP (B-cell progenitor)-ALL is a neoplasm of the B-cell lineage progenitor. BCP-ALL is caused and perpetuated by several mechanisms that provide the disease with its tumor potential and genetic and cytological characteristics. These pathological features are used for diagnosis and the prognostication of BCP-ALL. The BCP-ALL diagnostic protocol is well established. Firstly, it is necessary to demonstrate ≥ 20% lymphoblasts in bone marrow (BM) based on a BPM. Second, a hematopathological review is performed; it comprises a morphological assessment, and flow cytometric and genetic characterization.
- biomarkers
- circulating
- acute lymphoblastic leukemia
- BCP-ALL
1. Introduction
2. Diagnostic Biomarkers for BCP-ALL
The BCP-ALL diagnostic protocol is well established. Firstly, it is necessary to demonstrate ≥ 20% lymphoblasts in BM based on a BPM. Second, a hematopathological review is performed; it comprises a morphological assessment, and flow cytometric and genetic characterization. Once these studies are completed, a diagnosis according to the WHO classification can be made [12]. The BCP-ALL classification integrates morphology, immunophenotyping, and genetics/cytogenetics. There are no morphological features to distinguish between the BCP-ALL and TCP-ALL. Nevertheless, some lymphoblast characteristics are relevant: scant cytoplasm, size, and shape, wide relatively dispersed chromatin, and nuclear, and nucleolar peculiarities [34][59]. The immunophenotyping in BCP-ALL show some markers as almost always positive, namely CD19, cCD79a, cCD22, CD22, CD24, PAX5, and TdT; CD20, CD34, CD13, and CD33 expression is variable. Finally, among the genetic abnormalities BCR—ABL1, KMT2A-rearranged, ETV6-RUNX1, hyperdiploidy, hypodiploidy, IGH/IL3, TCF3-PBX1, BCR-ABL1-like, and iAMP21 can be mentioned [5]. The following circulating biomarkers have shown promise for either the diagnosis or prognosis of the illness.2.1. Proteins Type BCP-ALL Biomarkers
Proteins are helpful biomarkers because they have multiple crucial functions, making them central in biological systems [35][36][48,60]. In an individual manner, tumor necrosis factor α (TNF-α) is among the most studied biomarkers for ALL. TNF-α is a cytokine that induces apoptosis by inhibiting the activity of caspases [37][61]. Ahmed et al. evaluated its levels in serum from adult patients with ALL, comparing patients who received chemotherapy to those who did not (Table 1). Based on receiver operating characteristic (ROC) curve analysis, TNF-α showed good ALL diagnostic utility, an area under the ROC curve (AUC) of 0.94, a sensitivity of 91.7%, and a specificity of 100% [3]. These reports are consistent with the findings reported by Aref et al., who found an increase in TNF-α levels among patients newly diagnosed and in remission compared with controls [38][62]. Ahmed et al., was able to confirm this using an ROC curve, even though Aref’s cohort of patients had both BCP-ALL and BCP-CLL. These findings indicate that TNF-α can be a valuable tool in diagnosing ALL [3][38][39][3,62,63]. Table 1 summarizes the markers whose diagnostic values for ALL and/or BCP-ALL have been determined.| Biomarker | Blood Sample | Leukemia Type | Area under the ROC Curve | Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Reference |
|---|---|---|---|---|---|---|---|---|
| Smad 7 | Serum | ALL | 0.81 | 63 | 100 | 100 | 73 | |
| TGF-β1 | Serum | ALL | 0.79 | 57 | 93 | 89.5 | 68 | [16] |
| Smad 7 TGF-β1 miR-181a |
Serum | ALL | - | 100 | 93 | 93.7 | 100 | |
| IGF-I | Serum | ALL | - | 60.6 | 73.3 | - | - | [40][64] |
| IGF-II | Serum | ALL | - | 72.2 | 73.3 | - | - | |
| IGFBP-2 | Serum | ALL | - | 72.2 | 86.7 | - | - | |
| IGFBP-3 | Serum | ALL | - | 93.9 | 93.9 | - | - | |
| Anti-9-0AcSGs | Serum | ALL-GI | - | 98.9 | 92.1 | 96.8 | 97.2 | [41][65] |
| Anti-9-0AcSGs | Serum | ALL-GI | - | 96.8 | 95.9 | 96.8 | 95.9 | |
| PF4 CTAP-II |
Serum | ALL | - | 91.8 | 90 | - | - | [7] |
| C3f | Serum | AL | 0.99 | 97 | 100 | - | - | [42][45] |
| TNF-α | Serum | ALL | 0.94 | 91.7 | 100 | - | - | [3] |
| Survivin | Serum | ALL | 0.98 | 90 | 80 | - | - | |
| p53 | Serum | AL | 0.8 | 52 | 100 | - | - | [43][66] |
| EGFR | Serum | AL | 0.93 | 73.9 | 95.8 | - | - | |
| Pseudouridine | Serum | ALL | - | 90 | 97.5 | - | - | [44][67] |
| ADAM 17 | Plasma | BCP-ALL | 0.98 | 100 | 100 | - | - | [45][68] |
| ATG3 | Plasma | BCP-ALL | 0.95 | 100 | 100 | - | - | |
| AC133 * | Whole blood | ALL | - | 100 | 100 | 100 | 100 | [46][69] |
| miR-181a | Serum | ALL | 0.93 | 86.5 | 93.3 | 92.8 | 87.5 | [16] |
| miR-146a | Plasma | BCP-ALL | 1 | 100 | 100 | - | - | [9] |
| mRNA Survivin | Whole blood | BCP-ALL | 0.85 | 95 | 95 | - | - | [47][70] |
| mRNA HLA-G | PBMC | ALL | - | 74 | 100 | - | - | [48][71] |
| miR-125b-1 | Serum | ALL | 0.85 | 83.7 | 100 | - | - | [49][72] |
| miR-203 | Serum | ALL | 0.87 | 97.7 | 87 | - | - | |
| miR-100 | PBMC | ALL | 0.87 | 82.7 | 100 | - | - | [15] |
| miR-196a | PBMC | ALL | 0.537 | 46.6 | 100 | - | - | |
| miR-146a | PBMC | ALL | 1 | 100 | 100 | - | - | |
| miR-511 | Plasma | BCP-ALL | 1 | 100 | 100 | 1 | 1 | [50][73] |
| miR-34a | Plasma | BCP-ALL | 0.98 | 92 | 100 | 1 | 0.70 | |
| miR-22 | Plasma | BCP-ALL | 0.91 | 79 | 100 | 1 | 0.54 | |
| miR-26a | Plasma | BCP-ALL | 0.91 | 79 | 100 | 1 | 0.47 | |
| miR-221 | Plasma | BCP-ALL | 0.92 | 83 | 100 | 1 | 0.54 | |
| miR-223 | Plasma | BCP-ALL | 0.93 | 89 | 100 | 1 | 0.64 | |
| miR-21 | PBMC | ALL | 0.565 | 44 | 55 | - | - | [51][74] |
| miR-26 | PBMC | ALL | 0.464 | 54 | 50 | - | - | |
| miR-148a | PBMC | ALL | 0.719 | 74 | 79 | - | - | |
| miR-133b | PBMC | ALL | 0.669 | 70 | 60 | - | - | |
| miR-24 | PBMC | ALL | 0.785 | 72 | 81 | - | - | |
| miR-92a | PBMC and plasma | ALL | 0.99 | - | - | - | - | [52][75] |
| miR-92a | Plasma | ALL | 0.755 | 41.5 | 100 | 100 | 36.7 | [6] |
| miR-638 | Plasma | ALL | 0.86 | 54.7 | 100 | 100 | 42.9 | |
| miR-125b | PBMC | ALL | 0.99 | 98 | 96.7 | - | - | [53][76] |
| mRNA-Bcl-2 | PBMC | ALL | 0.9 | 96.7 | 70 | - | - | |
| miR-128b | PBMC | ALL | - | 75 | 87.5 | - | - | [54][77] |
| cf-DNA levels | Plasma | ALL, AML | 0.79 | 65 | 100 | - | - | [55][50] |
| cf-DNA integrity | Plasma | ALL, AML | 88 | 78 | 90 | - | - |
2.2. RNA Type BCP-ALL Biomarkers
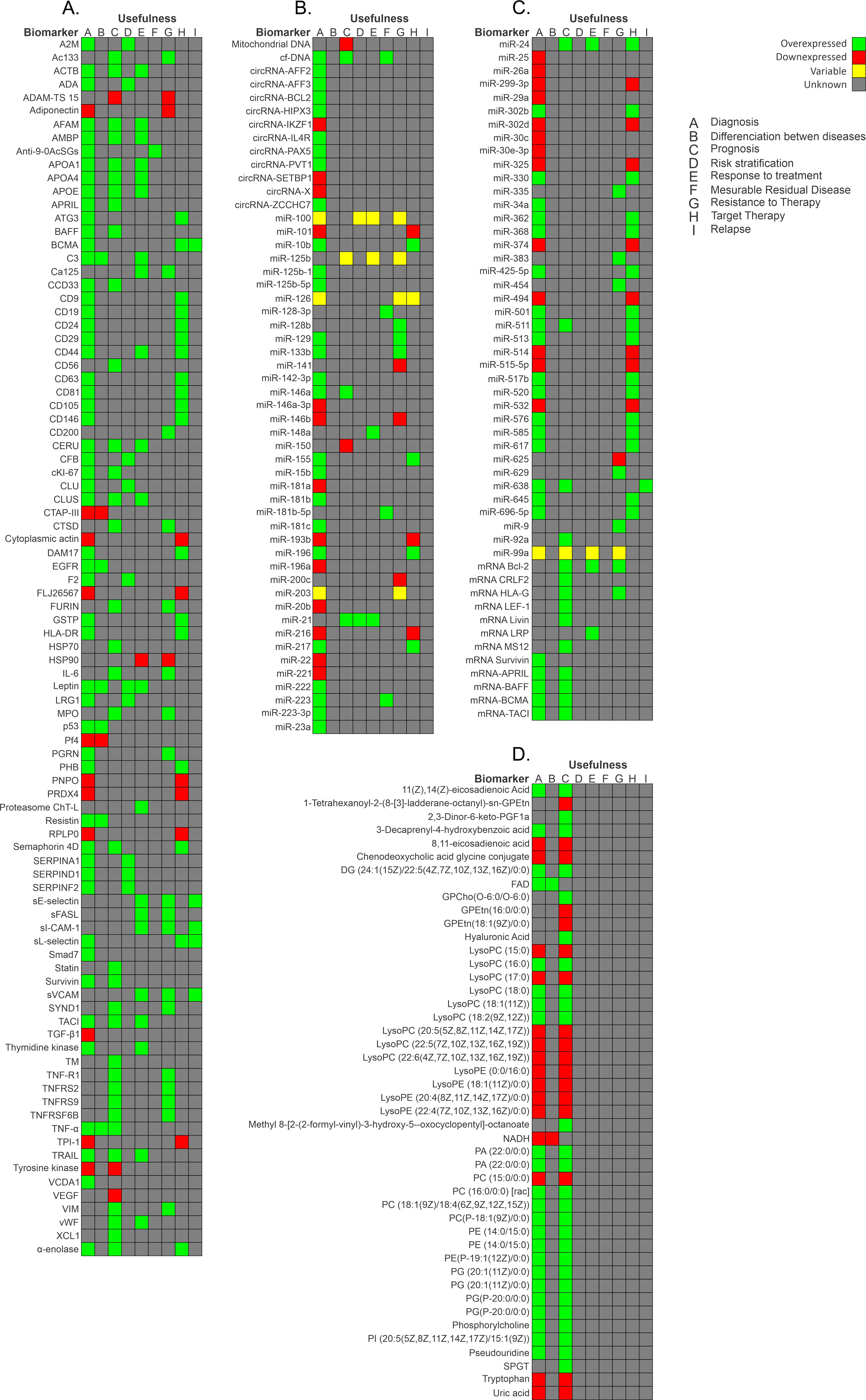
RNA can generate numerous proteins through protein-coding regions. Most genomic sequences can be transcribed into protein-coding RNA. In contrast, the non-coding parts are transcribed to produce non-coding RNAs (ncRNAs) [62][63][84,85]. Based on their size, location, and interacting partners, ncRNAs are classified into several types: transfer RNAs (tRNAs), ribosome RNAs (rRNAs), small nucleolar RNAs (snoRNAs), small nuclear RNAs (snRNAs), microRNAs (miRNAs), small interfering RNAs (siRNAs), long non-coding RNAs (lncRNAs), circular RNA (circRNAs), Piwi-interacting RNAs (piRNAs), and enhancer RNAs (eRNAs) [62][84]. It has been discovered that numerous microRNAs are essential in the initiation, progression, and metastasis of cancer [64][86]. miRNAs are stable in serum and display distinct expression patterns in healthy individuals and those with cancer. Hence, they are suitable biomarkers for cancer detection and prognosis [32]. Furthermore, one miRNA can modulate the expression of several genes, and there are many miRNA expression patterns in patients with ALL [65][87]. In a study carried out in Mexico, the authors evaluated the diagnostic usefulness of the circulating miRNA expression profile in plasma samples from patients with BCP-ALL. miR-511 showed the highest mean overexpression (FC: 159.5, p = 0.002), while miR-199a-3p was the most under-expressed (FC: −13.48, p < 0.001). ROC curves analysis provided good values for miR-511 (cut-off = 9.458, specificity 1, sensitivity 1, AUC = 1), miR-34a (cut-off = 7.179, specificity 1, sensitivity 0.92, AUC = 0.98), miR-222 (cut-off = −0.1325, AUC = 0.91), miR-26a (cut-off = 2.073, AUC = 0.91), miR-221 (cut-off = −0.1861, AUC = 0.92), and miR-223 (cut-off −4.309, specificity 1, sensitivity 0.89, AUC = 0.93). Moreover, pathway analysis revealed that mainly Wnt, MAPK, TGF-beta, p53, Jak-STAT, NOTCH, and B- and T-cell receptor signaling pathways are activated by the evaluated miRNAs (miR-511, miR-19b, miR-195, miR-565, miR-34a, miR-222, miR-363, miR-181a, miR-181c, miR-199a-3p, miR-340, miR-335, miR-99b, miR-221, miR-744, miR-223, miR-26a, miR-224, and miR-151-3p). These miRNAs have value as markers for diagnosis and to identify therapeutic targets for BCP-ALL [50][73]. CircRNAs are a type of non-coding RNA and represent a recent research hotspot in the field of RNA. circRNAs form covalently closed loop structures with neither 5′–3′ polarities nor polyadenylated tails and lengths between 100 to thousands of nucleotides [66][67][88,89]. circRNAs can modulate miRNA-target expression, acting like miRNA axes. Moreover, they can interact with RNA-binding proteins and regulate cellular processes [68][90]. Circular RNA (circRNA) expression in PBMCs of pediatric patients with BCP-ALL has also been evaluated [68][90]. After the comparison of circRNA expression profiles in B- and T-cells, and monocyte populations, which was validated by reverse transcriptase quantitative polymerase chain reaction (RT-qPCR), four circRNAs were more highly expressed in healthy donors than in patients with BCP-ALL: circIL4R (p < 0.0001), circZCCH7 (p = 0.0307), and circX (intergenic) (p = 0.076). Furthermore, circPVT1 (p = 0.0002), circHIPK3 (p < 0.0001), circPAX5 (p < 0.0001), and circAFF3 (p = 0.0115) were overexpressed in patients with BCP-ALL. The expression of the target set of circRNAs according to BCP-ALL cytogenetic subtype showed circAFF2 was highly expressed in TCF3-PBX1 (p = 0.0252), BCP-ALL, and to a lesser extent in ETV6- RUNX1 BCP-ALL (p = 0.021). CircBCL2 (intronic) was upregulated in ETV6-RUNX1 fusions (p = 0.0166), circSETBP1 and circX (intergenic) were significantly reduced in MLL rearranged samples (p = 0.0274 and p = 0.0472, respectively), and circIKZF1 was lower in BCP-ABL and hyperdiploid leukemias than in the ETV6-RUNX1 subtype (p = 0.0154), in which the expression was conserved at levels comparable to B-cells. Overall, circRNAs have the potential to regulate specific cell functions, cell differentiation, maturation stages, and the growth of leukemic cells. As circRNA deregulation has been observed in patients with BCP-ALL, based on their cytogenetic subtype and previous cancers, circRNAs could potentially serve as markers for BCP-ALL. However, further research is necessary to determine the exact role that circRNAs play in leukemogenesis and their potential as markers for the disease [68][69][90,91]. Figure 1 shows a summary of the biomarkers associated with BCP-ALL grouped as protein markers (Figure 1A), nucleic acid molecules (Figure 1B,C), metabolites (Figure 1D), and according to the different stages of disease.